Abstract
Objectives: To evaluate the efficacy and safety of GnRH-α pre-treatment with HIFU ablation for diffuse adenomyosis.
Methods: From January 2014 to December 2016, 61 patients were divided into two groups. Twenty-three patients with larger uteri received pre-treatment with GnRH-α and were then subjected to HIFU, and 38 patients underwent HIFU alone. The technical parameters included treatment time, sonication time, average sonication power, treatment intensity, total energy, non-perfusion volume (NPV) and NPV ratio. Intra-, post-procedural complaints, the relief rate of dysmenorrhoea and clinical effectiveness were followed up.
Results: Although the lesion volume in the HIFU + GnRH group was larger than in the HIFU-only group, higher NPV, NPVR%, treatment intensity and total energy with shorter treatment and sonication times in the HIFU + GnRH group were obtained than that in the HIFU-only group. Significant differences were evident for NPV, NPVR%, average power, and total intensity energy (p < 0.05), but not for other parameters between the two groups (p > 0.05).
Although no differences in the intra-, post-procedural VAS scores, in the adverse effects and in the relief rate or clinical effectiveness were apparent between the two groups (p > 0.05), the relief rate was better in the HIFU + GnRH group than that in the HIFU group from 6 to 12 months after treatment. Self-comparison of differences occurred in the two groups before and after treatment (p < 0.05) and were between the two groups after only 6 months (p < 0.05).
Conclusion: The combination of HIFU with GnRH-α is more effective than HIFU alone for ablation of diffuse adenomyosis. Moreover, the GnRH-α pre-treatment with HIFU is safe.
Introduction
Adenomyosis is a common benign gynaecologic disorder whereby ectopic endometrial and stromal tissues invade into the myometrium and grow diffusely, and the condition is often associated with hypertrophy and hyperplasia of muscle tissue that occur with changing hormone levels during the menstrual cycle [Citation1]. The main complaints include dysmenorrhoea, menorrhagia and infertility, which negatively affect the patient’s routine, life and daily work, and serious distress can ruin the patient’s physical and psychological health and fertility. Diffuse adenomyosis uteri are commonly enlarged and occupy the pelvis. Due to the diffuse growth and lack of a clear boundary between the normal myometrium and lesion, laparotomy and laparoscopic reduction of the glandular muscle cannot clearly and completely excise the lesion, and until now, no other methods have been developed.
Gonadotropin-releasing hormone (GnRH) is a neuro-hormone secreted by the hypothalamus that is essential for regulating human reproduction. GnRH-α causes pituitary GnRH receptor desensitisation and leads to temporary amenorrhoea, which is also called reversible drug oophorectomy. GnRH-α may improve symptoms caused by adenomyosis by suppressing cell proliferation, inducing apoptosis and diminishing the volumes of the uterus and lesion [Citation2,Citation3]. Meanwhile, GnRH-α has an inhibitory effect on the ovaries and leads to similar complaints during menopause; moreover, the most worrisome outcome in women afflicted with adenomyosis is re-occurrence after withdrawal of the medicine.
Ultrasound-guided high-intensity focussed ultrasound (HIFU) is a non-invasive treatment modality that can focus on the lesion and stimulate cellular necrosis through thermal ablation of the cellular shape [Citation4]. Satisfaction has been achieved with HIFU thermal inactivation of fibroids and adenomyosis [Citation5–8]. Recently, HIFU ablation of adenomyosis has generated great interest, especially in patients who wish to retain their uteri and fertility. Technical difficulties and relevant side effects have occurred with one-time HIFU ablation in several women with enlarged uteri. Explorations toward improving the effectiveness of the thermal procedure will be valuable in combination with other options, such as pre-treatment with GnRH-α [Citation9], enhancement of the ablation effect of a microbubble contrast agent known as “SonoVue” [Citation10] and intravenous injection of oxytocin during HIFU ablation [Citation11]. Although the 6-month clinical outcomes combining GnRH-a with HIFU ablation of adenomyosis have significantly improved [Citation12], studies of the 12-month clinical remission, the efficacy and safety of different HIFU technical parameters for sonicating diffuse adenomyosis in large uteri pre-treated with GnRH-α are lacking [Citation13].
