Abstract
Objective: To investigate the factors which may cause thermal injury of abdominal wall structures in ultrasound-guided high-intensity focussed ultrasound (USgHIFU) ablation of uterine fibroids.
Method: A total of 892 patients with uterine fibroids diagnosed on contrast-enhanced magnetic resonance imaging (MRI) scans received HIFU ablation and follow-up MRI scanning. After therapy, thermal injury to the skin was assessed via measurement of skin redness, blisters, subcutaneous nodules and to the abdominal wall structures via measurement of signal intensity on T2-weighted MRI images. A total of 151 patients were assigned to the injury group, 741 patients were assigned to the non-injury group. The relationship between patient and treatment parameters and injury were analysed using univariate and multiple logistic regression analyses.
Results: Univariate logistic regression revealed that sonication time, sonication time per hour, total energy deposited, distance from uterine fibroid ventral side to skin, volume of uterine fibroids, abdominal wall scar, abdominal wall thickness and body mass index (BMI) all affected whether thermal injury occurred (p < 0.05). Subsequently, multiple logistic regression analysis revealed that total energy (p = 0.000, OR = 2.228, 95% CI 1.831–2.712), abdominal wall scar (p = 0.019, OR = 1.639, 95% CI 1.085–2.477) and abdominal wall thickness (p = 0.000, OR = 1.562, 95% CI 1.313–1.857) were significantly correlated with thermal injury.
Conclusion: Multiple logistic regression analysis revealed that abdominal wall thickness, total energy and abdominal wall scar were the most significant influencing factors that influenced minimal thermal injury of abdominal wall structures in USgHIFU ablation of uterine fibroids.
Introduction
Uterine fibroids which are a common benign gynecological tumour and a range of treatment options are available [Citation1], including surgery, medication, uterine arterial embolisation (UAE) and ultrasound-guided high-intensity focussed ultrasound (USgHIFU) ablation. The latter approach has the advantage of being non-invasive, in that US waves generated outside the body are focussed on the targeted tissue inside the body, where they produce hyperthermia, cavitation and mechanical effects that induce tissue necrosis at the focal point. The hyperthermia effect is primary and the temperature at the focal point can reach between 60 and 100 °C within 0.5–1.0 s causing local ablation via coagulation necrosis of the protein in the lesions [Citation2]. The safety and efficacy of HIFU treatment has been demonstrated in many studies [Citation3–5].
HIFU is used extensively for treatment of uterine fibroids with minimal reports of any complications. Tissue damage within the US pathway is, however, still possible. The lowest temperature that can cause a skin burn is 44 °C [Citation6,Citation7], whereas the temperature at the focus of the HIFU ablation for uterine fibroids can reach 60–100 °C. According to one study with large sample size the incidence of skin burn was 0.26% [Citation4]. Previous studies of potential HIFU-related complications are based mostly on clinical reports of pain rating and discomfort and appearance of the skin, and subcutaneous fat, muscles and corresponding connective tissues, extra-peritoneal fat and peritoneum which may all lie in the US pathway and be injured by HIFU energy deposition are rarely assessed. Of particular interest are the changes in the layers and structures of the abdominal wall in patients with previous surgical scars in the abdominal wall. While there have been reports of skin injury caused by HIFU, which is considered relevant to the abdominal wall scars [Citation4,Citation8,Citation9], there have been no direct studies of potential thermal injury to abdominal wall structures.
The goal of the present study was to investigate the factors that influence the extent of thermal injury to both the skin and abdominal wall structures that may occur during HIFU. In particular, to assess if there is a relationship between injury to the skin and deeper structures and corresponding reports of pain and discomfort. The results obtained are likely to improve assessment procedures prior to performing HIFU, better predict treatment risk factors and provide best post-operative care.
Materials and methods
Patients
Women with uterine fibroids who received USgHIFU at the First Affiliated Hospital of Chongqing Medical University (CMU) from January 2013 to December 2015 were included in this study which was approved by the Research Ethics Committee at CMU and all patients gave fully informed written consent. Inclusion criteria were: women of child-bearing age before menopause; the patients had complete pre-operative and post-operative magnetic resonance imaging (MRI) scans and uterine fibroids were diagnosed by MRI; for patients with abdominal surgical scars the area of image blurring due to acoustic attenuation was no more than 15 mm, as assessed by US. Patients with adenomyosis were excluded.
