Abstract
Context: No defined pre-treatment factors are able to predict the response to radiofrequency ablation (RFA) of an autonomously functioning thyroid nodule (AFTN).
Objective: Primary endpoint was to evaluate the success rate of RFA to restore euthyroidism in a cohort of adult patients with small solitary AFTN compared with medium-sized nodules. Secondary endpoints included nodule volume reduction and rate of conversion from hot nodules to cold using scintiscan.
Methods: This was a 24-month prospective monocentric open parallel-group trial. Twenty-nine patients with AFTN were divided into two groups based on thyroid volume: 15 patients with small nodules (<12 mL) in group A and 14 patients with medium nodules (>12 mL) in group B. All patients underwent a single session of RFA and were clinically, biochemically, and morphologically evaluated at baseline and at 1, 6, 12 and 24 months after treatment.
Results: After RFA, there was greater nodule volume reduction in group A compared with group B (p < 0.001 for each follow-up point). In group A, there was a greater increase in TSH levels than in group B at 6 (p = 0.01), 12 (p = 0.005), and 24 months (p < 0.001). At 24 months, the rate of responders was greater in group A than in group B (86 vs. 45%; p < 0.001). In group A, 86% of nodules converted from hot to cold compared with 18% in group B (p < 0.001).
Conclusions: A single session of RFA was effective in restoring euthyroidism in patients with small AFTNs. Nodule volume seems to be a significant predictive factor of the efficacy of RFA in treating AFTN.
Introduction
Approximately 10% of solitary thyroid nodules are scintigraphically ‘hot’; half of these are autonomously functioning. Radioiodine and surgery are both effective in the treatment of pre-toxic and toxic thyroid nodules [Citation1–3]. Radioiodine using 131I is considered first-line therapy in the majority of patients because normalisation of thyroid function is attained in most cases, and the reduction in nodule volume is about 30–45% within one to two years [Citation4,Citation5]. The use of RFA has been tested in hot nodules, as determined by thyroid scintiscan, using both the fixed-electrode procedure [Citation6–9] and the ‘moving-shot’ technique [Citation10]. Control of thyroid hyperfunction and reduction in nodule volume has been reported in both non-controlled and controlled patient series [Citation7–11].
Indications for treatment of the Korean Society of Thyroid Radiology (KSThR) include patients with benign thyroid nodules with nodule-related compressive symptoms and/or cosmetic concerns, or with autonomously functioning thyroid nodules (AFTN). Nodule size is not considered a specific criterion for RFA, although the authors declare that “patients with nodules with a maximum diameter >2 cm that continue to grow over time, may be considered for RFA” [Citation12]. Even the experts’ panel in the first Italian opinion statement indicate RFA for AFTNs hot/warm at scintiscan, either toxic or pre-toxic [Citation13].
A major limitation of RFA is partial ablation of the margin of the hyperfunctioning lesion, that may be followed by regrowth of the nodule [Citation10,Citation14]. Wider or more complete ablation of the nodule margins is required to prevent regrowth of the toxic nodule, thus preventing recurrent hyperthyroidism during long-term follow-up [Citation10,Citation15]. Furthermore, some studies with RFA are sometimes hampered by a low level of clinical evidence because they are based on small trials with multiple treatment sessions and short-term follow-up [Citation8,Citation15].
No studies thus far have focussed on finding the predictive factors for treatment response. Recently, some authors [Citation16] have speculated that the percentage of nodule volume reduction at 12 months could be related to treatment response, with a greater volume reduction indicating a higher probability of hyperthyroidism remission. In fact, the available data indicate that whatever the energy is used, smaller nodules, both hot and cold, have a higher rate of volume reduction compared to larger nodules [Citation17–20]. It follows that the initial volume of a functioning nodule might play a decisive role in restoring euthyroidism. In light of these data, we enlisted a group of patients affected by small hot nodules and compared them with patients with medium nodules in a prospective, observational cohort study.
Our primary endpoint was to evaluate the success rate of RFA in restoring euthyroidism in the two groups at the first and second year after the procedure. The secondary endpoints include volume reduction and conversion rate from hot to cold using thyroid scintiscan.