Materials and methods
Materials
This study is a retrospective cohort study. From January 2014 to December 2016, 61 infertile women with diffuse uterine adenomyosis took part in the research at the HIFU department of the Center for Minimally Invasive Surgical Gynecology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Forty-three patients were included in the prospective study, 29 were included in the HIFU-only group, and 17 were included in the HIFU + GnRH group. All participants signed written informed consent, and the study was approved by the institutional ethical review board before the procedure.
Patients and inclusion criteria
Women with symptoms of menstrual pain and/or prolonged heavy bleeding were diagnosed with adenomyosis confirmed by ultrasound and/or MRI. The study cohort was divided into two groups. The HIFU-only group was ablated with HIFU, had an average age of 41.24 ± 7.07 years, and had a mean body mass index (BMI) of 23.91 ± 0.1 kg/cm2. Among the 38 patients, a horizontal scar was evident in 15, and a vertical scar was evident in 2. The HIFU + GnRH group was ablated with HIFU after pre-treatment with GnRH-α, had an average age of 41 ± 3.53 years, and had a mean BMI of 23.01 ± 2.76 kg/cm2. Among the 23 patients, a horizontal scar was evident in 12 ().
Table 1. Baseline characteristics of the patients with adenomyosis.
The inclusion criteria were as follows:
women over 18 and less than 50 years of age;
symptoms of prolonged heavy bleeding and/or menstrual pain, except when diseases of the cervix involving vaginal and endometrial lesions were present;
a diffuse uterine unilateral wall dimension greater than or equal to 3 cm that was apparent by acoustic imaging; and
normal communication with the doctor during the procedure.
The exclusion criteria were as follows:
acute pelvic inflammatory disease or
a suspected malignant uterine tumour.
GnRH-α pre-treatment
In the outpatient service, preconditioning with administration of GnRH-α one to three months before HIFU was recommended for women with uterine volumes larger than 300 cm3. Leuprorelin (Takeda, Jap.) was administered by subcutaneous injection at a dose of 3.75 mg per injection from one to three months. Trans-abdominal or transvaginal ultrasound was performed to measure the size of the uterus and lesion before and within one month of initiation of the medicine. Ultrasound-guided HIFU was performed within one month.
Pre-HIFU ablation preparation
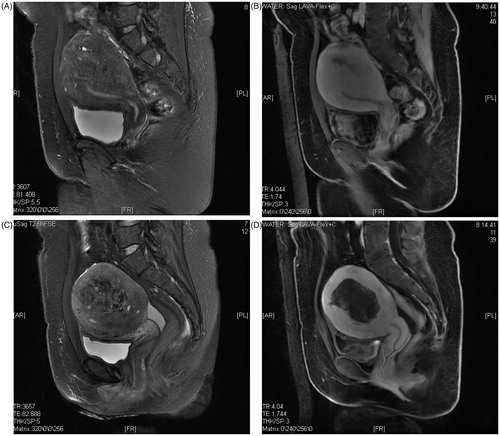
Relevant pre-surgical examinations were performed in addition to MRI () and/or ultrasonic examination to indicate the location, extent and vascular supply of the lesion. All women were instructed by specially assigned nurses to consume mild food for bowel preparations three days prior to becoming inpatients. Coloclysis was completed before the morning of the treatment day after 12 h of fasting. Skin preparations, degassing and degreasing were performed before treatment to diminish skin trauma. A urinary catheter was inserted to maintain a full bladder via irrigation with degassing warm saline to move away the bowel occupying the therapeutic acoustic pathway during the procedure. Intramuscular injection of atropine sulphate and tropisetron were performed 30 min before therapy to reduce secretions and avoid emesis [Citation8].
