Abstract
Antimicrobial chemotherapy and surgery are classical methods for treating infectious diseases. However, there is a need for alternative methods to cure infections caused by antibiotic-resistant pathogens, recurrent or chronic infections, and unreachable local infections in which the use of drugs or surgery is anatomically and physically restricted. Several micro-organisms are known to be sensitive to mild hyperthermia, and this sensitivity is one of the potential benefits proposed for the host during an episode of fever. Additionally, some immunological or biophysical changes occur during hyperthermia. These changes may be useful for eliminating thermo-susceptible microbial pathogens using local heat therapy. There are several experimental studies proposing the use of hyperthermia to treat local infections. The infected organs or tissues may be heated up to a temperature that can inhibit invading microorganisms. Here, it is hypothesised that local heat therapy may become an alternative or adjuvant method for curing local infections. Here, we highlight the potential for local hyperthermia in the treatment of bacterial infections caused by thermo-susceptible pathogens in a systematic plan. If the proposed thermal-microbiology concepts and local thermal therapies can be adapted to clinical microbiology and infectiology, new medical fields, such as thermo-microbiology and thermo-infectiology, may be created in the future.
Introduction
Heat therapy methods are being used in different fields of medicine, including physical medicine and oncology [Citation1,Citation2]. During local heat therapy, the application device can be focussed on the selected organ or tissue to increase the local temperature [Citation3,Citation4]. Commonly used means for inducing hyperthermia include radiofrequency applicators [Citation5], microwave applicators [Citation6], hot water baths [Citation7], lasers [Citation8], magnetic fluids [Citation9] and high intensity focussed ultrasound [Citation10]. Mild hyperthermia is a therapeutic technique in which cancerous tissue is heated above body temperature to induce a physiological or biological effect but is often not intended to directly produce substantial cell death. The goal is to obtain temperatures of 40–45 °C for time periods up to 1 h [Citation11,Citation12]. In contrast, ablative hyperthermia commonly uses temperatures higher than 55 °C but for shorter durations of 20 s to 15 min [Citation13]. Scientists claim that due to the effects of this hyperthermia, immunological and biophysical changes occur in the targeted tissue, and these changes can be useful for the treatment of some tumours [Citation3,Citation14].
It is known that natural hyperthermia or fever is a neuroendocrine adaptive mechanism of our body against invading microbial agents. During an infection, our body cannot selectively heat the target tissues, and thus it tries to increase the temperature of the whole body, which can be considered a general response. Therefore, pathogenic microorganisms may be inhibited if they are sensitive to higher temperatures [Citation15–17]. Similar to the effects of this natural fever, local heat therapy may help us eliminate thermo-susceptible microbial pathogens in specific organs or tissues. Mild hyperthermia, especially, may be very useful to treat local infections caused by bacterial or fungal microorganisms with regard to the type of infected tissue.
Generally, antibiotics are used to overcome infections. However, sometimes antibiotics cannot defeat multi-drug-resistant bacteria [Citation18,Citation19]. Additionally, sometimes microbial pathogens can protect themselves in tissues with low blood drainage. Micro-organisms can form biofilms or protect themselves in abscess formations [Citation20–22]. As a result, in some cases, including local infections, neither antibiotics nor the immune response can diffuse into the infected organs or tissues at optimum concentrations. In addition, clinicians face numerous recurrent or chronic infectious diseases that cannot be easily cured. Local infections, such as osteomyelitis, bronchiectasis and abscess formations, are sometimes difficult to treat by antimicrobial agents or surgery [Citation21,Citation23,Citation24]. Therefore, as an alternative, local thermal therapies should be evaluated for the treatment of infectious diseases. In the literature, there are several reports indicating the potential of local heat therapy to treat bacterial infections [Citation25–30]. However, these studies do not recommend analysing sensitivity or resistance of the pathogen before starting treatment.