Pre-operative preparation
Patients were instructed to eat an easily digestible and no-gas-producing diet for 3 d before the USgHIFU treatment. An enema was performed on the morning of the surgery and the skin of the anterior abdominal was shaved from the umbilicus to the superior border of the symphysis pubis and also degreased and degassed. A urinary catheter was inserted and bladder volume regulated by filling with degassed normal saline.
HIFU ablation
USgHIFU ablation was performed using the Model-JC Focused Ultrasound Tumor Therapeutic System (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China). The operating frequency of the US transducer was 0.8 MHz and energy was adjustable in the range 0 to 400 W. Circulating degassed water was used as the coupling medium and the focal region was 1.5 mm × 1.5 mm × 10.0 mm. A B-mode US (Esaote MyLab70, Genoa, Italy) probe operating at 3.5 MHz contained within USgHIFU system provided real-time image guidance during HIFU ablation. The patient was placed in a prone position on the US therapy bed for the USgHIFU treatment. The abdominal wall was in full contact with the degassed water in the tank, and the water temperature was set below 15 °C. In addition, a water balloon filled with cold degassed water was placed between the transducer and the anterior abdominal wall. USgHIFU was performed under intravenous sedation using fentanyl and midazolam hydrochloride. However, patients remained conscious and were thus able to report any discomfort or pain during the treatment procedures which were recorded. The energy for the ablation was adjusted based on the patients’ tolerance and the grey scale changes of the target area on the US image used to guide the procedure. Furthermore, the ablation effect was assessed post-operatively using contrast-enhanced US. The skin in the US pathway was examined immediately after the procedure and skin redness, blisters, burst and subcutaneous nodules recorded. Patients were then followed-up regularly to record any adverse events. The severity of any complications was recorded according to the Society of Interventional Radiology (SIR) classification system [Citation10].
Magnetic resonance imaging evaluation
MRI was performed on a 3.0 T MRI system (General Electric Company, Chicago, IL, USA) before and 1–2 d after the USgHIFU ablation. For T1-weighted imaging acquisition, parameters were repetition time (TR) 502 ms, echo time (TE) 12 ms, slice thickness 4 mm and slice interval 1 mm and for T2-weighted imaging corresponding values were TR 4000 ms, TE 98 ms, slice thickness 6 mm, and slice interval 0.875 mm. The T1-weighted imaging was performed both before and after administration by injection of the contrast agent gadodiamide (Omniscan, 0.5 mmol/ml) at a dose of 15 to 20 ml.
Calliper measurements were obtained for the targeted fibroids and uterus on the T2-weighted image before and after treatment and volume calculated using the formula for an ellipsoid as 0.5233 × D1 (longitudinal diameter) × D2 (anteroposterior diameter) × D3 (transverse diameter) [Citation11]. The thickness of the anterior abdominal wall and the distance from the anterior abdominal wall to the ventral side of the largest layer of the fibroid were also measured. The non-perfused volume (NPV) was also measured for each of the targeted fibroids on the post-contrast T1-weighted MR image. Abdominal wall thickness, presence or absence of abdominal wall scar and the distance from the ventral side of each treated fibroid to the skin surface were also recorded on the T2-weighted image.
Assessment the thermal damage of the abdominal wall structures
The T2-weighted post-operative MR images were also reviewed in order to detect any abnormal signals or image appearance of the abdominal wall. Patients were assigned to injury and non-injury groups based on whether thermal injury was detected for any of the abdominal wall structures.
Demographic and treatment data
Demographic data were collected for all patients, including age and body mass index (BMI). The USgHIFU treatment parameters including sonication time(s), sonication time per hour (s/h) and total energy (kJ) and post-operative incidence of pain in the sacro-coccygeal joint, vaginal bleeding and discharge, lower limb paresthaesia, urinary retention, fever and gross haematuria were also recorded.