Ultrasound-guided HIFU ablation process and management after the HIFU procedure
The treatment equipment was produced by JC200D (ultrasound-guided high-intensity focussed ultrasound therapeutic system, Chongqing Haifu Medical Corporation Ltd.). The ultrasound-guided device (Mylab79, Esaote, Italy), which was situated at the centre of the tank, offered real-time guidance according to the echo change during treatment. The therapeutic probe, which was 20 cm in diameter with a frequency of 0.8 MHz, emitted the highest energy at 400 W. The sizes of the focal regions were 1.5 mm × 1.5 mm × 8 mm. Adjustment of the volume of the full bladder and a degassed balloon were performed to push away the bowel occupying the acoustic pathway.
Patients were prone on the table tank, which was full of chilled degassed water (temperature below 15). Intravenous injection of Fentanyl (0.8–1 µg/kg) and Midazolam hydrochloride (0.02–0.03 mg/kg) was performed to attain sedation and analgesia and repeated at 30–40-min intervals so that all patients were kept conscious and reactive to the doctor’s instructions during the process. Ultrasonic imaging was used to map the ablated area and the near-by tissues around the uterus in real-time. Sonication was limited to the range of the lesions and started from deep in the lesion to the surface, with a safe distance of 10–15 mm away from the boundary of the lesions and endometrium. First, confirmation that there was no bowel or other foreign matter in the acoustic pathway was obtained. The treatment plan was developed according to the segmentation of the 5-mm sequence. Then, sequence-by-sequence sonication was commenced as a ratio of 1:3 with an energy of 300 W or 400 W, which produced a temperature from 65 °C to the highest at 100 °C for 10 min after intravenous bolus injections of SonoVue () [Citation8]. The rhythm of adjustment was based on the patient’s vital signs and remedy reaction. Sonication was not completed until the whole lesion echotextures had enhanced or changed under ultrasonic real-time monitoring. The results were evaluated in view of the non-perfused range of contrast-enhanced ultrasound (CEUS) ().
Figure B1–2. (B1) CEUS image before the HIFU procedure. (B2) CEUS image immediately after the HIFU procedure.
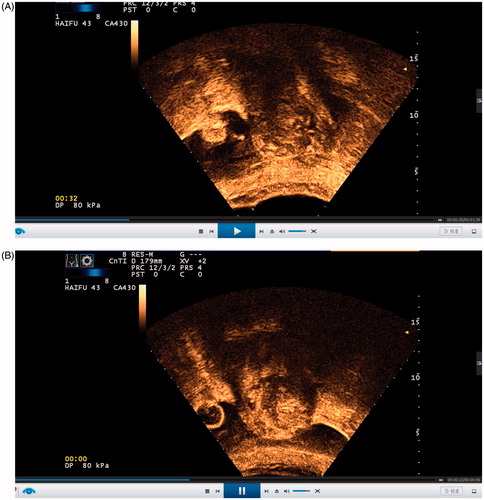
Before the beginning of sonication, intravenous injection of SonoVue (Bracco Suisse SA, Switzerland) was performed to evaluate the lesion range and its vascular perfusion compared with that of the uterine myometrium and to anticipate difficulties with thermal sonication. Before the end of sonication, CEUS was performed to map the lesion non-perfusion or necrotic area ablated by HIFU and to assess the ablation effect. CEUS was performed by injecting 1.5 ml of a mixture in which 59 mg of SonoVue was dissolved in 5 ml of normal saline, with a subsequent injection of 5 ml of normal saline as soon as possible by intravenous bolus sequentially every time. In real-time, every sequence was screened. Sometimes, if the results of the CEUS were not satisfactory, sonication would continue, and the CEUS was performed again to assess non-perfusion in the lesion. The volume of the adenomyotic lesion, the non-perfused volume (NPV) and the NPV ratio (NPVR %) were calculated with the following equation for the prolate ellipsoid: volume = 0.5233 × a × b × c, where a, b, and c indicate the longitudinal dimension, anterior–posterior dimension and transverse dimension, respectively.
At the end of treatment, patients remained in a prone position to allow the treated square of the skin to soak in cool water and to diminish the heat in the focal area for approximately 30 min after irrigation of the bladder with cold normal saline. Patients were permitted to leave unless they experienced signs of discomfort, whereby they were kept in the nursing ward in a prone position for two hours. All patients were discharged the next morning. Per the usual protocol, prophylactic antibiotics were prescribed orally for 3 to 7 days.