The goal of this opinion piece is to focus attention to the potential importance of local thermal therapies in clinical microbiology and treatment of infectious disease. Additionally, we recommend analysis of every clinical isolate with a thermobiogram to determine an isolate specific diagnosis (thermo-sensitive or resistant) and isolate specific treatment. If the use of these thermal therapies in infectiology can be confirmed in pre-clinical models or in the clinic, new medical fields, such as thermo-microbiology and thermo-infectiology, may be created in the future.
Findings that support the therapeutic effects of heat therapy
Thermal inhibition of pathogens by the direct effect of heat
In classical microbiology, micro-organisms are grouped into categories according to their temperature ranges for growth. These are psychrophiles (<20 °C), mesophiles (20–45 °C), thermophiles (40–80 °C) and extreme thermophiles (about 100 °C). For any organism, the minimum and maximum growth temperatures define the range over which growth is possible. Growth is slower at low temperatures because enzymes work less efficiently and also because lipids tend to harden and there is a loss of membrane fluidity. Growth rates increase with temperature until the optimum temperature is reached, and then the rate falls again. The optimum and limiting temperatures for an organism are a reflection of the temperature range of its enzyme systems, which in turn are determined by their three-dimensional protein structures. Once the optimum value is passed, the loss of activity caused by denaturation of enzymes causes the rate of growth to fall away sharply [Citation31]. Bacteriostatic antibiotics like macrolides and tetracyclines inhibit the growth of bacteria but do not kill [Citation32]. Similarly, hyperthermia may act on pathogens by inhibiting their growth.
The direct effect of local hyperthermia treatment may be the main mechanism for inhibiting a pathogen that is constitutively susceptible to hyperthermia. Based on classical bacteriology, it is known that some species of the genera Acinetobacter, Bordetella, Neisseria, Achromobacter, Pseudomonas, Stenotrophomonas, Burkholderia and Nocardia are constitutively susceptible to hyperthermia and cannot grow at temperatures between 42 °C and 45 °C [Citation33–36].
Clinical microbiologists build and use identification tables and matrices to identify pathogenic micro-organisms. Morphological properties (bacteria colony shape), cell shape, cell size, staining type, nutritional and atmospheric requirements, cultural characterisation, and transformation of chemical compounds by bacterial enzymes are used to classify bacterial species. These data are collected in the literature to form matrices which can later be used to identify bacterial isolates. By analysing positivity and negativity of these parameters, clinical microbiologists find the name of the bacterial isolate. Clinical microbiologists also perform heat tolerance tests at higher temperatures such as 41 °C or 45 °C. This is a compound of cultural characterisation methods such as the pigment production test. For testing, tubed media known to support the growth of organism (simple media or the type of media in which the strains were isolated from the patient) are inoculated lightly and incubated at different temperatures until growth occurs in the tube. Generally 24 or 48 h incubation is enough for testing [Citation37]. Growth at high temperature (heat tolerance) is an important parameter of these matrices. In a matrix study, most of the species of the genus Enterococcus could not grow at 50 °C and a few species (two out of 19 species) of this genus could not grow at 45 °C [Citation38]. According to another matrix study on Vibrionaceae, 59% of Vibrio spp. could not grow at 40 °C while 41% were variable or negative [Citation39]. In another study, some species of Campylobacter and Helicobacter could not grow at 42 °C [Citation40]. In a study investigating 933 Gram-negative bacteria, some species of the genera Acinetobacter, Aeromonas, Citrobacter, Enterobacter, Escherichia, Klebsiella, Morganella, Proteus, Providencia, Salmonella, Serratia, Shigella, Vibrio and Yersinia were susceptible to a temperature of 42 °C after one night incubation on nutrient agar [Citation41]. These bacteria are responsible for various local and systemic infections. Escherichia coli is an important cause of haemorrhagic colitis and haemolytic uraemic syndrome. Also it causes extraintestinal disease including bacteraemia, neonatal meningitis, urinary tract infections, and intra-abdominal