Materials and methods
Study population and design
This 24-month, prospective, monocentric, open parallel-group trial compared the efficacy of a single session of RFA to restore euthyroidism in a cohort of adult patients with solitary AFTN. All patients refused or had contraindications to radioiodine therapy or surgery.
All patients had to meet the following inclusion criteria: presence of a solitary AFTN, as assessed by serum thyroid stimulating hormone (TSH) and 99 m technetium (Tc) pertechnetate thyroid scintigraphy; age greater than 18 years; Thy2/Tir2 (or Bethesda II) cytology on ultrasound-guided fine-needle aspiration biopsy; no history of methimazole treatment; calcitonin levels within normal limits; refusal of or contraindications to surgery or radioiodine therapy. The exclusion criteria were: presence of a multinodular goitre; willingness to undergo radioiodine therapy; Thy3/Tir3 (Bethesda III) cytology; presence of a malignant thyroid nodule; history of radioiodine therapy or thermal ablation; previous neck or trunk external beam radiotherapy; pregnancy.
Between January 2014 and July 2015, a total of 29 patients at S. M. Goretti Hospital in Latina, Italy, were enrolled and were treated with a single session of RFA. Using criteria employed in previous studies [Citation18,Citation20–22], patients were divided into two groups based on thyroid volume at baseline: patients with small nodules made up group A (nodule volume <12 ml), and those with medium nodules made up group B (nodule volume >12 ml).
All patients were evaluated clinically, biochemically and morphologically before RFA (at baseline) and at 1, 6, 12 and 24 months after treatment. After treatment with RFA, if the patients had hyperthyroidism (low-serum TSH levels and normal or elevated serum-free triiodothyronine [FT3] and serum-free thyroxine [FT4]), they were treated with antithyroid medication (methimazole) to achieve normal thyroid function. In this study, ‘responders’ were defined as patients with clinical and biochemical evidence of a euthyroid state after RFA, without methimazole therapy, at the end of the study. The local ethical committee approved the study protocol and all the patients signed an informed consent statement allowing their anonymized information to be used for data analysis. Patient records were anonymized and de-identified prior to analysis.
Of the 29 patients who provided written informed consent, 26 subjects had reached the two-year time-point. One subject who belonged to group A (male, 44 y, toxic nodule, 6.2 ml) and two subjects who belonged to group B (1. male, 28 y, toxic nodule, 21 ml; 2. male, 45 y, pre-toxic, 22 ml) voluntary withdrew from the clinical trial, but they were included in this analysis at 1, 6, 12 months after treatment.
Clinical evaluation
At each visit, the patient’s medication history; current complaints, including symptoms of hyperthyroidism or hypothyroidism; nodule-related symptom score; and cosmetic score were recorded. The nodule-related symptom score was obtained by asking all patients to rate pressure symptoms on a 10 cm visual analogue scale. The cosmetic score was obtained using the following scale: (1) no palpable mass; (2) no cosmetic problem but a palpable mass; (3), cosmetic problem with swallowing only; (4) an easily visible mass. Complications and side effects were recorded at each visit and were consistent with the standardised terminology and reporting criteria proposed by the International Working Group on image-guided tumor ablation and by the Technology Assessment Committee of the Society of Interventional Radiology [Citation23,Citation24]. Major complications were events leading to significant morbidity and disability; all other complications were classified as minor. Side effects were defined as undesired consequences of the procedure that rarely, if ever, resulted in significant morbidity.
Biochemical evaluation
Thyroid function was monitored using TSH, FT3, and FT4 measurement. Chemiluminescent enzyme immunoassay (Architect i4000 SR Immunoassay; Abbott Core Laboratory, Abbot Park, IL, USA) was used to determine serum thyrotropin (normal range, 0.5–4.9 mIU/L), serum FT3 (normal range, 1.7–3.7 pg/mL), serum FT4 (normal range, 0.7–1.7 pg/mL), and serum antithyroid peroxidase antibody levels (normal range, 0–35 IU/mL). Immunoradiometric assay (Architect i4000 SR Immunoassay; Abbott Core Laboratory, Abbot Park, IL, USA) was used to determine serum calcitonin levels (normal range, 0–10 pg/mL). Coagulation testing (prothrombin time, activated partial thromboplastin time) was also performed.