Outcome measurements
The parameters that were used to evaluate the efficiency of ultrasound-guided HIFU thermal ablation were as follows: NPV, NPVR%, treatment time, sonication time, treatment intensity, average sonication power and total energy. Pre-manual CEUS was performed to define the perfusion and volume of the adenomyotic lesion, and a post-manual CEUS was performed to evaluate NPV. The NPVR% was calculated as the NPV divided by the lesion volume. Successful treatment with HIFU was defined as an occurrence of an NPV of no less than 1 cm3 in the planned ablation zone [Citation14].
All adverse events and complications in the patients were recorded by a nurse both during and after the procedure to examine the safety of HIFU. All patients were kept in the hospital for observation for one night after treatment and discharged. Follow-ups were performed by staff via telephone to register all post-surgical symptoms and complaints. One month and/or three months after treatment, routine check-ups were performed by evaluating the uterine volume and NPV by MRI () and/or ultrasound. A follow-up visit was scheduled within the first three days, at one week, and at two weeks via phone call. Meanwhile, the VAS, remission rate and clinical effectiveness were evaluated at 3 months, 6 months and 12 months after HIFU ablation respectively.
Based on the Society of Interventional Radiology (SIR) clinical application guidelines, the regulations for complications that were designated by the International Association of Interventional Radiotherapy were clarified. Grades A to B were considered minor, and grades C to F were considered major [Citation14].
Statistical tests
Normally distributed data are expressed as the mean ± standard deviation. Independent Student’s t-tests were used to compare the lesion and uterine volumes, NPV, NPVR%, treatment time, sonication time, acoustic power and total energy between the two groups. χ2 tests were utilised to compare the intra-procedural side effects, post-procedural complaints, VAS, relief rate and clinical effectiveness between the two groups. SPSS version 20.0 software (IBM Corporation, Armonk, NY) was used for data analysis. p < 0.05 was considered statistically significant.
Results
Baseline characteristics of the patients with adenomyosis
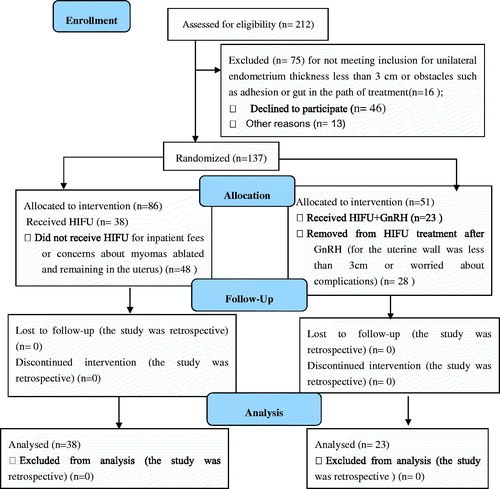
As shown in , 212 patients with menorrhagia participated in localising ultrasonic travel with ultrasound. Among those patients, 75 cases were excluded. Sixteen did not meet inclusion for unilateral endometrium thickness less than 3 cm or had obstacles such as adhesion or gut in the path of treatment, 46 declined to participate, and 13 for other reasons. Among 137 participates, 86 were allocated to the HIFU-only group, and 38 received HIFU. Forty-eight did not receive HIFU for inpatient fees or concerns about the myomas ablated staying in the uterus. Fifty-one were allocated to the HIFU + GnRH group, 23 received HIFU+GnRH, and 28 were removed from HIFU treatment after GnRH for the uterine wall was less than 3 cm or due to worries about complications. All participants completed follow-up at 1 month, those lost-to-follow-up were 4, 4 and 6 in the HIFU + GnRH group, and 1, 3 and 4 in the HIFU-only group at 3 months, 6 months and 12 months, respectively. Sixty-one patients with adenomyosis were included in the study, and these patients were divided into the two groups: the HIFU-only group with 38 cases and the HIFU + GnRH group with 23 cases. There were no statistically significant differences in the patient demographics or diffuse adenomyosis uteri dimensions between the two groups. In the HIFU + GnRH group, a statistically significant difference was found in the volume of the uterus (452.48 ± 280.31 cm3 vs 297.94 ± 137.57 cm3) before and after treatment with GnRH-α (p < 0.05) ().
