Abstract
Purpose: This study aimed to evaluate the safety and effectiveness of microwave-ablation-assisted liver resection (MW-LR) and clamp crushing liver resection (CC-LR) in cirrhotic patients with hepatocellular carcinoma (HCC).
Materials and methods: From July 2005 to January 2015, cirrhotic HCC patients who underwent CC-LR (n = 191) or MW-LR (n = 112) were retrospectively analysed. We compared morbidity, mortality, disease-free survival (DFS) time and overall survival time between the CC-LR and MW-LR groups.
Results: The blood loss volume was significantly higher in the CC-LR group (mean of 752 ml) than that in the MW-LR group (mean of 253 ml, p < 0.001). The abdominal abscess rate was higher in the MW-LR group (8.9%) than that in the CC-LR group (3.1%, p = 0.029). The 30-day mortality rate (1.5% vs. 0.8%) and postoperative complication rate (32.9% vs. 25.0%) were both similar between the CC-LR and MW-LR groups. MW-LR provided a survival benefit over CC-LR at 1, 3 and 5 years in the entire population (93.5% vs. 87.0%, 77.0% vs. 62.5% and 50.0% vs. 36.5%, respectively; p = 0.003). In a subgroup analysis, MW-LR provided a survival benefit over CC-LR for Barcelona Clinic Liver Cancer stage A (BCLC-A) HCC (p = 0.026) and stage B (BCLC-B) HCC (p = 0.035) patients and provided DFS benefits for BCLC-A HCC patients (p = 0.036).
Conclusions: MW-LR is a safe and feasible procedure for HCC patients with a cirrhotic liver history.
Introduction
Hepatocellular carcinoma (HCC) is a frequently occurring cancer and is the third most common cause of cancer death worldwide [Citation1]. Most HCCs are associated with chronic hepatitis and cirrhosis induced by infection with hepatitis B or C virus [Citation2,Citation3]. For cirrhotic patients with HCC, surgical resection remains the preferred curative modality. However, the liver reservoir in cirrhotic patients is usually insufficient, and mortality and morbidity after liver resection (LR) are common [Citation2,Citation3].
The Barcelona Clinic Liver Cancer (BCLC) staging system is a widely accepted system for HCC staging. This system considers three variables, namely, tumour burden, liver function status and health status [Citation4–6]. In the BCLC staging system, LR is recommended for BCLC stage A (BCLC-A) HCC. Transarterial chemoembolisation (TACE) is recommended for BCLC stage B (BCLC-B) or C (BCLC-C) HCC. However, controversy exists regarding the treatment of BCLC-B or BCLC-C HCC with LR [Citation7,Citation8]. Many researchers have reported that LR is feasible for large nodular HCCs with a diameter >5 cm and is more effective than TACE in treating BCLC-B HCC [Citation9]. In contrast, some studies have shown that the mortality and morbidity rates of LR were high for large HCCs [Citation3].
Patients with normal livers can tolerate a remnant liver volume of 20–30%. However, patients with underlying cirrhosis can only tolerate a remnant liver volume of 40–50%. Unfortunately, most HCC cases involve underlying cirrhosis. In addition, liver transection can be difficult under cirrhotic conditions. The traditional LR procedure is called clamp crushing LR (CC-LR) and is commonly performed with the Pringle procedure [Citation2,Citation10], which is an operative manoeuvre during liver surgery that involves intermittent clamping of the inflow vascular pedicle. However, inflow occlusion can lead to ischaemic reperfusion injury of the liver, especially in cirrhotic patients [Citation2]. Recent innovations, such as the Cavitron Ultrasonic Surgical Aspirator (CUSA), bipolar diathermy, staplers and LigaSure, are used to prevent bleeding during parenchymal transection. However, all the above-mentioned devices are unsuitable under cirrhotic conditions [Citation2]. Many studies have shown that LR for HCC treatment results in a mean blood loss of 600–1000 ml and that the Pringle manoeuvre is necessary intraoperatively [Citation10–12]. Blood loss, transfusion and inflow occlusion are risk factors for postoperative morbidity and mortality and are related to tumour recurrence in HCC [Citation12–14]. Nevertheless, an ideal LR technique with minimal blood loss and improved resection margins and without the use of Pringle procedures does not exist.
