Abstract
Background: Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) benefits selected patients with peritoneal mesothelioma. We present the outcomes of this treatment strategy in a UK peritoneal malignancy national referral centre.
Methods: Observational retrospective analysis of data prospectively collected in a dedicated peritoneal malignancy database between March 1998 and January 2016.
Results: Of 1586 patients treated for peritoneal malignancy, 76 (4.8%) underwent surgery for peritoneal mesothelioma. Median age was 49 years (range 21–73 years). 34 patients (45%) were female. Of the 76 patients, 39 (51%) had low grade histological subtypes (mostly multicystic mesothelioma), and 37 (49%) had diffuse malignant peritoneal mesothelioma (DMPM; mostly epithelioid mesothelioma). Complete cytoreduction was achieved in 52 patients (68%) and maximal tumour debulking (MTD) was performed in 20 patients (26%); the remaining 4 patients (5%) underwent a laparotomy with biopsy only. HIPEC was administered in 67 patients (88%). Median overall (OS) and disease-free survival (DFS) after CRS was 97.8 (80.2–115.4) and 58.8 (47.4–70.3) months, respectively. After complete cytoreduction, 100% overall survival was observed amongst patients with low-grade disease. Ki-67 proliferation index was significantly associated with survival outcomes after complete cytoreduction for DMPM and was an independent predictor of decreased survival.
Conclusion: With adequate patient selection (guided by histological classification and Ki-67 proliferation index) and complete cytoreduction with HIPEC, satisfactory outcomes can be achieved in selected patients with peritoneal mesothelioma.
Introduction
The incidence of mesothelioma in developed countries varies from 14 to 35 cases per million per year and peritoneal mesothelioma accounts for 10–30% of all cases [Citation1,Citation2]. Diffuse malignant peritoneal mesothelioma (DMPM) is a rare and fatal primary neoplasm of the serosal lining of the peritoneum with increasing incidence worldwide [Citation3,Citation4].
The aetiology, presentation, diagnostic and therapeutic management of peritoneal mesothelioma have been summarised in a recent review [Citation5]. Several environmental and genetic risk factors contributing to the development of peritoneal mesothelioma have been identified [Citation6–10]. Importantly, a proportion of patients who develop peritoneal mesothelioma, particularly cystic mesothelioma, have no definite history of exposure to asbestos [Citation11,Citation12].
Clinical presentations of DMPM are non-specific [Citation1,Citation12]. Patients usually present with signs and symptoms that reflect a diffuse progressive abdominal condition, including ascites and obstructive symptoms [Citation5].
Standard initial investigation is by CT imaging and tumour specimens for histological assessment may also be obtained by CT guided biopsy [Citation13]. Although CT and MRI have some utility in diagnosis, the extent of small bowel serosal involvement is often underestimated. Diagnostic laparoscopy has the added advantage of allowing direct visualisation of the peritoneal cavity and the assessment of the absence, or extent, of small bowel involvement which helps to select patients amenable to operative intervention. Ascites, if present, can also be drained at laparoscopy.
Until recently, systemic chemotherapy was considered standard treatment for both pleural and peritoneal mesothelioma. However, in peritoneal mesothelioma response rates after systemic chemotherapy are limited and associated with substantial toxicity [Citation5,Citation14,Citation15]. Despite the development of new immunotherapy agents such as checkpoint inhibitor PD-1 and new gene-targeted therapies, there has been little change in overall outcomes over the last decade.
Surgery can be of benefit in selected patients, particularly where complete tumour removal can be achieved. Complete removal of macroscopic disease, by cytoreductive surgery (CRS) using peritonectomies and multivisceral resection, if necessary, combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to result in good outcomes and long-term survival [Citation16]. If the small bowel is seen to be extensively involved at laparoscopy then CRS and HIPEC is unlikely to be of benefit. An exception to this is where there is a large omental cake and recurrent ascites, whereby a major tumour debulking with omentectomy (and, in patients with intestinal obstruction, total colectomy with ileostomy) combined with HIPEC may give good palliation by suppression of ascites [Citation1,Citation17]. The presence and extent of mesothelioma at crucial anatomical sites, such as the portal structures or small bowel serosa, limits the ability to achieve complete cytoreduction [Citation18].