Table 2. Comparison of the volumes of the uterus and lesions in the pre-treatment group before and after treatment with GnRH.
Comparison of the HIFU technical parameters between the two groups
As shown in , HIFU ablation was achieved in all 61 patients successfully. Although the lesion volume of 297.94 ± 137.57 cm3 in the HIFU-GnRH group was larger than the lesion volume of 268.86 ± 112.70 cm3 in the HIFU-only group, the process required less treatment time (80.22 ± 18.38 min vs. 86.66 ± 29.7 min) and less sonication time (983.65 ± 159.10 s vs. 1002.45 ± 787.01 s), with a lower average sonication power of 398.26 ± 0 W versus 399.08 ± 0.71 W and less total energy at 392.73 ± 63.64 KJ versus 412.22 ± 315.23 KJ. However, a higher treatment intensity of 747.65 ± 43.13 s/h, versus 724.88 ± 268 s/h was required in the HIFU + GnRH group than in the HIFU-only group, and no differences existed between the groups except for the average sonication power and treatment intensity.
Table 3. Comparison of HIFU outcomes between the HIFU group and pre-treatment group.
Side effects and complaints between the two groups
As shown in the , 100% (61/61) of the patients were willing to receive HIFU therapy again, and the mean visual analogue scale (VAS) was 3.75 ± 2.12 intra-procedurally. Between-group differences did not exist in intra-procedural or post-procedural VAS scores for pain. During the procedure, all adverse events were minor, and there were familiar symptoms such as 93.4% (57/61) had focused regional pain, 57.5% (3/61) had sacral tail pain and 34.4% (21/61) had skin thermalgia. Only 8.7% (2/23) of cases had groyne pain in the HIFU + GnRH group. The number of patients with lower limb radiation pain was similar between the groups at 10.5% (4/38) in the HIFU-only group and 4.4% (1/23) in the HIFU + GnRH group. Intravenous injection of 10 mg of dexamethasone was performed to anticipate radiation pain during the operation, and the maximum dose was set to 30 mg according to the number of occurrences of low body emission pain. No significant differences emerged in the two groups for discomfort (perineum regional pain, urethral pain, anal distention, etc.).
Table 4. Comparison of intra-procedural and post-procedural adverse effects between the HIFU group and pre-treatment group.
Immediately after treatment, 82% (50/61) of patients did not have abdominal pain, and the mean VAS was 3.54 ± 2.12; only 19.7% (12/61) of the patients with abdominal pain had their pain relieved approximately 2 h after intramuscular injection of tramadol. Occurrences of discomfort in the sacral tail were evident in 34.2% (13/38) of patients in the HIFU-only group and 26.1% (6/23) of patients in the HIFU + GnRH group. Vaginal discharge in the HIFU-only and HIFU + GnRH groups accounted for 15.8% (6/38) and 21.7% (5/23) of patients, respectively. No differences were found between the two groups (p > 0.05). A total of 19.67% (12/61) cases received nominal therapy involving 21.05% in the HIFU-only group and 17.39% in the HIFU + GnRH group.
Comparison of VAS, remission rate and clinical effectiveness between the two groups.
As shown in the and , the VAS scores of 3.3 ± 0.71 versus 3.2 ± 2.83, 3.94 ± 0 versus 3 ± 0.71, 4.44 ± 0.71 versus 5 ± 2.83 at 3, 6 and 12 months after treatment, respectively, were less than the VAS scores of 7.92 ± 0.71 versus 8.29 ± 0.71 before treatment. Statistically significant differences existed between the HIFU-only and HIFU-GnRH groups only 6 months after treatment (p < 0.05). Self-comparison differences were found in the two groups before and after treatment (p < 0.05). Although no difference appeared in the relief rate or clinical effectiveness after treatment (p > 0.05), the relief rate of (57.84 ± 7.07)% versus (63.69 ± 38.3)%, (50.53 ± 0.47)% versus (75.87 ± 30.5)%, (56.15 ± 19.45)% versus (56 ± 2.95)%, and the clinical effectiveness of 91.89% versus 73.9%, 85.29% versus 90%,79.41% versus 80% (p > 0.05), were all better in the HIFU + GnRH group than in the HIFU-only group 6 months after treatment.