Statistical analysis
Statistical analyses was performed using SPSS versionb22.0 (IBM, Chicago, IL). All measurements were expressed as mean ± SD. Thermal injury of the abdominal wall structures was considered a dependent variable, and eight influencing factors (i.e. abdominal wall thickness, BMI, abdominal wall scar, volume of the fibroid, distance from the fibroid ventral side to the skin, sonication time(s), sonication time per hour [s/h] and total energy [kJ]) was a covariate. The different influencing factors were subjected to univariate binary logistic regression analysis (α = 0.05, β = 0.1). The significant variables were further tested by a multiple binary logistic regression analysis. The χ2 test and Fisher’s exact tests were used to compare the incidence of the post-operative complications. p < 0.05 was considered significant.
Results
Characteristics of the patients
A total of 892 patients were recruited with an average age of 39.1 ± 6.4 years old and of these 151 patients showed evidence of injury to the abdominal wall and were assigned to the injury group and 741 to the no-injury group which is an incidence of 16.9%. None of the patients with evidence of abdominal wall injury had visible evidence of skin injury after the USgHIFU treatment. However, skin redness in the abdominal wall was noted in five patients (0.6%) and subcutaneous nodules in one patient (0.1%). For the remaining 145 patients, regions of hyperintense signal were observed in the abdominal wall on T2-weighted images (16.3%), while no abnormalities were observed on the T1-weighted images (.
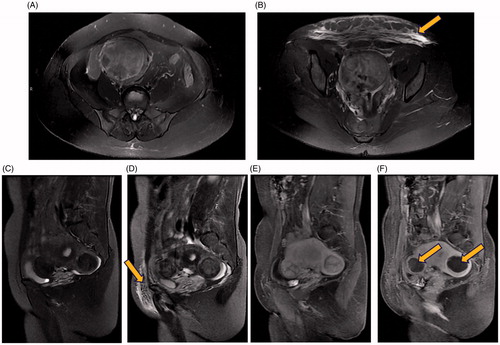
Figure 1. Pre-operative and post-operative MRI. T2-weighted images of a 51-year-old woman with uterine fibroids but no abdominal wall scar. (A) Axial view before HIFU treatment, indicating no abnormal signals in the abdominal wall; (B) Hyperintense signals on the axial view after HIFU treatment (indicated by the arrowhead); T2-weighted images and contrast-enhanced MRI images of a 41-year-old case with uterine fibroids and an abdominal wall scar. (C) Sagittal view before HIFU treatment, indicating no abnormal signals in the abdominal wall; (D) Hyperintense signals in the sagittal view after HIFU treatment (indicated by the arrowhead); (E) Sagittal contrast-enhanced image before HIFU treatment; (F) Sagittal contrast-enhanced image after HIFU treatment, uterine fibroids located in the anterior wall and posterior wall of the uterus were almost completely ablated with non-perfusion area in the fibroids (indicated by the arrowhead).
Further comparisons showed that abdominal wall thickness, BMI, distance from ventral side of fibroid to skin surface and average volume of targeted fibroid were higher in the injury group compared to the no-injury group. The number of patients with an abdominal wall scar was greater in the injury group (31.8%) compared to the no-injury group (22.5%) ().
Table 1. Patient baseline characteristics.
Evaluation of the therapeutic response
USgHIFU was successful in all 892 cases and comparison of efficacy between the injury and no-injury groups is shown in . The mean sonication time(s), sonication time per hour (s/h) and total energy (KJ) were higher in the injury group compared with the no-injury group. The average NPV of 76.8 ± 20.5% in the injury group is slightly lower than in the non-injury group (80.2 ± 17.1%). However, ablation was effective in both groups.
Table 2. Treatment results of HIFU for patients.