Peritoneal mesothelioma is traditionally categorised into either low-grade peritoneal mesothelioma (LGPM) or DMPM. LGPM includes multicystic mesothelioma and well-differentiated papillary mesothelioma (WDPM) [Citation19,Citation20]. Though multicystic mesothelioma has historically been described as a benign disorder, its behaviour suggests that recurrence is likely, unless radical resectional surgery is combined with HIPEC [Citation13,Citation21]. DMPM includes epithelioid, biphasic and sarcomatoid subtypes [Citation22,Citation23]. Ki-67 proliferation index, which has been shown to correlate with prognosis in a number of tumours [Citation24,Citation25], has recently been demonstrated to influence outcome after CRS and HIPEC in patients with DMPM [Citation26–28].
Here, we report the outcomes of a treatment strategy of CRS and HIPEC in patients with both low grade and DMPM and demonstrate the association of clinicopathological parameters with survival outcomes.
Methods
Patient and surgical procedures
Prospectively collected data in a dedicated peritoneal malignancy database between March 1998 and January 2016 were analysed. Patients undergoing surgery for peritoneal mesothelioma were included.
Suitability for surgery was assessed by a combination of clinical history, physical examination, CT scan and tumour marker analysis. At laparotomy, disease extent was quantified using the Peritoneal Cancer Index (PCI) [Citation29]. The intra-operative strategy was to remove all visible tumour (complete cytoreduction); however, patients in whom a complete cytoreduction was not considered achievable (due to tumour extent and distribution on pre-operative investigations), were offered a planned maximal tumour debulking (MTD) procedure to achieve palliation of symptoms (mainly refractory ascites and obstructive symptoms) [Citation30–32]. Complete cytoreduction was defined as CC0/1 (CC0 – no residual disease, CC1– esidual nodules up to 2.5 mm, CC2–nodules up to 2.5 cm and CC3–nodules >2.5 cm) and MTD as CC2/3 [Citation32]. In patients who underwent a complete cytoreduction, a variety of peritonectomy procedures were used in combination with visceral resections, to remove all visible tumour or, if not possible, to reduce residual tumour deposits to less than 2.5 mm, the estimated maximum direct penetration of locally applied chemotherapy. As per the institutional policy, all patients with a rectal anastomosis were defunctioned with loop ileostomy.
HIPEC was administered at 42–43 °C for 1 h by an open abdominal perfusion technique using a combination of doxorubicin (15 mg/m2) and cisplatin (50 mg/m2). In selected patients, in addition to HIPEC, early postoperative chemotherapy (EPIC) using doxorubicin (3 mg/m2) and cisplatin (20 mg/m2) was administered for a maximum of 4 days following surgery. EPIC was administered to patients who had complete cytoreduction, were haemodynamically stable during and after operation and had no, or one, bowel anastomosis only.
Routine follow-up for patients who had complete cytoreduction was by annual CT and tumour marker assessment or at shorter intervals if clinically indicated. Tumour markers measured were CEA, CA19.9 and CA125. Though not specific to mesothelioma, if markers are elevated prior to surgery, measurement is useful in monitoring peritoneal disease recurrence [Citation33–36].
Histopathological analysis
After referral, histopathology of previously resected specimens and ascitic fluid cytology, if available, was carefully reassessed by a histopathologist with a special interest in peritoneal mesothelioma. Specimens resected during definitive laparotomy in our unit were similarly assessed by a dedicated pathologist.
