Abstract
Purpose: To confirm the effectiveness of laser ablation on toxic nodules in a large population with three years of follow-up.
Material and methods: Between 2009 and 2014, we treated 82 patients with hyperthyroidism related to the presence of a toxic nodular goitre. Patients were pre-treated pharmacologically with methimazole prior to single session of laser ablation (LA) and then followed up every 3 months with FT4 and TSH blood tests as well as ultrasound examination of the nodules treated.
Results: All patients responded to the treatment. The median nodule volume decreased from 12 ml (range 5–118 ml) to 5 ml (range 1.2–40 ml) after three years (p < 0.001). The percentage of patients who discontinued methimazole therapy was reduced by increasing the initial volume of the toxic nodule. In nodules with a volume less than 5 ml, all patients were able to suspend methimazole; this percentage was reduced to 90.2% in nodules with a volume between 5 and 15 ml, 61.1% in those with volume 15–25 ml and only 28.5% in nodules larger than 25 ml. We had no major complications but only moderate pain and fever in the evening, a few hours after ablation therapy in 10% of treated patients.
Conclusions: Single session of LA of toxic thyroid nodules is effective and safe, especially in nodules with a volume under 15 ml.
Introduction
Nodular thyroid disease is very frequent in iodine-deficient areas, affecting at least 50% of the population. Approximately 10% of solitary nodules are scintigraphically hot, half of which are autonomously functioning [Citation1,Citation2]. Thionamides normalise free triiodothyronine (FT3), free thyroxine (FT4) and serum thyrotrophin (TSH) concentrations before definitive therapy. Radioiodine and surgery are both effective in the treatment of pretoxic as well as toxic thyroid nodules (TTNs) [Citation3]. After the publication of a feasibility study [Citation4] in the early 2000s, Døssing et al. in 2002 [Citation5] and Pacella et al. in 2004 [Citation6] introduced ultrasound-guided laser photo-coagulation (LA) for the treatment of benign thyroid nodules. Thereafter, numerous studies have been published confirming the safety and efficacy of LA therapy [Citation5–25]. Some studies have also been published regarding the effectiveness of LA in toxic nodules [Citation6,Citation7,Citation12,Citation26–28]. However, many of the later studies were performed on a small series of patients, and sometimes on an individual case (1–26), with a limited follow-up period (6–12 months). Our main endpoint was to evaluate the ability of a single session of LA to restore a condition of euthyroidism without any pharmacological treatment. The second endpoint was to evaluate the volume reduction with a single session of LA. We thus retrospectively evaluated 82 toxic nodules treated with a single session of LA and with a follow-up period of 3 years.
Materials and methods
Patients selection
The study was conducted in compliance with the Helsinki Declaration and the local Bioethics Committees. Informed consent for each procedure was obtained from all patients. In this retrospective study, we evaluated all patients with toxic nodules treated by single session of LA during the period 2009–2014. The characteristics of the treated patients are summarised in . Inclusion criteria were: high levels of FT3 and/or FT4 and suppressed TSH values; normal circulating levels of thyroglobulin, calcitonin, anti-thyroglobulin antibodies and antimicrosomal antibodies; evidence of a single hot nodule at scintigraphy, with suppression of extranodular thyroid tissue uptake by 99mTc; benign cytologic examination; high surgical risk or refusal of surgical or other conventional treatments (radioiodine therapy, PEI); no history of neck irradiation and surgery. All patients were treated with methimazole, achieving a condition of euthyroidism with TSH between 2 and 4 uUI/ml prior to laser treatment. We evaluated only solid or predominantly solid (with a fluid component ≤20% of its volume) thyroid nodules [Citation6,Citation13,Citation21]. Using criteria employed in previous studies by other authors [Citation14,Citation21,Citation25,Citation29,Citation30], patients were divided into four groups based on thyroid volume at baseline: 0–5 ml (very small nodules), 5–15 ml (small nodules), 15–25 ml (medium volume nodules) and >25 ml (large nodules) ().
Table 1. Population studied.
Clinical and ultrasound evaluation
Nodule-related symptom score and cosmetic score were recorded at each visit. The nodule-related symptom score was obtained by asking all patients to rate pressure symptoms on a 10-cm visual analogue scale. The cosmetic score was obtained using the following scale: 1, no palpable mass; 2, no cosmetic problem but a palpable mass; 3, cosmetic problem with swallowing only; 4, a readily observable cosmetic problem [Citation31].