infections. Salmonella and Shigella cause enteritis and enteric fever. Yersinia is the causative agent of plague and gastroenteritis [Citation42]. Acinetobacters are opportunistic pathogens that cause infections in the respiratory tract, urinary tract and wounds; they also cause septicaemia [Citation43]. Other genera are members of the Enterobacteriaceae family like Escherichia coli and they cause similar infectious diseases [Citation42]. In this study, 23% of Acinetobacter calcoaceticus, 100% of Citrobacter, Shigella, Yersinia, Vibrio, Serratia, Enterobacter and Escherichia species (including E. coli) were susceptible to a temperature of 42 °C. However, most Klebsiella species could grow well at 42 °C except Klebsiella pneumoniae subsp. rhinoscleromatis and Klebsiella terrigena. Four Proteus species were found 100% susceptible to a temperature of 42 °C while 99% of Proteus mirabilis species were resistant [Citation41]. Another study performed by Holmes et al. [Citation44] revealed that some species of the genera Acinetobacter, Brucella, Bordetella, Kingella, Moraxella, and Pseudomonas were susceptible to a temperature of 42 °C on nutrient agar plates. Pseudomonas aeruginosa is responsible for infections of the respiratory tract, urinary tract, skin and soft tissues, ears, and eyes, as well as bacteraemia and endocarditis. Moraxella may cause pulmonary infections [Citation43]. Bordetella species are responsible for pertussis and respiratory tract infections [Citation45]. In the study, Holmes et al. showed that, 99% of Pseudomonas aeruginosa, 77% of Acinetobacter calcoaceticus and 75% of Brucella species were resistant to a temperature of 42 °C. The results were very variable (1–99% resistance) for Bordetella, Moraxella genera and non-aeruginosa Pseudomonas species [Citation44]. According to a study investigating slowly growing Mycobacteria, most of the species, including Mycobacterium tuberculosis, were susceptible to temperatures of 42 °C and 45 °C, but they could grow well at 25 °C [Citation46]. It was interesting that 94% of M. tuberculosis strains were found sensitive to 42 °C temperature, while 92% of the M. avium strains were resistant and grew well.
After isolating a strain from an infection site and determining its thermal susceptibility in microbiological culture in the laboratory, the target tissue or organ can be heated up to an optimum temperature by a thermal device in the clinic. If the strain is constitutively sensitive to hyperthermia, the pathogenic micro-organism will not be able to grow, and it can be eradicated from the site of infection.
Additionally, combinations of heat therapy with antimicrobial drugs may have synergistic effects on infections with different kinds of isolates. Even if there are some reports investigating the synergistic effect of hyperthermia on the efficacy of some antibiotics, these studies are limited to a few bacterial strains (especially Staphylococcus aureus) and they do not recommend performing heat synergy tests before starting hyperthermia-plus-chemotherapy treatment [Citation29,Citation30]. One bacterial species, such as S. aureus, may have several biotypes with different physical and biochemical characteristics [Citation47]. So, we strongly recommend testing each clinical isolate by using synergy tests before starting treatment.
Thermal inhibition of pathogens by the indirect effect of heat
Vascular changes and oxygenation status in hyperthermia
Heat induces a prompt increase in blood flow accompanied by the dilation of vessels and an increase in the permeability of the vascular wall in normal tissues [Citation2,Citation48–50]. At the same time, increased blood temperatures result in a reduced affinity of haemoglobin for oxygen and thus a rightward shift of the Oxygen–Haemoglobin Dissociation Curve. When the temperature of muscle tissue is higher than 37 °C, oxygen can be unloaded from haemoglobin more easily (lowered oxygen affinity). These changes lead to an overall increase in oxygen availability and improved oxygenation status [Citation51]. Oxygen is necessary for cellular survival and is lethal to some bacteria, especially anaerobes [Citation52]. Heating tumours to higher temperatures typically causes a transient increase in perfusion during heating, followed by vascular collapse; however, such vascular collapse generally occurs at temperatures that cause a substantial blood flow increase in certain normal tissues [Citation53]. Increased blood flow and permeability of the vascular wall may help antibiotics and components of the immune system easily diffuse through the target tissue.