For determination of the Ki-67 proliferation index, monoclonal mouse anti-human Ki-67 antigen clone MIB-1 obtained from Dako was used. Positive control tissue from tonsil was stained in parallel with the study cases. The result of Ki-67 immunoreactivity for all assessments was expressed as the percentage of tumour cells with positive nuclear staining. Ki-67 expression was measured by two histopathologists with an interest and experience in peritoneal malignancy.
Statistical analysis
Continuous variables are presented with median and range, and categorical data with frequency and percentage. The Mann–Whitney U-test was used for continuous data and Pearson’s chi-square test for categorical data was used to detect differences between the groups where applicable. Statistical significance of the survival was analysed with the log-rank (Mantel-Cox) test and Kaplan–Meier curves. Multivariate analysis was performed with the Cox proportional hazards model. A p value of less than 0.05 was considered significant. Data were analysed using SPSS software (version 24) (IBM, Armonk, NY).
Results
Study population
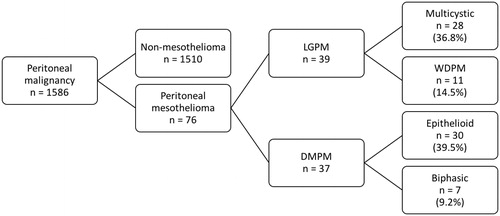
Between March 1998 and January 2016, 1586 patients underwent surgery for peritoneal malignancy; of these, 76/1586 (4.8%) underwent surgery for peritoneal mesothelioma. The study population and distribution of histological subtypes are shown in .
Demographic and surgical characteristics, as well as Ki-67 proliferation index of the study population are shown in . Ki-67 proliferation index data was available for 63/76 patients (83%); of the 52 patients undergoing a complete cytoreduction, data on Ki-67 proliferation index was available for 46 (89%). Patients with DMPM had significantly greater disease extent (as quantified by PCI) and higher Ki-67 proliferation index than those with LGPM.
Table 1. Demographic and surgical characteristics of 76 peritoneal mesothelioma patients.
Surgical treatment
Of the 76 patients undergoing laparotomy for peritoneal mesothelioma, a complete cytoreduction (CC0 or CC1) was achieved in 52 (68%); MTD was performed in 20 patients (26%) and 4 patients (5%) had a biopsy procedure only. Of the 52 patients with a complete cytoreduction, CC0 was achieved in 37 (71%) and CC1 in 15 (9%). As shown in , complete cytoreduction rates were significantly lower in patients with DMPM (18 patients; 49%) than in those with low-grade disease (34 patients; 87%). Amongst the patients with low-grade disease, rates of complete cytoreduction were significantly higher for multicystic versus WDPM (93 versus 55%; p = 0.006).
HIPEC was administered in 67 patients (88%), with 57 treated with cisplatin and doxorubicin, while the remaining 10 patients received mitomycin C. Overall 50/52 patients with complete cytoreduction received HIPEC; the remaining two had major haemodynamic instability which prevented safe HIPEC administration. Of the 20 patients undergoing MTD, 17 received HIPEC to suppress re-accumulation of ascites [Citation30–32]. Median operative time was 420 min (range 30–720) with median estimated blood loss of 1 L (range 0.2–2.5). EPIC was administered in 11 patients (15%). Median postoperative length of stay was 15 days (range 5–62). There was no 30-day postoperative mortality. Mortality within 90 days occurred in 2/76 patients (2.6%), one of whom was revealed to have pleural mesothelioma on post-mortem examination. 5 patients (7%) developed Clavien-Dindo grade III/IV complications.
Survival outcomes
Median follow-up time was 34 months (range 6–152). For the entire study population, median overall survival (OS) was 97.8 months (95% CI: 80.2–115.4), with 3- and 5-year OS rates of 75.8 and 67.7%, respectively. Median disease-free survival (DFS) was 58.8 months (95% CI: 47.4–70.3) with 3- and 5-year DFS of 67.5 and 60.4%, respectively.