Ultrasonography was performed using a 7.5–12-MHz linear probe equipped with colour Doppler and power Doppler modules (Esaote My Lab 50, Genua, Italy). Nodule volume and the percentage of volume reduction were calculated using the following equations: volume percentage (ellipsoid equation): volume percentage = length × width × depth ×0.525; volume reduction percentage: percentage of volume reduction = [(initial volume − final volume) × 100]/initial volume [Citation32].
LA procedure
Treatment procedures followed those previously applied and published elsewhere by Pacella et al. [Citation6]. We do not perform any local and/or general anaesthesia or conscious sedation [Citation33]. LA was performed under sterile conditions and US guidance. The number of 21-gauge (G) applicators (up to four) to be inserted was based on nodule size. The needles were positioned into the target toxic thyroid nodule along its longest axis, at a distance of about 1 cm from the caudal portion of the capsule. Subsequently, a laser 300-μ quartz fibre was inserted into the lumen of the needle, leaving 5 mm of the fibre extending from the needle; patients were treated via a 1064 nm continuous-wave neodymium yttrium–aluminum–garnet laser (Modilite Elesta, Calenzano, Florence, Italy). We used a continuous output power of 3 W for a variable time until the interstitial hyperechoic area produced by thermal ablation reached the periphery and a variable energy of 1400–1800 Joules was delivered. During the procedure the needle was moved up from the initial position 1 cm at a time, to reach a distance of 1 cm from the cranial portion of the capsule. At each step, a variable energy of 1400–1800 Joules was delivered on the basis of the extent of the hyperechoic area produced by photocoagulation. After LA, patients were asked whether they would repeat the treatment again, as a marker of tolerability.
Follow-up
Serum levels of FT3, FT4, TSH were evaluated one week before and after the treatment, and every 3 months thereafter for a total of three years. The ultrasonographic and colour/power examinations of the thyroid were performed before the LA session and every 3 months in the first year, and then once a year for 3 years. The volume of the nodules was measured by the same investigator, blinded for treatment, using the ellipsoid (formula above described). Patients were considered responsive to therapy if treatment with methimazole was reduced or discontinued. Volume reduction was considered a secondary endpoint.
The complications and side effects, as well as the terminology used, were evaluated in agreement with the “International Working Group on Image-Guided Tumor Ablation and the Technology Assessment Committee of the Society of Interventional Radiology” [Citation34,Citation35].
Statistical analysis
Values for quantitative variables are expressed as median and range. The Wilcoxon test was used to check whether the change in volume between every two consecutive follow-up visits were statistically significant. Statistical analysis was performed using SPSS (SPSS, Chicago, IL).
Results
The results are summarised in . There was a significant correlation between thermal energy delivered and nodule reduction (p < 0.001). The median nodule volume decreased from 12 (range 5–118) to 5.1 ml (range 1.2–39 ml) after 12 months (p < 0.001) and to 5 ml (range 1.2–40 ml) at the final evaluation (p < 0.001). Two optic fibres were placed (median 2, range 1–4); total energy delivered was 7200 (range 3600–18,400) at the output power of 3 W (see ).
Figure 1. Longitudinal scan of toxic nodule before (AP 23 mm × TR 32.5 × LG 39 mm; volume 15.2 ml) and after 6 months (AP 15 mm × TR 22.5 mm × LG 29; volume 5.1 ml) of single session of LA.
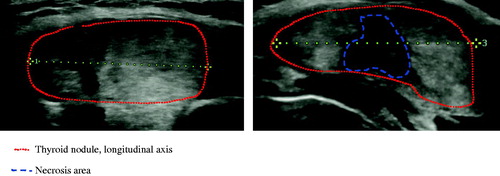
Table 2. Volume reduction and hormonal changes at different times.
Table 3. Correlation between initial volume of toxic nodule and therapeutic response (suspension of methimazole) with volume reduction ratio.
Statistical regression analysis showed a correlation between the initial nodule volume and the outcomes after a single session of LA therapy (p = 0.01), with a large effect in nodules smaller than 15 ml ().