Procedure
A radiofrequency generator (Medtronic Cool-tip RF Ablation System, E-Series; Covidien, Minneapolis, MN, USA) with a 17-gauge, 15 cm electrode with a 1 cm active tip was employed. The same operator carried out all procedures under ultrasonographic guidance, using the same scanner as for the initial diagnostic evaluation. The intra-observer coefficient of variation for sonographic volume assessment was previously defined as 4% [Citation20]. For local anaesthesia at the puncture site, we used 2–5 ml of 2% mepivacaine (Carbosen), and 3 ml of ropivacaine (Naropine; Fresenius Kabi USA, LLC, Lake Zurich, IL, USA). All patients were prescribed 4 mg intravenous prednisone to reduce post-treatment oedema. Anxiolytic drugs were never used prior to the procedure; per the suggestions of other authors, we never induced deep sedation [Citation12]. On the basis of our previous experience, we used the transisthmic approach along the short axis of the nodule, and the nodules were managed using the moving-shot technique as reported by Baek et al. [Citation11,Citation25]. We applied a variant of this technique, using 60 W of radiofrequency outpower and adequate exposure time, to induce some transient multiple hyperechoic zones as a sign of the effectiveness of the ablation. We calculated the treatment time for every RFA session from the initial insertion of the radiofrequency needle into the thyroid nodule to the final assessment of the treatment session.
Ultrasound evaluation
Ultrasonography was performed using a 7.5 to 12 MHz linear probe equipped with colour Doppler and power Doppler modules (Technos MPX; Esaote My Lab 50, Novarium SRL, Rapallo, Italy).
We enrolled only solid thyroid nodules or predominantly solid (with a fluid component ≤30% of its volume) [Citation20]. We classified nodules as spongiform, hysoechoic and hyperechoic [Citation12,Citation26,Citation27].
Nodule volume and the percentage of volume reduction were calculated using the following equations: volume percentage (ellipsoid equation): volume percentage = length × width × depth × 0.525; volume reduction percentage: percentage of volume reduction = [(initial volume-final volume) × 100]/initial volume [Citation12].
Statistical analysis
Statistical analyses were performed using IBM-SPSS Statistics software, version 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. IBM Corp., Armonk, NY). Descriptive statistics (e.g. median, mean, standard deviation, range) were computed on thyroid volume and other clinical variables. As transformed data did not conform to a log-normal distribution, non-parametric tests were used to compare the means between groups: the Mann–Whitney U-test for two independent samples and the Kruskal–Wallis test for multiple comparisons. The Wilcoxon test was used to compare related samples. Logistic regression was used to explain the relationship between binary variable “responders” and the independent variables age, sex, basal volume and TSH. The significance level was defined as p ≤ 0.05.
Results
Baseline characteristics
The 29 patients included in this study had a mean age of 51.41 ± 15.5 years. There were 11 women and 18 men. Group A (small nodules) contained 15 patients, and group B (medium nodules) contained 14 patients. In group A, 5 and 10 subjects had respectively toxic and pre-toxic nodules. In group B, 4 and 10 subjects had respectively toxic and pre-toxic nodules. The patient characteristics, the clinical data and the ultrasound features at baseline are summarised in and .
Table 1. Main baseline characteristics of the study population.
Table 2. US characteristics of the study population.