LR using heat coagulative necrosis through microwave (MW) energy or radiofrequency (RF) energy has been widely adopted worldwide [Citation15–21]. Compared with traditional LR, this precoagulated-assisted LR has shown some advantages of less blood loss, lower transfusion rates and fewer Pringle procedures. Precoagulated-assisted LR is recommended for cirrhotic patients [Citation19], but its use remains controversial [Citation22–24]. Some studies have reported that precoagulated-assisted LR causes severe postoperative complications, such as abdominal abscess, biliary fistula and biliary injury [Citation22–24]. The safety of microwave-assisted LR (MW-LR) in cirrhotic patients with HCC requires further clarification. MW- or RF-assisted LR results in minimal blood loss and improved resection margins. Therefore, this procedure may theoretically favour the survival of HCC patients. The long-term outcomes after MW-LR for cirrhotic patients with HCC also need to be elucidated. In the past 11 years, MW-LR has been performed in some cases of HCC in our unit. In the present study, the efficiency and safety of MW-LR were retrospectively assessed in cirrhotic patients with HCC.
Methods
Ethics statement
Written informed consent for data collection and the use of devices was obtained from all patients. The patients were sufficiently informed of the risks, benefits and alternatives to hepatic resection. The study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki (as revised in Brazil in 2013). All procedures were approved by the Ethical Committee of Renmin Hospital of Wuhan University.
Patients
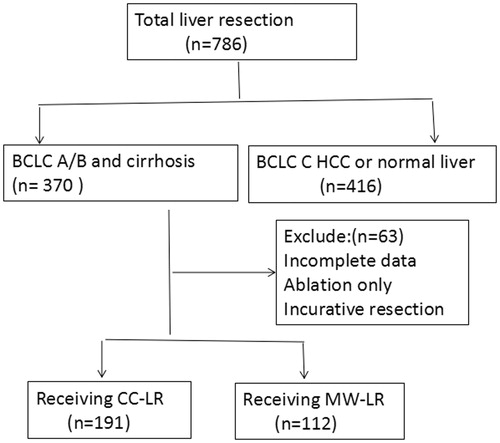
From July 2005 to January 2015, a total of 786 HCC patients underwent curative LR at the Hepatobiliary Department of Renmin Hospital of Wuhan University. Among these patients, only those with BCLC-A/B HCC and cirrhosis were included in the retrospective analysis (). We also excluded patients who received noncurative LR or only local ablation or those with incomplete follow-up data. The remaining patients were treated with either CC-LR or MW-LR. All patients underwent careful preoperative assessments of their conditions through spiral computed tomography (CT), magnetic resonance imaging (MRI) and/or positron emission tomography. Preoperative discussions were conducted for all cases. HCC was confirmed after LR by a histopathological examination of surgical samples in all patients. We retrospectively analysed prospectively collected data, including demographic details, the nature and number of tumours, surgical procedures, intraoperative data, postoperative complications, 30-day hospital mortality rates, disease-free survival (DFS) and overall survival (OS) rates. The inclusion criteria for the operations were as follows: curative intention of resection, no extrahepatic metastasis, no main vascular invasion, cirrhosis, Child–Pugh liver function A and BCLC-A/B HCC.
BCLC-A HCC was characterised by a single nodule with a diameter <5 cm or two to three masses with diameters <3 cm without extrahepatic metastasis and main vascular invasion. By contrast, BCLC-B was characterised by a single nodule with a diameter >5 cm, two to three masses with diameters >3 cm, or >3 masses with any diameter. The masses had to be devoid of extrahepatic metastasis and main vascular invasion. Finally, BCLC-C HCC was characterised similarly to BCLC-B HCC, but with main vascular invasion [Citation6].
Surgical procedure
Surgery was performed in cirrhotic patients with BCLC-A/B HCC. An adequate remnant liver volume, as determined by CT or MRI, was >50% for HCC patients with cirrhosis or severe fatty liver disease. The operation was performed by two surgeons (Z. Chen and Y. Ding) with more than 20 years of experience in liver surgery who were equally skilled in both MW ablation and liver surgery. All cases were randomly assigned numbers preoperatively for Group A (even numbers) or Group B (odd numbers). The patients in Group A were informed about the benefits and risks of MW-LR and were assigned to the MW-LR group upon request. Otherwise, the patients were assigned to the CC-LR group. The patients in Group B were assigned to the CC-LR group. Among 191 patients who were assigned to the MW-LR group, 132 (69.1%) accepted the assignment.