The percentage of patients who discontinued methimazole therapy was reduced by increasing the initial volume of the toxic nodule. Patients who could not suspend drug therapy reduced dosage of methimazole by 53% (range 30–72%). In fact, all patients who had nodules with a volume less than 5 ml were able to suspend methimazole; in nodules with a volume between 5 and 15 ml, the dose was reduced to 90.2% in those with volume 15–25 ml, to 61.1%, and in nodules larger than 25 ml, only to 28.5%.
The patients with very small or small nodules did not have significant compressive symptoms and/or cosmetic damage signs. In patients with volume at baseline greater 15 ml (18 patients with nodules ranging in volume between 15 and 25 ml and seven patients with volume >25 ml at baseline), the presence of local symptoms decreased from 36% to 11% of cases (p < 0.001) and evidence of cosmetic signs from 76% to 8% of cases (p < 0.001) [Citation50]. More in detail, local symptoms decreased from 42% to 11% in medium-sized nodules (p < 0.001), and from 80% to 19% in large nodules (p < 0.001). Evidence of cosmetic score decreased from 56% to 8% in medium-sized nodules (p < 0.001), and from 73% to 14% in large nodules (p < 0.001).
Complications
The results of this study confirm the low incidence of major complications already reported in previous studies [Citation36–38]. The single session of LA was well tolerated, as demonstrated by the fact that all patients would accept a new LA. No patient had major complications; only two side effects were reported in the first 24 h: moderate pain (11/82, 13.4%) and fever (5/82, 6%).
Discussion
The prevalence of toxic nodules is about 4–9% in iodine-deficient regions. They can be solitary nodules or a part of multinodular goitre. Treatment is indicated for nodular size, compression of adjacent structures, hyperthyroid symptoms or to avoid progression to hyperthyroidism (annual risk 4%) [Citation23].
Current treatments include radioiodine and surgery. Therapy with 131I causes a reduction in volume of about 50% and in the risk of hypothyroidism of at least 10% [Citation39]. Surgical intervention is usually reserved for large nodules [Citation40].
Some studies have shown the effectiveness of LA in treating small solitary and mild hyperfunctioning nodules [Citation7,Citation12,Citation27,Citation28,Citation41,Citation42]. Instead, the favourable results are inconsistent in toxic goitres or large AFTNs, and the normalisation of thyroid function usually requires repeated treatment sessions [Citation6,Citation16,Citation27]. All these studies highlight the importance of complete ablation of toxic thyroid nodules (TTNs) to restore euthyroidism steadily over time. Also, in case of combined treatment (131therapy followed by LA) in patients with TTNs <5 cm, a single session of LA alone is sufficient to achieve complete ablation of the nodule and simultaneously induce normalisation of TSH without resorting to the subsequent administration of radioiodine [Citation43]. It is therefore essential to completely destroy hyperfunctioning area or achieve a very high percentage of coagulation zone with consequent marked reduction of hot thyroid tissue with a single or multiple sessions of ablation. Of course, this result is more easily accessible in case of nodules with volume around 10–15 ml, while repeated sessions are necessary in large TTNs, with increased cost and discomfort [Citation42]. In the latter case, the major limitation is the risk of incomplete and insufficient ablation of the external border of the hyperfunctioning lesion, followed by regrowth of AFTN and relapse of hyperthyroidism at long-term follow-up [Citation6,Citation27].
These studies have been conducted predominantly on a small series of cases and for a limited follow-up period. The rate of normalisation of TSH in patients with multinodular goitre treated with LA was between 47% and 87% (cured patients) within 6–12 months. The energy was delivered in multiple sessions (1–9) [Citation6,Citation12,Citation27] or with multiple cycles of treatment [Citation16]. The rate of patients with euthyroidism ranged from 50% to 88% [Citation12,Citation16].
The same variability is present in studies performed with radiofrequency. Many studies have described the effects of radiofrequency technique on hot thyroid nodules in nonhomogeneous populations previously treated with radioiodine therapy, with or without surgery [Citation44,Citation45], with multiple radiofrequency sessions [Citation46–49] or with different devices and techniques [Citation44,Citation45,Citation50]. The increase of TSH appears variable in the various studies while the clinical remission ranges from 24% to 82% [Citation44–47,Citation49–51].