Thyroid function
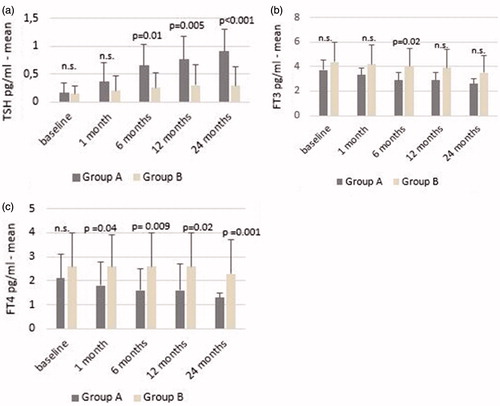
The mean TSH value for the entire study population increased between study baseline and 24 months after RFA (0.15 ± 0.17 mIU/mL vs. 0.63 ± 0.48 mIU/mL; p < 0.001). In group A, the increase over group B started to become statistically significant after the first month (p = 0.01 at six months; p = 0.005 at 12 months; p < 0.001 at 24 months) (). In the entire study population, FT3 levels decreased between baseline and 24 months after RFA (4.0 ± 1.31 pg/mL vs. 3.0 ± 1.0 pg/mL; p < 0.001). No significant difference was detected in FT3 values between group A and group B except at six months (). In the entire study population, FT4 levels decreased between baseline and 24 months (2.4 ± 1.2 pg/mL vs. 1.8 ± 1.0 pg/mL; p = 0.006) ().
At the end of the study period, the rate of responders was greater in group A than in group B (86% vs. 45%; p < 0.01). At 24 months, 86% of nodules in group A converted to cold compared with 18% in group B (p < 0.001).
A logistic regression analysis has been applied to evaluate the relationship between the dependent binary variable “responder” and the independent variables such as sex, age, volume at baseline and TSH at baseline. The analysis shows that the baseline volume represents the only significant variable (p = 0.001). The Hosmer–Lemeshow statistic indicates that the model adequately fits the data (p = 0.32) and the model correctly classifies 73.4% of the subjects.
We did not find any significant association between the response to treatment and the ultrasonographic characteristics of the nodules.
Nodule volume
The change in nodule volume over time is shown in . In the entire study population, there was a significant decrease in nodule volume between baseline and 24 months after RFA (p < 0.001 for each time point vs. baseline). After RFA, there was a larger volume reduction in group A (68%, 75%, 82% and 84% after 1, 6, 12 and 24 months compared with baseline) than in group B (54%, 61%, 67% and 68% after 1, 6, 12 and 24 months; p < 0.001 for each time point). The mean rate of volume reduction was higher in responders compared with the other subjects (81% ± 8% vs. 70% ± 14%; p = 0.04).
Table 3. Thyroid nodule volume over the time.
Table 4. Symptom and cosmetic score over the time.
Symptom and cosmetic score ()
In the entire study population, the symptom score decreased during the follow-up period (p < 0.004 for trend). The symptom score was significantly higher in group B than in group A (p < 0.001 at all observation points). The cosmetic score also improved between baseline and 24 months in the entire study population (p = 0.001 for trend) and difference between the two groups are significant only at baseline (p = 0.001).
Complications and safety
During RFA, most patients reported mild pain and a sensation of heat in the neck that radiated to the chest, teeth, shoulder and head. No major complications were encountered, such as voice change, haematoma formation, infection, fever or skin burning.
Logistic regression
Logistic regression analysis shows that basal volume is a predictor to explain the relationship with dependent binary variable responder. A negative correlation between responder and basal volume (p = 0.005) was revealed as shown in . Hosmer–Lemeshow statistic indicates a good fit to data (p = 0.322); the model correctly classifies 75.9% of the observations.
Table 5. Multivariate logistic regression analysis to explain the relationship between binary variable “responders” and the independent variables “basal volume”.
Discussion
In our prospective study, we evaluated the ability of RFA to restore euthyroidism in a cohort of adult patients with small solitary AFTN compared with medium-sized nodules and secondly, we investigated the nodule volume reduction and rate of conversion from hot to cold nodules. At the end of the study period, we demonstrate that a single RFA session is effective in restoring euthyroidism in patients with AFTN, mainly in those patients with small nodules. Nodule volume appears to be a significant predictive factor of the efficacy of RFA in treating AFTN.
In an Italian consensus statement regarding RFA of thyroid nodules, the authors give indication to thermoablation treatment of hot/warm nodules either toxic or pre-toxic, when surgery and radioiodine are contraindicated or declined or in the case of large (volume >20 ml) AFTN, for whom combined RF treatment plus radioiodine could induce faster and greater improvement in local symptoms, reducing radioiodine-administered activity, if compared with radioiodine alone.