Table 5. Comparison of the VAS and the relief rate between the HIFU-only group and HIFU + GnRH group from 3 months to 12 months after treatment.
Table 6. Comparison of the clinical effectiveness between the HIFU-only group and HIFU + GnRH group from 3 months to 12 months after treatment.
Discussion
HIFU is a non-invasive therapy that is widely used in the treatment of solid tumours, including hepatic cancer and osteosarcoma [Citation4,Citation15]. Since the 1990 s, the use of HIFU to ablate fibroids in gynaecologic disease has been widespread [Citation7]. Additionally, explorations HIFU ablation for placental implantation, caesarean scar pregnancy and adenomyosis has been valuable. Homogeneous hyperintensive leiomyomas on T2-weighted MR imaging are difficult to ablate with single HIFU because thermal energy is difficult to accumulate within cellular leiomyoma [Citation16,Citation17]. The GnRH-α pre-treatment may improve the efficiency of HIFU thermal ablation for leiomyoma [Citation9].
The efficacy and safety of HIFU sonication on adenomyosis has been shown in several studies [Citation18–21]. Multiple noteworthy issues remain as to how to rapidly improve HIFU ablation for adenomyosis, especially with enlarged uteri [Citation22]. GnRH-α is an analogue of the gonadotrophin-releasing hormone agonist, which inhibits ectopic endometrial growth. In the gynaecology clinic, GnRH-α is used to improve the symptoms of adenomyosis and alleviate menstrual bleeding and dysmenorrhoea.
Until now, few reports have described HIFU ablation combined with GnRH-α pre-treatment for diffuse adenomyosis with a volume greater than 300 cm3 and clinical follow-up after 12 months. Our study showed that after treatment with GnRH-α, the sizes of most uteri and lesions were clearly reduced and that vascular flow decreased, as observed in other studies [Citation2,Citation3]. The MR enhancement images or CEUS images showed that the non-perfused area indicated temporary ischaemic infarction in some lesions. This issue may be due to a decrease in microcirculation or cell apoptosis. The exact mechanism should be further investigated and elucidated. Diffuse uterine adenomyosis does not have a clear boundary in the uterine muscle wall. The HIFU treatment must be targeted to the lesions. The treatment plan was developed on the basis of MR and ultrasonic imaging or CEUS imaging, where the lesions are cut to 5-mm pieces with real-time ultrasound guidance. First, because of the characteristics of thermal transmission, the focal spot should be kept between 10 mm and 15 mm away from the capsule and/or endometrium for safety. Second, under sonication, oxytocin is injected intravenously routinely and causes the blood vessel to contract so that the vascular flow in the myometrium is reduced. When the hyper-echo appears, termination of oxytocin instillation should occur to control thermal propagation over time. Meanwhile, the sonication intensity and time must be adjusted according to the greyscale change.
Our pilot study showed that preconditioning with GnRH-α improved the NPV and NPVR% of HIFU thermal ablation significantly. Preconditioning not only lessens the size of the lesion but may also change the pathological characteristics and internal pelvic microenvironment that induce the deposition of HIFU thermal energy. Otherwise, compared to earlier cases that had undergone follow-up evaluations and observations, the clinical symptoms showed significant improvements, although the NPV obtained for several lesions was lower than in the plan anticipated. Thus, the remission of the patient’s symptoms was not completely relevant to the range of lesions ablated. The exact mechanism has not been clearly elucidated and needs to be discussed further. This phenomenon reminds us that close attention should be paid not only to the NPV but also to the safety and patient’s self-perceptions. Although there was no obvious difference between the two groups in uterine and lesion volumes, sonication time, treatment volume and total energy, HIFU required less treatment time, less sonication time, and less average power and obtained a larger NPV, treatment intensity and total energy in the HIFU + GnRH group than in the control group. Between-group differences in treatment time, total energy and treatment intensity were found. Thus, pre-treatment enhanced the efficiency of ablation and simultaneously improved the safety of thermal treatment during HIFU.