CC-LR was performed following the technique described by Zhou et al. [Citation25]. Briefly, a modified right or bilateral subcostal incision was performed under general anaesthesia. The peritoneal cavity was examined, and intraoperative ultrasound was performed to reveal any previously undetected lesions. The liver was then mobilised based on lesion size and site. The Pringle manoeuvre was carried out for 15 min each time at 5-min intervals. A drain was placed at the resection site.
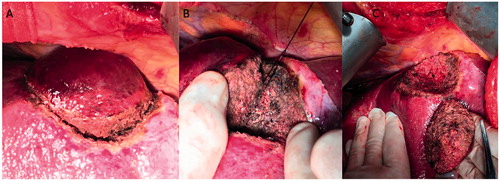
MW-LR was performed similarly to Habib’s technique () [Citation26]. In brief, the liver was mobilised as performed with the CC-LR procedure. An intended resection line was marked 2 cm away from the tumour edge on the liver capsule with diathermy. Subsequently, coagulated necrosis was induced along the intended resection line using the MW probe and a 2450-MHz MW generator (ECO Microwave System Co., Ltd., Nanjing, China). The probe contained 15-cm needle electrodes and two coaxial cannulas through which chilled water was circulated during ablation to prevent tissue carbonisation. After complete ablation, the coagulated tissues of the liver overlapped and formed a coagulated zone surrounding the tumour. The coagulated liver tissue was easily removed with gentle crushing by forceps or a clamp. Then, the vessels were separately dissected and ligated. Finally, liver parenchymal transection was completed with a gentle crushing transection along the intended line. The method left a 1-cm area of coagulative tissue around the tumour sample and a 1-cm coagulative zone along the LR margin. A drain was placed at the resection site.
Figure 2. MW-LR procedure. (A) Parenchymal transection after precoagulation with a microwave probe along the intended line marked 2 cm away from the edge of a tumour on the liver capsule. (B) Dissection and ligation of vessels across the transection plane. (C) The sample and surgical margin after transection.
Postoperative complications were graded in accordance with the Clavien-Dindo classification, and complications of grade 2 or above were analysed [Citation27]. Biliary leakage was defined as either biliary drainage five days after surgery or biliary collection confirmed by percutaneous drainage. Abdominal abscess was defined as a fluid collection with an inflammatory reaction that required percutaneous drainage.
Follow-up
Follow-up examinations consisted of ultrasonography and/or helical CT of the liver and serial assessments of alpha-fetoprotein (AFP) levels 1 month after surgery, every 3 months in the first 2 years and every 6 months thereafter. The diagnoses of tumour recurrence and distant metastasis were based on cytohistology or the non-invasive diagnostic criteria for HCC used by the European Association for the Study of the Liver and were defined as the appearance of a new lesion with radiological features that are characteristic of HCC. In patients who showed resectable intrahepatic metastasis after initial treatment, LR was performed when feasible based on liver function and remnant liver volume, which were evaluated according to the same criteria as those used at the time of initial resection. If LR could not be performed because of poor liver function or other unfavourable factors, TACE, MW or RF ablation, or sorafenib therapy was applied.
The width of the resection margin was defined as the shortest macroscopic distance from the edge of the tumour to the line of transection. The narrow margin group consisted of patients with a margin width less than 1 cm. The wide margin group of patients had a margin width of 1 cm or more. These two groups were compared for recurrence rates. Resection was deemed curative if macroscopic tumour clearance was achieved. Patients were classified into those with a negative (R0) or a positive (R1) microscopic margin. Non-curative cases were excluded with macroscopic residual tumour (R2) and the following resections: (1) LR with simultaneous local ablation of HCC that could not be removed surgically; and (2) LR with portal and/or hepatic venous thrombus. A positive microscopic margin (R1) was defined as the presence of tumour cells at the transection plane as detected by histological examination in the CC-LR group or the presence of tumour cells at the transection plane beyond the coagulative zone in the MW-LR group.