After complete cytoreduction, median OS was 127.0 months (95% CI: 111.0–143.0) with 3- and 5-year survival rates of 87.6 and 79.7%, respectively; however, even after MTD median OS of 43.2 months (95% CI: 20.0–66.4), i.e. nearly 4 years, was achieved, with 3- and 5-year survival of 45.0 and 36.1%, respectively. Survival rates after MTD were significantly higher in patients with low-grade mesothelioma versus DMPM, with median OS 85.1 months (95% CI: 28.2–142.0) and 29.0 months (95% CI: 14.3–43.7), respectively. Of the 5 patients with low-grade mesothelioma not undergoing a complete cytoreduction, 4 patients died of disease progression during follow-up.
Survival after complete cytoreduction
Amongst the 52 patients undergoing a complete cytoreduction, OS and DFS were significantly lower in DMPM patients as compared to those with low-grade mesothelioma, as outlined in and . All patients with low-grade disease, who had complete cytoreduction, were alive at the end of follow-up. Overall and disease-free survival rates did not differ significantly between patients with multicystic and WDPM.
Figure 2. Kaplan–Meier curves for overall (A) and disease-free (B) survival for patients with low-grade peritoneal mesothelioma (LGPM) and diffuse malignant peritoneal mesothelioma (DMPM).
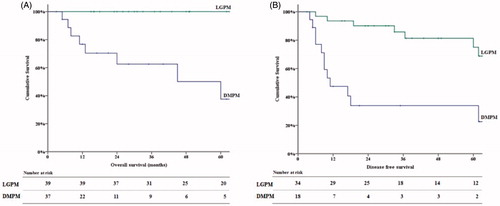
Table 2. Survival outcomes after complete cytoreduction by histological subtype.
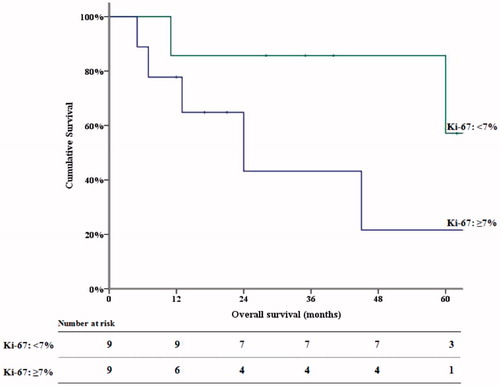
The effect of PCI and Ki-67 proliferation index on survival following complete cytoreduction in DMPM patients was investigated. Median values (used as cut-off values) for PCI and Ki-67 proliferation index in this group were 15 and 7%, respectively. PCI had no significant association with survival outcomes. In contrast, Ki-67 proliferation index was significantly associated with OS, as shown in . In patients with Ki-67 proliferation index <7%, mean OS was 70.0 months (95%CI: 47.5–92.0), with 3-year and 5-year OS of 86.5 and 59.0%, respectively. In patients with Ki-67 proliferation index ≥7%, mean OS was 32.8 months (95% CI: 15.0–50.0), with 3- and 5-year OS of 42.2% and 21.9%.
Multivariate analysis
Cox proportional hazard model was used to identify independent predictors of overall survival after complete cytoreduction in patients with DMPM. Gender, age, tumour markers, PCI and Ki-67 proliferation index were included in multivariate analysis. Ki-67 proliferation index was demonstrated to be an independent predictor of decreased overall survival (HR 3.4; 95% CI: 1.7–18.1; p = 0.040).
Discussion
Malignant mesothelioma, whether pleural or peritoneal, generally has a poor prognosis, and there are few treatment options for these patients [Citation12,Citation37]. Due to the rarity of the disease, histological diagnosis is challenging, and often delayed, and may require review by an experienced pathologist for expert pathological classification [Citation14,Citation38]. Once the diagnosis has been made there are few chemotherapy options with no new systemic drug regimes in the last decade since Vogelzang demonstrated that the addition of pemetrexed to cisplatin improved survival in patients with pleural mesothelioma [Citation14]. Systemic treatment using this combination of cisplatin and pemetrexed is associated with median overall survival of just 13 months [Citation39]. In the absence of effective chemotherapeutic agents, the only treatment to show any real promise has been CRS with HIPEC.