Risk factors of thermal damage to the abdominal wall structures
The eight potential influencing factors, namely, abdominal wall thickness, BMI, presence of abdominal wall scar, volume of the fibroid, distance from the fibroid ventral side to the skin, sonication time(s), sonication time per hour (s/h) and total energy (KJ), were subjected to a univariate unconditional logistic regression analysis. This showed that abdominal wall thickness (p = 0.000, OR = 1.570, 95%CI = 1.329–1.854), BMI (p = 0.000, OR = 2.097, 95%CI = 1.575–2.745), presence of abdominal wall scar (p = 0.016, OR = 1.602, 95%CI = 1.092–2.350), volume of the fibroid (p = 0.011, OR = 1.229, 95%CI = 1.048–1.441), distance from the ventral side of the fibroid to the skin surface (p = 0.046, OR = 1.175, 95%CI = 1.003–1.377), sonication time per hour (s/h) (p = 0.000, OR = 1.523, 95%CI = 1.287–1.801), sonication time(s) (p = 0.000, OR = 2.123, 95%CI = 1.764–2.556) and total energy (KJ) (p = 0.000, OR = 2.180, 95%CI = 1.807–2.630) were all significant factors (p < 0.05) ().
Table 3. Univariable logistic regression analysis of variables associated with thermal damage of the abdominal wall structures.
Subsequent multiple logistic regression analysis revealed that abdominal wall thickness (p = 0.000, OR = 1.562, 95%CI = 1.313–1.857), abdominal wall scar (p = 0.019, OR = 1.639, 95%CI = 1.085–2.477) and total energy (KJ) (p = 0.000, OR = 2.228, 95%CI = 1.831–2.712) (p < 0.05) were the significant variables ().
Table 4. Multiple logistic regression analysis of variables associated with thermal damage of the abdominal wall structures.
Intra-operative reaction and post-operative complications
Of the 892 patients, 403 patients (45.2%) reported feeling skin burn during USgHIFU. The prevalence was significantly higher in the injury group (91 of 151 patients, i.e. 60.3%) than the no-injury group (312 of 741patients, i.e. 42.1%) (p = 0.000). In addition, lower abdominal pain is a common symptom after USgHIFU and was reported by of 38.6% of patients, being 47.7% and 36.7% in the injury and no-injury groups, respectively, which is a significant difference (p = 0.012). The injury and no-injury groups showed no significant differences with regard to post-operative incidence of pain in the sacro-coccygeal joint, vaginal bleeding and discharge, lower limb paresthaesia, urinary retention, fever and gross haematuria (p > 0.05) ().
Table 5. Post-operative adverse reactions of patients [n (%)].
Discussion
Retrospective analysis of clinical reports, and US and MR images, has been performed for patients who received USgHIFU for uterine fibroids. A univariable logistic regression analysis revealed that the abdominal wall thickness, BMI, abdominal wall scar, volume of the fibroid, distance from the fibroid ventral side to the skin, sonication time, sonication time per hour and the total energy were associated with the thermal damage in the abdominal wall structures. A multiple logistic regression analysis showed that abdominal wall thickness, total energy and the presence of an abdominal wall scar were factors that influenced whether thermal injury occurred in abdominal wall structures. These findings are consistent with previous studies of the effects of ablation of uterine fibroids with USgHIFU on thermal injury to the abdominal wall structures. Notably, Xiong et al. [Citation8] reported on the significance of abdominal scars in the US pathway, which is supported by the report by Zhao et al. [Citation12] of increased risk of thermal injury in patients who had received abdominal wall liposuction, and Xiao et al. [Citation13] reported on the significance of sonication time and total energy (J). The present study is the first to report on the significance of abdominal wall thickness.
Subcutaneous fat and muscles are the main components of the abdominal wall. Previous studies show that US energy is converted to thermal energy, with the highest efficiency, at the fat/muscle interface [Citation14] and that fat tissues easily absorb thermal energy, which causes thermal injury. Furthermore, increases in abdominal wall thickness, BMI and fat content of the abdominal wall generate more thermal energy in the US pathway, thus potentially increasing thermal injury. In the present study, univariate logistic regression analysis showed that abdominal wall thickness and BMI were the factors causing thermal injury in abdominal wall structures during USgHIFU ablation of uterine fibroids, while multiple logistic regression analysis further indicated that abdominal wall thickness was also a significant factor. These findings led to the recommendation that some patients should exercise or control their diet in order to lose weight and reduce abdominal wall thickness, fat content of abdominal tissues and BMI before USgHIFU treatment so as to reduce the risk of thermal injury to abdominal wall structures.