Effects of temperature on biofilm formation
Bacteria in biofilms are surrounded by an extracellular matrix that might physically restrict the diffusion of antimicrobial agents. Nutrient and oxygen depletion within the biofilm causes some bacteria to enter a nongrowing (stationary) state in which they are less susceptible to growth-dependent antimicrobial killing [Citation20]. Hyperthermia produces an increase in nutrients and oxygen in the heated region by inducing a prompt increase in blood flow [Citation2,Citation48,Citation49]. This change may prevent bacteria from entering a nongrowing (stationary) state in the biofilms. A study showed that Salmonella had maximum biofilm formation capacity at 20 °C and that biofilm formation decreased at any other temperature [Citation54]. Ramli and co-workers compared the biofilm formation of Burkholderia pseudomallei at 30 °C and 37 °C. They showed that the biofilm mass and viable bacterial cells decreased at the higher temperature [Citation55]. Burkholderia pseudomallei causes localised, suppurative cutaneous infection accompanied by regional lymphadenitis, fever, and malaise [Citation43]. So, heating cooler parts of the body like skin up to a temperature of 37 °C may help treat superficial infections. O’Toole and co-workers investigated thermal mitigation of Pseudomonas aeruginosa biofilms and reported that temperature has a larger effect than exposure time on biofilm cell death [Citation28]. Park and co-workes showed that Pseudomonas aeruginosa biofilms were inactivated and viable cell number decreased using superparamagnetic iron oxide nanoparticles heating method [Citation56]. In an in vivo study, it was reported that the protection effect of biofilms on Methicillin-sensitive Staphylococcus aureus could be destroyed by magnetic nanoparticle-induced hyperthermia and therapeutic effect of systemic antibiotics could be enhanced [Citation57]. Studies performed by Chopra and co-workers support the hypothesis that alternating magnetic fields exposures can eradicate Pseudomonas aeruginosa biofilm on metal implants, and may enhance the effectiveness of conventional antibiotics [Citation27].
Elevation of immune response and cytokine induction by hyperthermia
During hyperthermia, CD8 + T lymphocytes and natural killer (NK) cells have been shown to be significantly elevated, thus resulting in a slight increase in the total lymphocyte count despite a decrease in CD4 + T lymphocytes [Citation58]. Shen and co-workers demonstrated that NK cell function was increased at temperatures of approximately 40 °C but was impaired at temperatures above 42 °C. Findings at in vitro temperatures below 41 °C revealed increased NK cell proliferation, which was accompanied by heat-shock response [Citation14,Citation59]. Hyperthermia was also shown to enhance antigen presentation and consequently the activity of dendritic cells in cancer patients [Citation60]. Dendritic cells are antigen-presenting cells that adapt immune responses by sensing cell damage or inflammation in the microenvironment [Citation61]. Increased dendritic cells and macrophages are all capable of endocytosis, phagocytosis and internalise microbial cells, viruses and inert particles. Tissue injury frequently serves as an entry point for infectious microorganisms. One mechanism by which the complement system directly kills microorganisms is the membrane attack complex, which involves insertion into the membrane of its target cell and creating a large transmembrane pore. Opsonophagocytosis is key to immunity to Gram positive bacteria and Gram negative organisms that cannot be killed by the lytic pathway of complement [Citation62]. In a study investigating the effects of mild hyperthermia at 40 °C, elevated temperatures caused immature dendritic cells to mature; this process specifically occurred through the elevation of intracellular levels of heat-shock protein 90, which enhanced the expression of co-stimulatory molecules and improved their ability to prime autologous naive CD8 + T lymphocytes [Citation63]. In another study in 2017, dendritic cell exposure to 39 °C caused the upregulation of 43 genes and the downregulation of 24 genes. Functionally, the up/downregulated genes are involved in post-translational modification, protein folding, cell death/survival, and cellular movement. The exposure of dendritic cells to 39 °C induced apoptosis/necrosis and resulted in an accumulation of insulin-like growth factor-binding protein-6, which may have a functional role in inducing the chemotaxis of monocytes and T lymphocytes [Citation64]. An understanding of the mechanisms regulating the immune response in hyperthermia will be important for treating patients with heat therapy.