Outcomes from a multi-institutional study of 401 patients with malignant subtypes of peritoneal mesothelioma (excluding multicystic and WDPM) demonstrated overall median survival of 53 months with 3 and 5 year survival rates of 60 and 47%, respectively. The predominant histological subtype was epithelioid which had the best survival after complete cytoreduction and HIPEC, if there were no lymph nodes involved [Citation16]. Baratti reported similar findings in 108 patients treated with CRS and HIPEC identifying a Ki-67 proliferation index of <10% as an indicator of prolonged survival [Citation2,Citation28]. A subset of 26 patients from the same multi-institutional group had multicystic mesothelioma. After a median follow up of 54 months all 26 were alive after complete cytoreduction with HIPEC [Citation12]. which is similar to our experience.
This current study reports outcomes of CRS and HIPEC in peritoneal mesothelioma, including low-grade histological types as well as DMPM. The patients in the current series included a relatively high proportion with low-grade lesions (multicystic and WDPM), and there were no patients with sarcomatoid mesothelioma. This distribution of cases reflects the pattern of referral to the unit. The results support the classification of multicystic and WDPM as low-grade and demonstrate that even these low-grade subtypes can recur after CRS and HIPEC.
The relatively high rate of incomplete cytoreduction in our series is caused by our strategy of routinely offering MTD to those patients who with refractory symptoms who are not considered candidates for a complete cytoreduction, in contrast to most previous reports, where such patients were not offered CRS at all. Our data show that, in patients in whom complete cytoreduction is not possible, a strategy of MTD still achieves survival outcomes superior than those achieved historically with palliative systemic chemotherapy [Citation40]. This is in keeping with previously demonstrated results of MTD in pseudomyxoma peritonei [Citation32]. The addition of HIPEC in the majority of patients undergoing debulking is based on previous findings in patients with refractory malignant ascites, where a combination of debulking and HIPEC significantly improved quality of life and ascites inhibition [Citation30,Citation31].
The present study shows that, in DMPM, outcomes after complete CRS and HIPEC is influenced by the Ki-67 proliferation index; patients with a proliferation index ≥7% had significantly decreased survival rates. Moreover, Ki-67 proliferation index was found to be an independent predictor of decreased overall survival after complete cytoreduction for DMPM. This has clear implications for patient selection and indications for either neo-adjuvant or adjuvant systemic chemotherapy in this patient population; moreover, this finding underlines the importance of determining Ki-67 proliferation index in pre-treatment biopsy specimens.
In contrast to Ki-67 proliferation index, PCI score was not associated with outcome after complete cytoreduction. This finding is in contrast to a recent study of 117 patients with DMPM, where PCI was found to be an independent predictor of survival after CRS and HIPEC, along with the Ki-67 proliferation index [Citation28]. However, this study included 23 patients with an incomplete cytoreduction in the survival analysis; it is possible that the negative association of PCI found in this study was due to the impact of PCI on completeness of cytoreduction. In our study, this effect is filtered out by limiting the survival analysis to patients undergoing a complete cytoreduction. However, the relatively small study population in our study may also have impacted the ability to identify any association between PCI and survival outcome.
The results also demonstrate that CRS and HIPEC is a relatively safe treatment in patients with peritoneal mesothelioma, with major morbidity occurring in approximately 7% of patients. This is in keeping with previously published rates of morbidity in our peritoneal malignancy institute [Citation41]. Some patients will develop recurrent disease and there is a role for repeat CRS and HIPEC in selected patients with favourable histopathology and a longer interval from surgery to recurrence [Citation42]. Long term intraperitoneal chemotherapy has also been proposed using indwelling intraperitoneal ports but we have no experience with this approach [Citation43]. New immunotherapy agents, such as checkpoint inhibitor PD-1, are showing some activity in solid tumours and may herald the next treatment modality for peritoneal mesothelioma [Citation44,Citation45]. Despite these novel agents, CRS and HIPEC is likely to continue as a key part of the management of selected patients with peritoneal mesothelioma. The results in this series suggest that CRS and HIPEC, even when complete cytoreduction is not achieved, is of benefit to patients with either low-grade or malignant mesothelioma and at present is the only treatment which has the potential for cure.