Many previous studies have described the effects of radiofrequency technique on hot thyroid nodules in non-homogeneous populations previously treated with radioiodine therapy, with or without surgery [Citation7,Citation9], with multiple RFA sessions [Citation15,Citation28–30] or with different devices and techniques [Citation7–9]. The heterogeneity of these studies explains, at least in part, the extreme variability of the rate of normalisation of thyroid function after percutaneous RFA, ranging from 24 to 82% [Citation7–10,Citation15,Citation28–30]. In the most recent study, the authors resorted to up to six treatment sessions to obtain a rate of normalisation of 82% [Citation30]. It is evident that, compared with radioiodine therapy, these procedures seem less cost-effective for treating AFTN. Other authors, in a comparison between RFA and surgery, pointed out that only a single treatment is cost-effective for treating hot nodules. But, in the same study, the authors pointed out that the euthyroidism rate 12 months after ablation was only 50% with a volume reduction of 75% [Citation28].
If we consider it true that the hypotheses of those authors who think that remission of functional symptoms is linked to the extent of volume reduction at 12 months (remission with an average reduction of 81%; improvement with an average reduction of 68%) [Citation16], it is highly likely that it is necessary to induce a coagulation zone as wide as possible in order to achieve a high percentage of volume reduction. This substantially limits the vital marginal tissue at the periphery of the nodule, thereby preventing regrowth of the nodule over time with recurrence of hyperthyroidism. This is clearly more easily achieved with nodules of smaller volume. Our data are consistent with this hypothesis. At the 24th month after treatment, the percentage of euthyroid patients in the small-nodule group was 86%, much higher than the 45% of patients with nodules larger than 12 ml. Conversion to cold nodule status was seen in 86% of patients with nodules smaller than 12 ml at baseline, compared with 18% of patients with nodules larger than 12 ml.
The results of our study, therefore, suggest that percutaneous treatment with RFA may be effective in restoring euthyroidism in patients with small AFTNs (defined as ranging in size from 10–13 ml). These results, unlike those of other studies [Citation16], were obtained using a single treatment session, assessing thyroid function before and after RFA treatment with scintigraphy in patients not previously treated with antithyroid medication and followed for a period of 24 months after RFA. Our results suggest that the volume of the nodule affects the treatment result, and it is this criterion that should be adopted in the future to successfully treat both pre-toxic and toxic small nodules. For medium and large nodules, the technique of choice must be radioiodine therapy or surgery.
With regard to laser technology, taking into account differences related to different energy, we noticed the same variability of outcomes we observe with RFA. In detail, the rate of normalisation of TSH in patients with multinodular goitre treated with LA were between 47–87% within 6–12 months. The energy was delivered in multiple sessions (1–9) [Citation31–33] or with multiple cycles of treatment [Citation34]. The rate of patients with euthyroidism ranged from 50 to 88% [Citation33,Citation34]. All these studies have been conducted predominantly on a small series of cases.
To date, there are no direct comparisons head-to-head between the two techniques. We think that the volume of the nodule at baseline should be the only predictive factor while the role of technique used should be quite secondary and irrelevant. Future studies on a large series of patients will be able to enlighten us on this particular application.
The main limitation of our study is its small sample size. Large patient series with long-term follow-up are needed to confirm the prominent role of the baseline nodule volume in RFA efficacy.
Conclusions
Minimally invasive hyperthermic techniques are increasingly used in daily clinical practice for percutaneous debulking of benign thyroid nodules. These techniques offer several advantages when compared with surgery: they are low-cost outpatient procedures, do not result in cervical scarring or loss of thyroid function and are nearly completely devoid of the risk of permanent complications. Our study shows that nodule size may be a significant predictive factor of the efficacy of RFA for treating AFTNs. In particular, we demonstrate that a single RFA session is effective in restoring euthyroidism in patients with AFTN, mainly in those patients with small nodules. Nodule volume appears to be a significant predictive factor of the efficacy of RFA in treating AFTN. The possibility of having another therapeutic option for the treatment of this disease, even if limited to functioning small nodules, could be a part of the present trend in managing nodular thyroid goitre, which suggests a customised therapeutic approach.