Recommendations for future studies and clinical use
Recommendations for infectiologists
Infectiologists should first know the maximum nonhazardous temperatures of each tissue and organ to ensure that they do not cause harm to the patient’s body [Citation65,Citation66]. Some tissues like skin, fat and muscle seem to be more resistant to thermal damage than organs like the kidney and liver [Citation67]. So, it might be easier to treat infections of these tissues. They should try to find the optimum temperatures and minimum effective application time periods for each infective agent according to the disease and anatomical location. Additionally, exploring the clinical benefits of adjuvant therapy with antibiotics is very important. If the effects of local heat therapy can be confirmed for infectious disease, thermo-infectiologists could apply heat therapy according to the isolated or estimated strain, tissue/organ type, and location and feature of the disease.
Recommendations for microbiologists
Thermo-microbiologists should first build thermal tables and classify the microbial agents according to their thermal susceptibilities or thermal resistances. By collecting these data, they could inform clinicians about the possible inhibitory temperatures of the suspected pathogens according to these tables.
In hospital laboratories, clinical microbiologists try to isolate and identify bacterial and fungal agents from patient samples using culturing methods. Afterwards, they perform antibiograms to determine the minimum inhibitory concentration (MIC) of an antimicrobial drug. If the MIC value is lower than a determined threshold, the strain is accepted as susceptible/sensitive to that antimicrobial [Citation68]. These threshold values (critical breakpoints) are determined by scientific committees for each bacterial/fungal species and antimicrobial drug according to the drug’s achievable concentrations [Citation68].
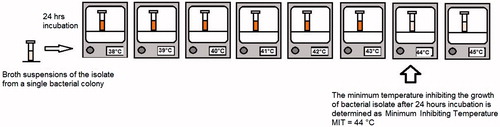
In clinical microbiology laboratories, microbiologists should determine the minimum inhibitory temperature (MIT) of each isolated microorganism by performing thermobiograms (similar to antibiograms).
Thermobiogram: To find the MIT of a strain, broth media suspensions of the same strain are placed in various incubators with increasing temperature levels (e.g., 37 °C, 38 °C, 39 °C, 40 °C, 41 °C, 42 °C, 43 °C, 44 °C, 45 °C) . After one night of incubation, the temperature value of the lowest-temperature incubator with no visible microbial growth can be used as the MIT. For testing, tubed media known to support the growth of microorganism (simple media or the type of media in which the strains were isolated from the patient) are inoculated lightly and incubated at different temperatures until growth occurs in the tube. Generally 24 or 48 h incubation can be enough for testing. As an example; nine brain heart infusion broth tubes (volume of 5 ml) are inoculated with 50 microliters of 24 h broth culture of Enterococcus faecalis. Then, each tube are incubated at different temperature levels from 37 °C to 45 °C. After one night of incubation the MIT can be determined as stated above ().
After performing thermobiograms and determining the MIT, microbiologists can decide whether the isolate is thermo-susceptible or thermo-resistant according to a threshold temperature. The main parameter that can affect the threshold temperature is tissue/organ type. Here, the threshold value is the maximum temperature to which a tissue or an organ can be heated without thermal damage. Therefore, microbiologists should also review classical and new data on thermal dosimetry [Citation65–67]. If the MIT of the strain is lower than threshold temperature of the infected tissue, the strain is considered thermo-susceptible. If the MIT of the strain is higher than the threshold temperature, it is considered thermo-resistant and cannot be inhibited by the direct effect of hyperthermia. It is important to note that a strain that is thermo-resistant for one tissue type may be thermo-susceptible for another tissue.
If the pathogenic microorganism is thermo-susceptible, it can be inhibited by heat and the infected patient can be treated by hyperthermia. The clinician should treat the patient with minimum thermal damage; therefore, determining the MIT will help avoid administration of an excessive thermal dose to the patient.