Disclosure statement
The authors declare no conflicts of interest pertaining to the subject of this manuscript.
References
- Yano H, Moran BJ, Cecil TD, Murphy EM. (2009). Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal mesothelioma. Eur J Surg Oncol 35:980–5.
- Baratti D, Kusamura S, Cabras AD, et al. (2013). Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer 49:3140–8.
- Mohamed F, Sugarbaker PH. (2002). Peritoneal mesothelioma. Curr Treat Options Oncol 3:375–86.
- Mohamed F, Cecil T, Moran B, Sugarbaker P. (2011). A new standard of care for the management of peritoneal surface malignancy. Curr Oncol 18:e84–96.
- Garcia-Fadrique A, Mehta A, Mohamed F, et al. (2017). Clinical presentation, diagnosis, classification and management of peritoneal mesothelioma: a review. J Gastrointest Oncol 8:915–24.
- Maurer R, Egloff B. (1975). Malignant peritoneal mesothelioma after cholangiography with thorotrast. Cancer 36:1381–5.
- Testa JR, Carbone M, Hirvonen A, et al. (1998). A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res 58:4505–9.
- Baumann F, Ambrosi JP, Carbone M. (2013). Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol 14:576–8.
- Liu S, Staats P, Lee M, et al. (2014). Diffuse mesothelioma of the peritoneum: correlation between histological and clinical parameters and survival in 73 patients. Pathology 46:604–9.
- Cleaver AL, Bhamidipaty K, Wylie B, et al. (2014). Long-term exposure of mesothelial cells to SV40 and asbestos leads to malignant transformation and chemotherapy resistance. Carcinogenesis 35:407–14.
- Dogan AU, Baris YI, Dogan M, et al. (2006). Genetic predisposition to fiber carcinogenesis causes a mesothelioma epidemic in Turkey. Cancer Res 66:5063–8.
- Chua TC, Yan TD, Deraco M, et al. (2011). Multi-institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. Br J Surg 98:60–4.
- Mohamed F, Sethna K, Elias D, Sugarbaker PH. (2003). Challenging and unusual cases: Case 3. Peritoneal cystic mesothelioma. J Clin Oncol 21:1419–20.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. (2003). Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–44.
- Simon GR, Verschraegen CF, Janne PA, et al. (2008). Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol 26:3567–72.
- Yan TD, Deraco M, Baratti D, et al. (2009). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 27:6237–42.
- Hompes D, D'Hoore A, Van Cutsem E, et al. (2012). The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol 19:2186–94.
- Bretcha-Boix P, Farre-Alegre J, Sureda M, et al. (2010). Cytoreductive surgery and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colonic origin: outcomes after 7 years' experience of a new centre for peritoneal surface malignancies. Clin Transl Oncol 12:437–42.
- Malpica A, Sant'Ambrogio S, Deavers MT, Silva EG. (2012). Well-differentiated papillary mesothelioma of the female peritoneum. A clinicopathologic study of 26 cases. Am J Surg Pathol 36:117–27.
- Chen X, Sheng W, Wang J. (2013). Well-differentiated papillary mesothelioma: a clinicopathological and immunohistochemical study of 18 cases with additional observation. Histopathology 62:805–13.
- Sethna K, Mohamed F, Marchettini P, et al. (2003). Peritoneal cystic mesothelioma: a case series. Tumori 89:31–5.
- Attanoos RL, Gibbs AR. (1997). Pathology of malignant mesothelioma. Histopathology 30:403–18.