Interestingly, the percentage of nodular volume reduction was the best parameter predicting a normalised serum TSH, whether laser ablation [Citation16] or radiofrequency ablation RFA is used [Citation51]. More in detail, the following parameters were taken into account: (i) percentage of nodular volume reduction; (ii) difference between nodular volume measured before treatment and after the last cycle (area of necrosis); (iii) total amount of energy delivered expressed in Joules. The correlation between the area of necrosis (as assessed by the absolute changes in nodular volume) and the total amount of energy delivered is also equally interesting [Citation16].
In this study examining only toxic nodules after single session LA we observed that the maximal volume reduction was reached after 6 months and not later, as described by other authors studying LA in non-toxic nodules [Citation15,Citation21].
Prior to treatment, we submitted patients to therapy with methimazole in order to attain euthyroidism in occasion of LA. This allowed us to reduce the risk of post-therapy thyrotoxicosis and to increase the total amount of energy delivered within the nodule. Methimazole therapy, in fact, reduced vascularisation of the nodule and therefore reduced heat dispersion, thereby increasing its effectiveness (unpublished personal observations).
The initial volume of the nodule also predicts the success of the treatment, understood as a possibility of suspending drug therapy. The response is 100% in nodules with a volume of less than 5 ml but remains very high, over 90%, in nodules with a volume less than 15 ml. For larger volumes, the therapeutic response is drastically reduced. It is likely that these less encouraging results obtained in large nodules are due to the residual functioning nodular tissue that is not destroyed by LA.
Some authors [Citation43] have also combined LA and radiometabolic treatment; in our opinion, because patients do not refuse a second treatment when it is indicated, radioiodine therapy could be avoided. In this case, however, the cost should be carefully weighed. Last but not least, this study has also demonstrated the safety of single session of LA.
In conclusion, this study confirms that the single session of LA of toxic nodules is very effective, especially in nodules with a volume less than 15 ml. Prospective multicenter studies with a large population are necessary to confirm our preliminary data and to determine whether the treatment of choice for the most voluminous nodules is radiometabolic or a second session of LA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hegedus L. (2004). Clinical practice. The thyroid nodule. N Engl J Med 351:1764–71.
- Burch HB, Shakir F, Fitzsimmons TR, et al. (1998). Diagnosis and management of the autonomously functioning thyroid nodule: the Walter Reed Army Medical Center experience, 1975–1996. Thyroid 8:871–80.
- Ferrari C, Reschini E, Paracchi A. (1996). Treatment of the autonomous thyroid nodule: a review. Eur J Endocrinol 135:383–90.
- Pacella CM, Bizzarri G, Guglielmi R, et al. (2000). Thyroid tissue: US-guided percutaneous interstitial laser ablation – a feasibility study. Radiology 217:673–7.
- Dossing H, Bennedbaek FN, Karstrup S, Hegedus L. (2002). Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation-initial experience. Radiology 225:53–7.
- Pacella CM, Bizzarri G, Spiezia S, et al. (2004). Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 232:272–80.
- Spiezia S, Vitale G, Di Somma C, et al. (2003). Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid 13:941–7.
- Papini E, Guglielmi R, Bizzarri G, Pacella CM. (2004). Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract 10:276–83.
- Dossing H, Bennedbaek FN, Hegedus L. (2005). Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules – a randomised study. Eur J Endocrinol 152:341–5.
- Cakir B, Topaloglu O, Gul K, et al. (2006). Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Invest 29:876–84.
- Gambelunghe G, Fatone C, Ranchelli A, et al. (2006). A randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Invest 29:RC23–6.
- Dossing H, Bennedbaek FN, Bonnema SJ, et al. (2007). Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol 157:95–100.
- Papini E, Guglielmi R, Bizzarri G, et al. (2007). Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 17:229–35.
- Valcavi R, Riganti F, Bertani A, et al. (2010). Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 20:1253–61.
- Dossing H, Bennedbaek FN, Hegedus L. (2011). Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol 165:123–8.
- Amabile G, Rotondi M, Pirali B, et al. (2011). Interstitial laser photocoagulation for benign thyroid nodules: time to treat large nodules. Lasers Surg Med 43:797–803.
- Gambelunghe G, Fede R, Bini V, et al. (2013). Ultrasound-guided interstitial laser ablation for thyroid nodules is effective only at high total amounts of energy: results from a three-year pilot study. Surg Innov 20:345–50.
- Gharib H, Hegedus L, Pacella CM, et al. (2013). Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 98:3949–57.