In previous matrix studies there were also data about heat tolerance, but they included only one or two temperature levels higher than 37 °C [Citation38–41,Citation44,Citation46]. It is not enough to analyse thermal susceptibility of a species using only one or two temperature parameters. We propose to build thermal tables using standard bacterial strains and clinical isolates. Heat tolerance test at the temperatures between 37 °C and 45 °C (thermobiogram) should be applied to all species isolated from patients. MIT values of each strain should be stored in a data bank. By this way, the average MIT value of each species can be determined and we can have a prescience to predict potential success of hyperthermia treatment as soon as we identify the bacterial isolate. After identifying the microorganism, these thermal tables (average MIT values of species) may be used according to tissue/organ type for planning hyperthermia treatment.
Since antibiotic therapy is often extended over many days/weeks for treatment of local infection, it is almost inevitable that local hyperthermia would be conducted in the presence of antimicrobial drugs. Since combined hyperthermia and antimicrobial chemotherapy are being performed, assays to determine sensitivity/synergy should be performed in a combined fashion. In a way similar to thermobiogram, microbiologists can perform antibiogram tests in various incubators with increasing temperature levels (e.g., 37 °C to 45 °C). If the MIC of the antimicrobial drug tested decreases at higher temperatures we can talk about synergism. In this case, some drug resistant strains may become drug susceptible because of the decrease of MIC, and it would be possible to cure these infections. As a second advantage, it might be possible to decrease antimicrobial chemotherapy dose of the patient if microbiologists can observe a significant decrease at MIC of the drug tested in the presence of hyperthermia.
Discussion and conclusion
O’Toole and co-workers showed that some bacterial strains are more thermally resistant than eukaryotic cells, which would make a thermal approach difficult to implement in practice [Citation28]. Hoiby and co-workers reported that some genetic and biochemical changes occur in biofilm–growing bacteria [Citation69]. It was also reported that biofilm forming salmonella strains exhibit enhanced thermal resistance compared to non-biofilm-forming ones [Citation70]. So it is important to test the thermal sensitivity of bacterial species also by using biofilm cultures since their thermal resistance could be higher compared to planktonic bacteria. As we analyse the general results of matrix studies in the Section “Thermal inhibition of pathogens by the direct effect of heat”, there are some pathogenic bacterial species which seem to be resistant to mild hyperthermia. Enterococcus faecalis and Enterococcus faecium are resistant to a temperature of 45 °C [Citation38]. Pseudomonas aeruginosa, Proteus mirabilis and most Klebsiella species are highly resistant to a temperature of 42 °C [Citation41,Citation44]. But in this hypothesis we underline the potential for clinical hyperthermia in the treatment of selected bacterial infections caused by thermo-susceptible pathogens.
Local hyperthermia may have indirect immunological and biophysical effects against some infections. However, the direct effect of local hyperthermia might be more significant than these indirect effects. After a short period of bacterial isolation and culturing, thermobiograms can easily be performed. If the pathogen is found to be constitutively susceptible to hyperthermia, it may be directly inhibited by local heat therapy.
Some studies supporting the immune effects of heat therapy have been performed on cancer patients [Citation60]. The use of this patient population may be a limitation on the immunologic effects of hyperthermia in our hypothesis. There are a few reports that evaluate the effects of hyperthermia against local viral infection [Citation71] and others that indicate the potential effects of magnetic hyperthermia (via magnetic nanoparticles) against bacterial pathogens [Citation72,Citation73]. Recently, some in vivo studies underlined the positive effects of local heat therapy against infections [Citation25–27]. However, these studies do not suggest the inhibition of specific microorganisms after a procedure that determines thermal sensitivity. There are no studies investigating the MIT and thermo-susceptibility of microbial pathogens. Therefore, well-organised in vivo and in vitro trials are needed to determine constitutively thermo-susceptible pathogens. Additionally, clinical studies are needed to prove the potential benefits of local hyperthermia against bacterial infections. Hereafter, other pathogenic microorganisms, such as fungi, viruses and parasites, should be investigated for survival or growth at higher temperatures. It is easy to culture fungal strains just like bacterial isolates. There are many correlation studies, reference antifungal susceptibility methods, and approved standards for fungal infections [Citation74,Citation75]. So, for fungal pathogens in particular, it is possible to perform thermobiogram and determine MIT values using methods that are similar to those for bacteria.