The site of intrahepatic recurrence was defined as follows: (i) recurrence at the transection plane, located less than 2 cm from the resection margin, regardless of any simultaneous recurrence in the distant liver remnant; (ii) recurrence somewhere other than the resection plane; and (iii) advanced recurrence, defined as recurrence with four or more lesions and/or portal vein invasion.
Study objective
Determining total survival time was the primary objective of this study. Survival time was defined as the time from the date of surgery to the date of death. The patients who were alive at the end of the follow-up were censored. The second objective of the study was determination of OS. The third objective of the study was assessment of DFS time, which was defined as the time between the date of surgery and the date of recurrence.
Statistical analysis
Continuous variables are expressed as the mean ± SD and were compared using Student’s t-test. Categorical variables were compared using the χ2, Fisher’s exact and Mann–Whitney U tests as appropriate. All statistical tests were two-sided, and a difference was considered statistically significant when p < 0.05. OS and DFS rates were calculated using the Kaplan–Meier method. Statistical analyses were performed using SPSS 19.0 statistical software for Windows (SPSS, Chicago, IL).
Results
Study population
During the study period, 786 consecutive patients with HCC underwent curative LR and were enrolled in the database. Among these patients, 370 exhibited BCLC-A/B HCC with cirrhosis. We excluded 41 patients (11.0%) who underwent local ablation or noncurative resection and 26 patients (8.5%) with incomplete data. The remaining 303 patients (81.8%) were enrolled in this study. Among these patients, 191 (51.6%) underwent CC-LR and 112 (30.2%) underwent MW-LR (). Among the 191 patients in the CC-LR group, 65 (34.0%) underwent surgery between 2005 and 2010 and 126 (66.0%) underwent surgery between 2011 and 2015. Meanwhile, 28.6% of the MW-LR patients underwent surgery between 2005 and 2010 and 71.4% underwent surgery between 2011 and 2015. There were no differences in the proportions of patients who underwent surgery during different periods between the CC-LR and MW-LR groups (p = 0.060) ().
Table 1. Comparison of the clinicopathological features between the MW-LR and CC-LR groups.
Clinical pathological data
The demographic and clinicopathological data of the 303 HCC patients are listed in . All clinical characteristics were similar between the two groups at baseline (). Age, gender distribution, surgical period, tumour number, tumour size, tumour aetiology, prothrombin time, Edmondson grade, total bilirubin, and the levels of AFP, albumin, and alanine aminotransferase showed no significant differences.
Morbidity and mortality
The 30-day mortality (1.5% vs. 0.8%) and postoperative complication rates (32.9% vs. 25.0%) were both similar between the CC-LR and MW-LR groups (). However, the abdominal abscess rate was higher in the MW-LR group (8.9%) than that in the CC-LR group (3.1%, p = 0.002). The pleural effusion rate was higher in the CC-LR group (10.4%) than that in the MW-LR group (2.6%, p = 0.024). The blood loss volume was significantly higher in the CC-LR group (mean of 752 ml) than that in the MW-LR group (mean of 253 ml, p < 0.001). The Pringle manoeuvre was less frequently used in the MW-LR group (29.4%) than in the CC-LR group (94.7%, p < 0.001). The blood transfusion rate was significantly lower in the MW-LR group (18.7%) than that in the CC-LR group (67.5%, p < 0.001). The narrow margin was significantly greater in the MW-LR group (79.5%) than that in the CC-LR group (47.1%, p = 0.000) (). The R1 resection rate was significantly greater in the CC-LR group (12.5%) than that in the MW-LR group (5.3%, p = 0.020) ().
Table 2. Comparison of operative variables and postoperative outcomes between the MW-LR and CC-LR groups.
Survival analysis in the entire population
During a mean follow-up period of 44.2 months (13–97 months), the OS rate was significantly better in the MW-LR group (n = 112) than that in the CC-LR group (n = 191) (). The 1-, 3- and 5-year OS rates of the patients in the MW-LR group were 93.5%, 77.0% and 50.0%, respectively, and the corresponding rates in the CC-LR group were 87.0%, 62.5% and 36.5%, respectively (p = 0.003). The median survival times were 50.9 and 36.7 months in the MW-LR and CC-LR groups, respectively.