Acknowledgements
We thank BioMed Proofreading® LLC for his help with the English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gharib H, Papini E. (2007). Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am 36:707–35.
- Hegedus L, Bonnema SJ, Bennedbaek FN. (2003). Management of simple nodular goiter: current status and future perspectives. Endocr Rev 24:102–32.
- Gharib H, Papini E, Paschke R, et al. (2010). American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest 33:51–6.
- Bonnema SJ, Hegedus L. (2012). Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev 33:920–80.
- Bahn RS, Burch HB, Cooper DS, et al. (2011). Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract 17:456–520.
- Spiezia S, Garberoglio R, Di Somma C, et al. (2007). Efficacy and safety of radiofrequency thermal ablation in the treatment of thyroid nodules with pressure symptoms in elderly patients. J Am Geriatr Soc 55:1478–9.
- Spiezia S, Garberoglio R, Milone F, et al. (2009). Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 19:219–25.
- Deandrea M, Limone P, Basso E, et al. (2008). US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 34:784–91.
- Faggiano A, Ramundo V, Assanti AP, et al. (2012). Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab 97:4439–45.
- Baek JH, Jeong HJ, Kim YS, et al. (2008). Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid 18:675–6.
- Baek JH, Kim YS, Lee D, et al. (2010). Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 194:1137–42.
- Na DG, Lee JH, Jung SL, et al. (2012). Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 13:117–25.
- Garberoglio R, Aliberti C, Appetecchia M, et al. (2015). Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound 18:423–30.
- Sim JS, Baek JH, Lee J, et al. (2017). Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia 33:905–10.
- Baek JH, Moon WJ, Kim YS, et al. (2009). Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 33:1971–7.
- Bernardi S, Stacul F, Michelli A, et al. (2017). 12-month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine 57:402–8.
- Valcavi R, Bertani A, Pesenti M, et al. (2008). Laser and radiofrequency ablation procedures. In: Baskin HJ, Duick DS, Levine RA, eds. Thyroid ultrasound and ultrasound guided FNA biopsy. New York (NY), USA: Springer, 191–218.
- Valcavi R, Riganti F, Bertani A, et al. (2010). Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 20:1253–61.
- Dossing H, Bennedbaek FN, Hegedus L. (2011). Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol 165:123–8.
- Cesareo R, Pasqualini V, Simeoni C, et al. (2015). Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab 100:460–6.
- Achille G, Zizzi S, Di Stasio E, et al. (2016). Ultrasound-guided percutaneous laser ablation in treating symptomatic solid benign thyroid nodules: our experience in 45 patients. Head Neck 38:677–82.
- Pacella CM, Mauri G, Cesareo R, et al. (2017). A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia 33:911–9.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol 25:1691–705 e1694.
- Jeong WK, Baek JH, Rhim H, et al. (2008). Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–50.
- Moon WJ, Baek JH, Jung SL, et al. (2011). Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 12:1–14.
- Kwak JY, Han KH, Yoon JH, et al. (2011). Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 260:892–9.
- Bernardi S, Dobrinja C, Fabris B, et al. (2014). Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol 2014:934595.
- Che Y, Jin S, Shi C, et al. (2015). Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol 36:1321–5.
- Sung JY, Baek JH, Jung SL, et al. (2015). Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid 25:112–7.
- Pacella CM, Bizzarri G, Spiezia S, et al. (2004). Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 232:272–80.
- Barbaro D, Orsini P, Lapi P, et al. (2007). Percutaneous laser ablation in the treatment of toxic and pretoxic nodular goiter. Endocr Pract 13:30–6.
- Dossing H, Bennedbaek FN, Bonnema SJ, et al. (2007). Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol 157:95–100.
- Amabile G, Rotondi M, Pirali B, et al. (2011). Interstitial laser photocoagulation for benign thyroid nodules: time to treat large nodules. Lasers Surg Med 43:797–803.