- Papini E, Rago T, Gambelunghe G, et al. (2014). Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab 99:3653–9.
- Gambelunghe G, Bini V, Stefanetti E, et al. (2014). Thyroid nodule morphology affects the efficacy of ultrasound-guided interstitial laser ablation: a nested case-control study. Int J Hyperthermia 30:486–9.
- Pacella CM, Mauri G, Achille G, et al. (2015). Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 100:3903–10.
- Mauri G, Cova L, Monaco CG, et al. (2017). Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia 33:295–9.
- Mauri G, Sconfienza LM. (2017). Percutaneous ablation holds the potential to substitute for surgery as first choice treatment for symptomatic benign thyroid nodules. Int J Hyperthermia 33:301–2.
- Negro R, Salem TM, Greco G. (2016). Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int J Hyperthermia 32:822–8.
- Pacella CM, Mauri G, Cesareo R, et al. (2017). A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia 33:911–9.
- Dossing H, Bennedbaek FN, Hegedus L. (2003). Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid 13:885–8.
- Barbaro D, Orsini P, Lapi P, et al. (2007). Percutaneous laser ablation in the treatment of toxic and pretoxic nodular goiter. Endocr Pract 13:30–6.
- Cakir B, Gul K, Ugras S, et al. (2008). Percutaneous laser ablation of an autonomous thyroid nodule: effects on nodule size and histopathology of the nodule 2 years after the procedure. Thyroid 18:803–5.
- Cesareo R, Pasqualini V, Simeoni C, et al. (2015). Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab 100:460–6.
- Achille G, Zizzi S, Di Stasio E, et al. (2016). Ultrasound-guided percutaneous laser ablation in treating symptomatic solid benign thyroid nodules: our experience in 45 patients. Head Neck 38:677–82.
- Baek JH, Lee JH, Valcavi R, et al. (2011). Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 12:525–40.
- Na DG, Lee JH, Jung SL, et al. (2012). Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 13:117–25.
- Gambelunghe G, Bini V, Monacelli M, et al. (2013). The administration of anesthetic in the thyroid pericapsular region increases the possibility of side effects during percutaneous laser photocoagulation of thyroid nodules. Lasers Surg Med 45:34–7.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol 25:1691–705. e1694.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Mainini AP, Monaco C, Pescatori LC, et al. (2017). Image-guided thermal ablation of benign thyroid nodules. J Ultrasound 20:11–22.
- Pacella CM. (2017). Image-guided thermal ablation of benign thyroid nodules. J Ultrasound 20:347–9.
- Papini E, Pacella CM, Misischi I, et al. (2014). The advent of ultrasound-guided ablation techniques in nodular disease: towards a patient-tailored approach. Best Pract Res Clin Endocrinol Metab 28:601–18.
- Bonnema SJ, Hegedus L. (2012). Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev 33:920–80.
- Bahn RS, Burch HB, Cooper DS, et al. (2011). Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract 17:456–520.
- Valcavi R, Bertani A, Pesenti M, et al. (2008). Laser and radiofrequency ablation procedures. In Baskin HJ, Duick DS, Levine RA, eds. Thyroid ultrasound and ultrasound guided FNA biopsy. 2nd ed. New York: Springer, 191–218.
- Rotondi M, Amabile G, Leporati P, et al. (2009). Repeated laser thermal ablation of a large functioning thyroid nodule restores euthyroidism and ameliorates constrictive symptoms. J Clin Endocrinol Metab 94:382–3.
- Chianelli M, Bizzarri G, Todino V, et al. (2014). Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab 99:E1283–6.
- Spiezia S, Garberoglio R, Milone F, et al. (2009). Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 19:219–25.
- Faggiano A, Ramundo V, Assanti AP, et al. (2012). Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab 97:4439–45.
- Baek JH, Moon WJ, Kim YS, et al. (2009). Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 33:1971–7.
- Bernardi S, Dobrinja C, Fabris B, et al. (2014). Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol 2014:934595.
- Che Y, Jin S, Shi C, et al. (2015). Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. Am J Neuroradiol 36:1321–5.
- Sung JY, Baek JH, Jung SL, et al. (2015). Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid 25:112–7.
- Deandrea M, Limone P, Basso E, et al. (2008). US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 34:784–91.
- Bernardi S, Stacul F, Michelli A, et al. (2017). 12-month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine 57:402–8.