Cesarean section is becoming increasingly common in almost all countries. Between 1990 and 2014 the annual rate of increase globally was on average 12.4% [Citation15] with the prevalence becoming as high as 50% in some countries. Consequently, an increasing number of patients will have surgical scars in the lower abdominal wall which lies in the US pathway and thus potentially increases the risk of thermal injury during USgHIFU treatment of uterine fibroids. Scar tissue contains fewer blood vessels and more connective tissue fibres than normal tissues. Therefore, it absorbs more ultrasonic energy and increasing the risk of thermal injury. MRI-guided US ablation is not recommended for uterine fibroids in patients with abdominal wall scars [Citation9,Citation16]. Of the 892 cases patients recruited to the present study, 215 patients had abdominal wall scars and a 1.639 times greater risk of thermal injury than in patients without abdominal wall scars. Two patients with abdominal scars presented with redness and swelling of the abdominal wall and one patient with an abdominal scar had a subcutaneous nodule after USgHIFU. All three patients had first degree burn injuries, and none had second or third degree burn injuries. The remaining patients with thermal injury in the abdominal wall structures had no visible abnormalities, and are classified entirely on the basis of the presence of regions of hyperintense signal on T2-weighted images. None of the patients required specific palliative treatment for the thermal injury, and no long-term complications were recorded during the follow-up period. Thus, USgHIFU was demonstrated to be a safe and effective treatment for uterine fibroids in patients with abdominal wall scars. Appropriate screening and monitoring of standardised treatment procedures are important countermeasures to reduce the risk of thermal injury to abdominal wall structures when using USgHIFU to treat uterine fibroids, especially in patients with abdominal wall scars.
Peng et al. [Citation17] reported that the factors that influence the energy required for US ablation were fibroid size, distance from the ventral side of the fibroid ventral to the skin surface, enhancement type on T1-weighted images and signal intensity of the T2-weighted images of the fibroid. The more energy that is used to achieve the ablation, the more energy will be potentially deposited in the abdominal wall structures along the ablation pathway, and the greater the risk of thermal injury. The univariate logistic regression analysis performed in the present study indicated that total energy was a significant factor contributing to the thermal damage to the abdominal wall structures. Therefore, for uterine fibroids which require high levels of thermal energy for ablation (e.g. fibroids presenting with hyperintense signal on T2-weighted images) gonadotropin-releasing hormone agonist (GnRHa) can be given pre-operatively [Citation18]. GnRHa reduces fibroid size, the blood supply to the fibroids and the total energy needed for US ablation, thus reducing the probability of thermal injury to abdominal wall structures. An intra-operative monitoring and assessment of ablation efficacy further helps prevent thermal injury to the abdominal wall structures.
Along with advances in US ablation technology and US imaging and monitoring procedures, the incidence of thermal injury to abdominal wall structures has decreased, but the risk still exists and is important to address. In particular, every step of the USgHIFU treatment procedure deserves particular attention, from patient preparation and skin preparation onwards. For example, Zhang et al. [Citation19] found that a degassed water balloon can be used to push the intestinal tract out of the US pathway and also serves as a reflective interface enabling a greater amount of energy to be deposited at the targeted fibroid. Unfortunately, however, the compression produced by the water balloon reduces blood circulation in the adjacent skin and subcutaneous tissues and lessening the ability of the skin to dissipate heat which may lead to skin injury. Consequently, the clinical protocol requires that the water balloon should be regularly partially deflated to relieve the compression of the abdominal wall. In our study, the degassed water balloon was applied in most of the treatments. However, since the study adopted a retrospective design, it was impossible to obtain reliable data on the pressure and position of the water balloon and the duration of the persistent compression. For this reason, the use of the degassed water balloon was not included as an influencing factor, and more research on the effect of the water balloon on skin injury is needed. Moreover, the frequency of skin burns is higher in patients with abdominal wall scars compared to those without. Intra-operative skin response may correlate to skin injury. It is reported that the lowest temperature to cause a skin thermal pain sensation is 43.2 °C [Citation20]. Any complaint of a skin burning sensation during USgHIFU should be considered a warning that indicates the need to adjust the position of the focus and to loosen the water balloon to reduce skin compression. The risk of a skin burn is reduced by the fact that, notwithstanding the position of the water balloon, the patient’s abdomen is in direct contact with cold degassed water as they lie prone for the treatment.