- Cerruto CA, Brun EA, Chang D, Sugarbaker PH. (2006). Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med 130:1654–61.
- Viale G, Giobbie-Hurder A, Regan MM, et al. (2008). Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 26:5569–75.
- Burkhart RA, Ronnekleiv-Kelly SM, Pawlik TM. (2017). Personalized therapy in hepatocellular carcinoma: Molecular markers of prognosis and therapeutic response. Surg Oncol 26:138–45.
- Deraco M, Cabras A, Baratti D, Kusamura S. (2015). Immunohistochemical evaluation of minichromosome maintenance protein 7 (MCM7), topoisomerase IIalpha, and Ki-67 in diffuse malignant peritoneal mesothelioma patients using tissue microarray. Ann Surg Oncol 22:4344–51.
- Pillai K, Pourgholami MH, Chua TC, Morris DL. (2015). Prognostic significance of Ki67 expression in malignant peritoneal mesothelioma. Am J Clin Oncol 38:388–94.
- Kusamura S, Torres Mesa PA, Cabras A, et al. (2016). The role of Ki-67 and pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DMPM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 23:1468–73.
- Jacquet P, Sugarbaker PH. (1996). Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–74.
- Facchiano E, Scaringi S, Kianmanesh R, et al. (2008). Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol 34:154–8.
- Valle M, Van der Speeten K, Garofalo A. (2009). Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: A multi-institutional retrospective analysis in 52 patients. J Surg Oncol 100:331–4.
- Dayal S, Taflampas P, Riss S, et al. (2013). Complete cytoreduction for pseudomyxoma peritonei is optimal but maximal tumor debulking may be beneficial in patients in whom complete tumor removal cannot be achieved. Dis Colon Rectum 56:1366–72.
- Alexander-Sefre F, Chandrakumaran K, Banerjee S, et al. (2005). Elevated tumour markers prior to complete tumour removal in patients with pseudomyxoma peritonei predict early recurrence. Colorectal Dis 7:382–6.
- Baratti D, Kusamura S, Martinetti A, et al. (2007). Circulating CA125 in patients with peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol 14:500–8.
- Baratti D, Kusamura S, Deraco M. (2009). Circulating CA125 and diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 35:1198–9.
- Di Fabio F, Aston W, Mohamed F, et al. (2015). Elevated tumour markers are normalized in most patients with pseudomyxoma peritonei one week after complete tumour removal. Colorectal Dis 17:698–703.
- Dayal S, Ghosh D, Moran B. (2014). Miscellaneous conditions of the peritoneal cavity–peritoneal tumors, pseudomyxoma, mesothelioma, fibroblastic reaction, cocoon, cystic lymphatic malformations, blue-bleb, and chylous ascites. Semin Pediatr Surg 23:363–8.
- Antman KH, Pomfret EA, Aisner J, et al. (1983). Peritoneal mesothelioma: natural history and response to chemotherapy. J Clin Oncol 1:386–91.
- Alexander HR, Jr, Burke AP. (2016). Diagnosis and management of patients with malignant peritoneal mesothelioma. J Gastrointest Oncol 7:79–86.
- Yan TD, Welch L, Black D, Sugarbaker PH. (2007). A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 18:827–34.
- Moran B, Cecil T, Chandrakumaran K, et al. (2015). The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal Dis 17:772–8.
- Sugarbaker PH, Yan TD, Stuart OA, Yoo D. (2006). Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 32:686–91.
- Kluger MD, Taub RN, Hesdorffer M, et al. (2010). Two-stage operative cytoreduction and intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma: Operative morbidity and mortality in phase I and II trials. Eur J Surg Oncol 36:997–1003.
- Wong RM, Ianculescu I, Sharma S, et al. (2014). Immunotherapy for malignant pleural mesothelioma. Current status and future prospects. Am J Respir Cell Mol Biol 50:870–5.
- Calabro L, Maio M. (2015). Immune checkpoint blockade in malignant mesothelioma. Semin Oncol 42:418–22.