Figure 3. OS and DFS curves of patients in the MW-LR and CC-LR groups. (A) MW-LR provided a survival benefit over CC-LR at 1, 3 and 5 years (93.5% vs. 87.0%, 77.0% vs. 62.5% and 50.0% vs. 36.5%, respectively; p = 0.003). (B) MW-LR has a similar DFS benefit to that of CC-LR (p = 0.103).
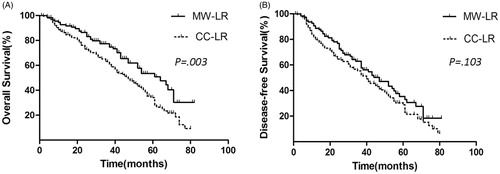
The DFS rate was not significantly different between the MW-LR group (n = 112) and the CC-LR group (n = 191) (p = 0.103) (). The 1-, 3- and 5-year DFS rates of the patients in the MW-LR group were 89.5%, 63.5% and 35.5%, respectively, and the corresponding rates in the CC-LR group were 80.0%, 54.5% and 30.0% (p = 0.103), respectively.
Patients were classified into narrow margin and wide margin subgroups within the CC-LR and MW-LR groups. Analyses revealed no significant correlation between the width of the resection margin (margin width of <1 cm vs. >1 cm) and postoperative 5-year DFS in any patient subgroup ().
Table 3. Subgroup analyses of the effects of resection margin width on 5-year disease-free survival.
Subgroup survival analysis according to BCLC classification
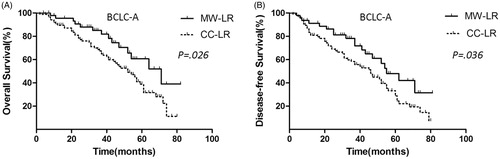
For BCLC-A HCC, the OS rate of the MW-LR group (n = 47) was significantly superior to that of the CC-LR group (n = 88) (). The 1-, 3- and 5-year OS rates of the patients in the MW-LR group were 96.1%, 85.0% and 49.9%, respectively, and the corresponding rates in the CC-LR group were 90.0%, 68.5% and 38.5%, respectively (p = 0.026).
Figure 4. OS and DFS curves of patients with BCLC-A HCC in the MW-LR and CC-LR groups. (A) MW-LR provided a survival benefit over CC-LR (p = 0.026). (B) MW-LR provided a DFS benefit over CC-LR (p = 0.036).
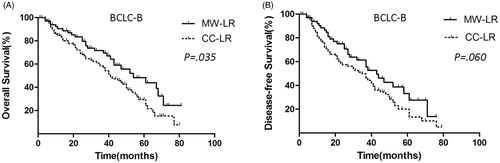
For BCLC-B HCC, the OS rate was significantly better in the MW-LR group (n = 65) than that in the CC-LR group (n = 103) (). The 1-, 3- and 5-year OS rates of the patients in the MW-LR group were 91.1%, 70.0% and 45.5%, respectively, and the corresponding rates in the CC-LR group were 85.2%, 59.1% and 30.5%, respectively (p = 0.035).
Figure 5. OS and DFS curves of patients with BCLC-B HCC in the MW-LR and CC-LR groups. (A) MW-LR provided a survival benefit over CC-LR (p = 0.035). (B) MW-LR has a similar DFS to that of CC-LR (p = 0.060).
For BCLC-A HCC, the DFS rate was significantly better in the MW-LR group (n = 47) than that in the CC-LR group (n = 88) (). The 1-, 3- and 5-year DFS rates of the patients in the MW-LR group were 91.1%, 72.0% and 47.5%, respectively, and the corresponding rates in the CC-LR group were 82.5%, 59.5% and 35.1%, respectively (p = 0.036).
For BCLC-B HCC, the DFS rate was not significantly different between the MW-LR group (n = 65) and the CC-LR group (n = 103) (). The 1-, 3- and 5-year DFS rates of the patients in the MW-LR group were 88.5%, 62.5% and 33.5%, respectively, and the corresponding rates in the CC-LR group were 80.0%, 45.1% and 20.5%, respectively (p = 0.060).