Some patients in our study were included in the prospective group [Citation12]. Our study focussed on diffuse adenomyosis with larger uteri and diffuse or focal lesions were included in the prospective study, but they made no difference [Citation12]. Although the results at the 6-month follow-up were similar to others, there was no difference in the VAS and remission rate of dysmenorrhoea in patients with adenomyosis between the two groups 12 months after treatment. The exact cause and the long-term clinical follow-up have to be explored further. Differences were found in the short term clinical symptoms due to the effect of GnRH-α hormone suppression. Meanwhile, no matter which HIFU modality was used, single or HIFU combined with GnRH-α, the VAS of dysmenorrhoea improved significantly compared to pre-treatment. In other words, HIFU treatment is an effective option that ablates the adenomyotic lesion.
Moreover, there were two kinds of common discomforts noted during the HIFU procedure: focal regional pain and sacrococcygeal pain. The uterus with adenomyosis is often oriented retro-posterior, and the lesion often stands at the posterior wall. All of these factors cause the uterus to nearly become positioned next to the sacral region. Then, during treatment, a tolerable level of pain was obtained under sedation analgesia or with adjustment of bladder filling, and the site and size of the water-filled balloon changed sacral tail soreness. Although many measures were taken to ameliorate the patients’ thermal discomfort in the sacral tail, there were still several cases that could not endure the procedure. Recently, when Buddhist music or light music was played during the procedure, pain relief in patients was more significant than before. The results must be assessed in comparison with control groups. During the ablation process, close attention was paid to protecting the skin of the treatment area. A prior abdominal surgical scar emerged in 47.5% (29/61) of cases. As a routine rule, we put down the transduced probe and allowed the skin in the abdominal region to soak in cool degassed water at sonication interval times of more than 1 h. These manoeuvres significantly lessened thermal damage to the skin. In our study, skin thermal burns never occurred. The incidences of focal pain were 100% (38/38) and 82.6% (19/23) in the HIFU-only group and HIFU + GnRH group, respectively. In the future, comprehensive modalities will promote patient satisfaction with HIFU sonication. Immediately after the procedure, low-level abdominal discomfort and sacral tail pain were still common complaints in the two groups, and the discomforts quickly diminished without intervention after a few hours. Only 19.7% (12/61) of patients took pain-killers. Between one and several weeks after treatment, both doctors and patients were perplexed by some mild, long-term vaginal discharge at a rate of 18% (11/61) of cases in the two groups. Because of the invasive growth of adenomyosis and its lack of a clear-cut boundary, there may be accompanying signs and symptoms during rehabilitation of the sonicated lesion. Further research is still required to explore and solve this troublesome effect. During the ablation process, attention should be paid to protecting the endometrial inner membrane and to preserving its continuity and integrity, with dominant hyper-echo diffusion. Almost 82% (50/61) of cases were observed without taking any precautions. There were no obvious differences in complications between the two groups during or after treatment. Thus, HIFU ablation is effective and safe for adenomyosis pre-treated with GnRH-α in the clinic, especially in women with large uteri.
The limitations of our pilot study are its small sample size and lack of multiple treatment modality comparisons. This study was a single-centre study without comparisons with other centres. Multiple confounding factors exist, including case or treatment modality selection bias. Therefore, a multicenter, randomised controlled trial will be a more effective way to evaluate the effect of preoperative GnRH-α preconditioning on HIFU ablation of adenomyosis and to elucidate the mechanism of its influence. A combination of comprehensive methods may have far-reaching effects for these patients.