Almost all of the adverse events in our study were classified as grade A according to the SIR system, very few cases were grade B and none of the cases had post-operative complications of grade C or above. The skin burn was of grade B in all the cases with symptoms, and all of the patients recovered spontaneously without any specific palliative treatment. Skin injury of grade C or D have also been reported after USgHIFU [Citation4,Citation21], and the need for palliative treatment was indicated. However, none of these cases had long-term complications. Along with the advances in US ablation technology and optimisation of monitoring equipment and procedures, complications will further decrease and USgHIFU has the full potential to become a truly non-invasive treatment.
Conclusion
The present study has shown that with appropriate screening and monitoring procedures, USgHIFU may be safely used for treatment of uterine fibroids in patients with an abdominal wall scar. In addition, we identified several factors, including, abdominal wall thickness, BMI, abdominal wall scar, fibroid size, distance from the ventral side of the fibroid to the skin surface, sonication time, sonication time per hour and the total energy, which are all associated with the presence of thermal injury to the abdominal wall structures. The abdominal wall thickness, the abdominal wall scar and the total energy for ablation were the most significant influencing factors that influenced the thermal damage of the abdominal wall. These influencing factors should be given careful consideration when screening for eligible patients and establishing and optimising protocols for USgHIFU ablation of uterine fibroids.
Disclosure statement
Zhibiao Wang is a shareholder of Chongqing Haifu. The other authors have no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Donnez J, Dolmans M. (2016). Uterine fibroid management: from the present to the future. Hum Reprod Update 22:665–86.
- Chen L, ter Haar G, Hill CR. (1997). Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol 23:921–31.
- Stewart EA, Gedroyc WM, Tempany CM, et al. (2003). Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol 189:48–54.
- Chen J, Chen W, Zhang L, et al. (2015). Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem 27:671–6.
- Chen J, Li Y, Wang Z, et al. (2017). Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG 125:354–64. Available from: https://doi.org/10.1111/1471-0528.14689.
- Martin NA, Falder S. (2017). A review of the evidence for threshold of burn injury. Burns 43:1624–39.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. (2003). Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 19:267–94.
- Xiong Y, Yue Y, Shui L, et al. (2015). Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia 31:777–83.
- Yoon S, Lee C, Cha SH, et al. (2008). Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol 18:2997–3006.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Orsini LF, Salardi S, Pilu G, et al. (1984). Pelvic organs in premenarcheal girls: real-time ultrasonography. Radiology 153:113–16.
- Zhao W, Chen J, Chen W. (2014). Effect of abdominal liposuction on sonographically guided high-intensity focused ultrasound ablation. J Ultrasound Med 33:1539–44.
- Xiao Y, Xu Y, Yang L, Jia W. (2015). Analysis of patients with abdominal injury by high intensity focused ultrasound in treatment of uterine leiomyoma. J Clin Ultrasound Med 17:297–300.
- Baker KG, Robertson VJ, Duck FA. (2001). A review of therapeutic ultrasound: biophysical effects. Phys Ther 81:1351–8.
- Betran AP, Ye J, Moller AB, et al. (2016). The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PLoS ONE 11:e0148343.
- Zaher S, Gedroyc WM, Regan L. (2009). Patient suitability for magnetic resonance guided focused ultrasound surgery of uterine fibroids. Eur J Obstet Gynecol Reprod Biol 143:98–102.
- Peng S, Zhang L, Hu L, et al. (2015). Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids. Medcine 94:e650.
- Funaki K, Sawada K, Maeda F, Nagai S. (2007). Subjective effect of magnetic resonance-guided focused ultrasound surgery for uterine fibroids. J Obstet Gynaecol Res 33:834–9.
- Zhang L, Chen W, Liu Y, et al. (2010). Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol 73:396–403.
- Stoll AM, Chianta MA, Piergallini JR. (1979). Thermal conduction effects in human skin. Aviat Space Environ Med 50:778–87.
- Leon-Villapalos J, Kaniorou-Larai M, Dziewulski P. (2005). Full thickness abdominal burn following magnetic resonance guided focused ultrasound therapy. Burns 31:1054–5.