Comparison of recurrence patterns in the CC-LR and MW-LR groups
During the follow-up period, recurrence developed in 134 patients (70.1%) in the CC-LR group and in 63 patients (56.3%) in the MW-LR group. There were no significant differences between the two groups with respect to the type of recurrence (p = 0.472) and the site of intrahepatic recurrence (p = 0.212) (). Tumours mostly reappeared within 5 years (162/197, 82.2%). Recurrence in the resection plane was more frequent in the CC-LR group (22.8%) compared with the MW-LR group (11.5%), but the difference was not significant (p = 0.212).
Table 4. Comparison of recurrence patterns in CC-LR and MW-LR patients who suffered from recurrence.
Discussion
Significant technical advances have been achieved for LR, including clamp crushing, the Pringle manoeuvre, the CUSA, staplers and bipolar diathermy. For noncirrhotic livers, LR procedures have been successfully performed with the abovementioned techniques. However, for cirrhotic livers, surgeons face a significant challenge regarding how to perform LR with minimal blood loss and limited inflow occlusion times. The clamp crushing technique should be carried out with inflow occlusion for 15 min each time, which could cause serious ischaemic reperfusion injury in the cirrhotic liver. The CUSA technique also has disadvantages and may cause heavy bleeding in the cirrhotic liver. Most HCCs are associated with chronic hepatitis and cirrhosis. A series of studies reported that LR for HCC resulted in blood loss as high as 600–1000 ml [Citation10–12]. Blood transfusion and inflow vascular clamping are risk factors for morbidity and mortality [Citation10–12]. Intraoperative blood loss also affects long-term survival in HCC [Citation10–12].
Precoagulated-assisted LR, including RF-assisted or MW-assisted techniques, have been reported in some studies with advantages of minimal blood loss and few inflow occlusions. RF-LR with a single-needle coagulator was first reported by Weber et al. [Citation26]. A bipolar coagulator was developed later, which is currently the most widely available tool for precoagulated LR [Citation19,Citation28–30]. MW-LR is another precoagulated method used by some surgeons. We also used this procedure in this study. RF-LR or MW-LR was successfully performed with minimal blood loss and limited Pringle manoeuvre times for cirrhotic patients with HCC in some reports [Citation15–19,Citation31]. In the present study, intraoperative blood loss was reduced to 253 ml. The Pringle procedure was used in 33 cases (29.4%), and blood transfusion was conducted in 21 patients (18.7%). Our results were consistent with those of other reports on precoagulated LR using RF or MW energy. Curro et al. reported RF-assisted LR with a mean blood loss of 165 ml in 55 consecutive cirrhotic patients with HCC [Citation19]. Sasaki reported their use of an MW tissue coagulator to perform LR. The group obtained a mean blood loss of 250 ml in 1118 cases of HCC (59% cirrhosis) [Citation15]. Therefore, MW-assisted LR with minimal blood loss is a safe procedure for HCC patients with cirrhosis.
Postoperative bile complications remain controversial in the performance of precoagulated LR. The rate of bile leakage varied from 0% to 10.5% in a series of studies in HCC patients with underlying cirrhosis () [Citation15–19,Citation31]. Another issue associated with coagulated-assisted LR is infectious complications. The rate of abdominal abscesses varies from 0% to 9.0% () [Citation15–19,Citation31]. In our study, the rate of biliary leakage was 7.1%, and the rate of abdominal abscess was 8.9%. With our method, each vessel across the transection plane was separately exposed and ligated because the coagulated liver tissue was fragile and could be easily removed by crushing with forceps or a clamp (). Our method differed from other precoagulated LR methods utilising RF energy in that transection of the liver parenchyma was performed with a scalpel [Citation19,Citation26,Citation28–30,Citation32]. The sealed biliary branch, without proper ligation, may reopen postoperatively and cause a biliary fistula. This occurrence can significantly increase the likelihood of abscess formation from necrotic liver tissue.
Table 5. Comparison of published outcomes of precoagulated-assisted liver resection for hepatocellular carcinoma patients with underlying cirrhosis.
In this study on cirrhotic patients with HCC, the mortality and morbidity rates were 1.5% and 32.9% in the CC-LR group, respectively, and 0.8% and 25.0% in the MW-LR group, respectively. Our mortality and morbidity rates were comparable to those in studies on patients with HCC, which varied from 10.9% to 42% and from 0% to 8%, respectively [Citation2–4,Citation9,Citation10]. Therefore, MW-assisted LR with minimal blood loss is obviously a safe procedure for LR in the context of cirrhosis.