Conclusions
In summary, pre-treatment with GnRH-α has a significant effect on enhancing the HIFU ablation rate while lowering risk during HIFU in enlarged diffuse adenomyosis. The use of this comprehensive method to manage diffuse adenomyosis with enlarged uteri, especially those with volumes above 300 cm3, is worth pursing further.
Disclosure statement
All authors are responsible for and have the same rights to the article. All authors have read and approved the article for publication and have no potential conflicts of interest to disclose.
Additional information
Funding
References
- Bird CC, McElin TW, Manalo-Estrella P. (1972). The elusive adenomyosis of the uterus-revisited. Am J Obstet Gynecol 112:583–93.
- Ueki K, Kumagai K, Yamashita H, et al. (2004). Expression of apoptosis-related proteins in adenomyotic uteri treated with danazol and GnRH agonists. Int J Gynecol Pathol 23:248–58.
- Khan KN, Kitajima M, Hiraki K, et al. (2010). Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod 25:2878–90.
- Wu F, Chen WZ, Bai J, et al. (2001). Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 27:1099–106.
- Stewart EA, Gedroyc WM, Tempany CM, et al. (2003). Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol 189:48–54.
- Stewart EA, Gostout B, Rabinovici J, et al. (2007). Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 110:279–87.
- Ren XL, Zhou XD, Yan RL, et al. (2009). Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: midterm results. J Ultrasound Med 28:100–3.
- Chen J, Chen W, Zhang L, et al. (2015). Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: A review of 9988 cases. Ultrason Sonochem Sonochem 27:671–6.
- Yang S, Kong F, Hou R, et al. (2017). Ultrasound guided high-intensity focused ultrasound combined with gonadotropin releasing hormone analogue (GnRHa) ablating uterine leiomyoma with homogeneous hyperintensity on T2 weighted MR imaging. Br J Radiol 90:20160760.
- Jiang N, Xie B, Zhang X, et al. (2014). Enhancing ablation effects of a microbubble-enhancing contrast agent (“SonoVue”) in the treatment of uterine fibroids with high-intensity focused ultrasound: a randomized controlled trial. Cardiovasc Intervent Radiol 37:1321–8.
- Zhang X, Zou M, Zhang C, et al. (2014). Effects of oxytocin on high intensity focused ultrasound (HIFU) ablation of adenomysis: a prospective study. Eur J Radiol 83:1607–11.
- Guo Y, Cheng J, Zhang Y, Duan H. (2017). Gonadotrophin-releasing hormone agonist combined with high-intensity focused ultrasound ablation for adenomyosis: a clinical study. BJOG Int J Obstet Gyn 124:7–11.
- Ye MZ, Deng XL, Zhu XG, Xue M. (2016). Clinical study of high intensity focused ultrasound ablation combined with GnRH-a and LNG-IUS for the treatment of adenomyosis. Zhonghua Fu Chan Ke Za Zhi 51:643–9.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). International Working Group on Image-Guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol 25:1691–705.
- Wu F, Wang Z, Chen W, et al. (2004). Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: early Chinese clinical experience. Ultrasound Med Biol 30:245–26.
- Cheng H, Wang C, Tian J. (2015). Correlation between uterine fibroids with various magnetic resonance imaging features and therapeutic effects of high-intensity focused ultrasound ablation. Pak J Med Sci 31:869–73.
- Zhao WP, Chen JY, Chen WZ. (2015). Effect of biological characteristics of different types of uterine fibroids, as assessed with T2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablation. Ultrasound Med Biol 41:423–31.
- Fukunishi H, Funaki K, Sawada K, et al. (2008). Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol 15:571–9.
- Yang Z, Cao YD, Hu LN, et al. (2009). Feasibility of laparoscopic high-intensity focused ultrasound treatment for patients with uterine localized adenomyosis. Fertil Steril 91:2338–43.
- Zhou M, Chen JY, Tang LD, et al. (2011). Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril 95:900–5.
- Feng Y, Hu L, Chen W, et al. (2017). Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: A retrospective cohort study. Ultrason. Sonochem 36:139–45.
- Gong C, Yang B, Shi Y, et al. (2016). Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: A retrospective study. Int J Hyperthermia 32:496–503.