The causes of HCC recurrence after LR are related to micrometastasis through the portal system and/or multicentric carcinogenesis, especially in patients with hepatitis. In this study, MW-LR provided survival benefits (93.5% vs. 87.0%, 77.0% vs. 62.5% and 50.0% vs. 36.5%, respectively; p = 0.003) over CC-LR at 1, 3 and 5 years in the entire population. MW-LR also provided DFS benefits (91.0% vs. 82.5%, 72.0% vs. 59.5% and 47.5% vs. 35.0%, respectively; p = 0.036) over CC-LR at 1, 3 and 5 years in the BCLC-A HCC subgroup. Our results are consistent with those of Sasaki, who used MW-LC in a study of 1118 HCC patients. Sasaki found that the 1-, 3- and 5-year recurrence-free survival rates were 84%, 56% and 40%, respectively, and the 3-, 5- and 10-year OS rates were 88%, 78% and 49%, respectively [Citation15]. Several reasons account for the differences in survival between CC-LR and MW-LR. First, multiple studies have shown that blood loss and blood transfusion are significantly related to tumour recurrence and OS among HCC patients. Therefore, MW-LR can decrease the recurrence rate of HCC via minimal intraoperative blood loss. Second, in the CC-LR group, the rotating, lifting and stretching of the liver parenchyma around the tumour increase the likelihood of developing distant tumour metastasis through the portal system. In addition, MW-LR does not require complete liver mobilisation and Pringle manoeuvres. Consequently, MW-LR more closely follows the no-touch principle of oncologic surgery compared with CC-LR. Finally, MW-LR produced a 1-cm necrotic area at the resection margin after transection of the liver parenchyma, decreasing the rate of a positive surgical margin in HCC.
The strategy based on precoagulated-assisted LR was effective in liver surgery, especially in cases with underlying cirrhosis as shown in . However, this technique is primitive, and these single needle devices should be promoted. The primary precoagulated target under RF or MW energy should be small vessels and liver parenchyma tissue rather than large branches of portal veins or hepatic veins. The large vessels should be ligated instead of being sealed by coagulation. Otherwise, overcoagulation may ensue and result in complications, such as bile leakage, abdominal abscess and liver damage. In addition, the creation of devices such as pencil-type RF electrodes rather than needle-like coagulator electrodes is also possible [Citation21].
Some limitations of this study must also be addressed. First, this study was retrospectively designed and probably involved selection bias. In the future, multicentric, randomised and controlled clinical studies with long follow-up periods should be carried out to verify the feasibility of using MW-LR in HCC patients with underlying cirrhosis. Second, this technique is primitive and time-consuming because it requires a series of applications to achieve a zone of coagulated necrosis along the intended line of transection. The probe is a needle electrode, which may cause bleeding when inserted into the vessels. We expect further improvements to this method in the future.
Acknowledgments
The authors acknowledge the kind advice of Prof. Kai-Wen Huang (Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine).
Disclosure statement
The authors report no conflicts of interest.
References
- Torre LA, Bray F, Siegel RL, et al. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65:87–108.
- Bruix J, Gores GJ, Mazzaferro V. (2014). Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63:844–55.
- Farges O, Malassagne B, Flejou JF, et al. (1999). Risk of major liver resection in patients with underlying chronic liver disease. A reappraisal. Ann Surg 229:210–15.
- Fonseca AL, Cha CH. (2014). Hepatocellular carcinoma: a comprehensive overview of surgical therapy. J Surg Oncol 110:712–19.
- Fan ST, Poon RT, Yeung C, et al. (2011). Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg 98:1292–300.
- Cillo U, Vitale A, Grigoletto F, et al. (2006). Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 44:723–31.
- Torzilli G, Donadon M, Marconi M, et al. (2008). Hepatectomy for stage B and stage C hepatocellular carcinoma in the Barcelona Clinic Liver Cancer classification: results of a prospective analysis. Arch Surg 143:1082–90.
- Ruzzenente A, Capra F, Pachera S, et al. (2009). Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg 13:1313–20.
- Ariizumi S, Kotera Y, Takahashi Y, et al. (2013). Impact of hepatectomy for huge solitary hepatocellular carcinoma. J Surg Oncol 107:408–13.
- Yamashita Y, Tsuijita E, Takeishi K, et al. (2014). Trends in surgical results of hepatic resection for hepatocellular carcinoma: 1,000 consecutive cases over 20 years in a single institution. Am J Surg 207:890–6.
- Yeh CN, Lee WC, Chen MF, Tsay PK. (2003). Predictors of long-term disease-free survival after resection of hepatocellular carcinoma: two decades of experience at Chang Gung Memorial Hospital. Ann Surg Oncol 10:916–21.
- Imamura H, Matsuyama Y, Tanaka E, et al. (2003). Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 38:200–7.
- Hirokawa F, Hayashi M, Asakuma M, et al. (2016). Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 25:24–9.
- Katz SC, Shia J, Liau KH, et al. (2009). Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 249:617–23.
- Sasaki K. (2015). Liver resection for hepatocellular carcinoma using a microwave tissue coagulator: experience of 1118 cases. WJG 21:10400.
- Li M, Zhang W, Li Y, et al. (2013). Radiofrequency-assisted versus clamp-crushing parenchyma transection in cirrhotic patients with hepatocellular carcinoma: a randomized clinical trial. Dig Dis Sci 58:835–40.
- Xia F, Wang S, Ma K, et al. (2008). The use of saline-linked radiofrequency dissecting sealer for liver transection in patients with cirrhosis. J Surg Res 149:110–14.
- Sandonato L, Soresi M, Cipolla C, et al. (2011). Minor hepatic resection for hepatocellular carcinoma in cirrhotic patients: Kelly clamp crushing resection versus heat coagulative necrosis with bipolar radiofrequency device. Am Surg 77:1490–5.
- Curro G, Jiao L, Scisca C, et al. (2008). Radiofrequency-assisted liver resection in cirrhotic patients with hepatocellular carcinoma. J Surg Oncol 98:407–10.
- Wang Q, Yan J, Feng X, et al. (2017). Safety and efficacy of radiofrequency-assisted ALPPS (RALPPS) in patients with cirrhosis-related hepatocellular carcinoma. Int J Hyperthermia 33:846–52.
- Quesada R, Poves I, Berjano E, et al. (2016). Impact of monopolar radiofrequency coagulation on intraoperative blood loss during liver resection: a prospective randomised controlled trial. Int J Hyperthermia 33:135–41.
- Lupo L, Gallerani A, Panzera P, et al. (2007). Randomized clinical trial of radiofrequency-assisted versus clamp-crushing liver resection. Br J Surg 94:287–91.
- Berber E, Siperstein A. (2008). Radiofrequency (RF)-assisted hepatectomy may induce severe liver damage. World J Surg 32:1897–8, 1899–900.
- Mitsuo M, Takahiro T, Yasuko T, et al. (2007). Radiofrequency (RF)-assisted hepatectomy may induce severe postoperative liver damage. World J Surg 31:2208–12, 2213–4.
- Zhou XD, Tang ZY, Yang BH, et al. (2001). Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer-Am Cancer Soc 91:1479–86.
- Weber JC, Navarra G, Jiao LR, et al. (2002). New technique for liver resection using heat coagulative necrosis. Ann Surg 236:560–3.
- Clavien PA, Barkun J, de Oliveira ML, et al. (2009). The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–96.
- Pai M, Frampton AE, Mikhail S, et al. (2012). Radiofrequency assisted liver resection: analysis of 604 consecutive cases. Eur J Surg Oncol (EJSO) 38:274–80.
- Kleinert R, Wahba R, Bangard C, et al. (2010). Radiomorphology of the Habib sealer-induced resection plane during long-time follow up: a longitudinal single center experience after 64 radiofrequency-assisted liver resections. HPB Surg 2010:1–7.
- Pai M, Jiao LR, Khorsandi S, et al. (2008). Liver resection with bipolar radiofrequency device: Habib 4X. HPB (Oxford) 10:256–60.
- Zhang F, Yan J, Feng XB, et al. (2015). Efficiency and safety of radiofrequency-assisted hepatectomy for hepatocellular carcinoma with cirrhosis: a single-center retrospective cohort study. WJG 21:10159–65.
- Ayav A, Bachellier P, Habib NA, et al. (2007). Impact of radiofrequency assisted hepatectomy for reduction of transfusion requirements. Am J Surg 193:143–8.