In the field there is a lack of studies comparing in vitro thermobiogram results with in vivo effects of hyperthermia. If we can find a correlation between thermobiogram results and in vivo effects of hyperthermia, this can provide very valuable information before starting treatment. The goal of antimicrobial susceptibility testing is to predict the in vivo success or failure of antibiotic therapy. Clinical Laboratory Standards Institute (CLSI) published interpretation criteria for the antibiogram test, which has come from extensive testing and clinical correlation [Citation68,Citation74,Citation75]. In the future, thermobiogram studies could be performed and organised by international committees in a similar way to predict the in vivo success or failure of heat therapy against microbial pathogens.
This hypothesis highlights the potential of clinical hyperthermia in the treatment of local infections caused by constitutively thermo-susceptible pathogens and suggests new methods and concepts, such as thermobiogram and the MIT. We hope that this opinion piece will inspire medical scientists to study different fields in thermal medicine, infectiology and clinical microbiology.
Disclosure statement
The authors report no conflicts of interest.
References
- Hurwitz M, Stauffer P. (2014). Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Semin Oncol 41:714–29.
- Giombini A, Giovannini V, Di Cesare A, et al. (2007). Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. Br Med Bull 83:379–96.
- Hegyi G, Szigeti GP, Szász A. (2013). Hyperthermia versus oncothermia: cellular effects in omplementary cancer therapy. Evid Based Complement Alternat Med 2013:672873.
- Lee SY, Lee NR, Cho DH, et al. (2017). Treatment outcome analysis of chemotherapy combined with modulated electro-hyperthermia compared with chemotherapy alone for recurrent cervical cancer, following irradiation. Oncol Lett 14:73–8.
- Paulides MM, Bakker JF, Neufeld E, et al. (2007). Winner of the ‘New Investigator Award’ at the European Society of Hyperthermia Oncology Meeting 2007. The HYPERcollar: a novel applicator for hyperthermia. Int J Hyperthermia 23:567–76.
- Juang T, Stauffer PR, Neuman DG, Schlorff JL. (2006). Multilayer conformal applicator for microwave heating and brachytherapy treatment of superficial tissue disease. Int J Hyperthermia 22:527–44.
- Boreham DR, Gasmann HC, Mitchel RE. (1995). Water bath hyperthermia is a simple therapy for psoriasis and also stimulates skin tanning in response to sunlight. Int J Hyperthermia 11:745–54.
- McNichols RJ, Kangasniemi M, Gowda A, et al. (2004). Technical developments for cerebral thermal treatment: water-cooled diffusing laser fibre tips and temperature-sensitive MRI using intersecting image planes. Int J Hyperthermia 20:45–56.
- Jordan A, Wust P, Fahling H, et al. (2009). Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia 25:499–511. 1993.
- Partanen A, Yarmolenko PS, Viitala A, et al. (2012). Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia 28:320–36.
- Viglianti BL, Stauffer P, Repasky E, et al. (2010). Hyperthermia. In: Hong W, Bast R Jr, Hait W, Kufe DW, Holland JF, Pollock RE, et al, editors. Holland Frei cancer medicine. Shelton, CT: Peoples Medical Publishing House-USA, 528–40.
- Issels RD, Lindner LH, Verweij J, et al. (2010). Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 11:561–70.
- Wood BJ, Ramkaransingh JR, Fojo T, et al. (2002). Percutaneous tumor ablation with radiofrequency. Cancer 94:443–51.
- Hildebrandt B, Wust P, Ahlers O, et al. (2002). The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43:33–56.
- Casadevall A. (2016). Thermal restriction as an antimicrobial function of fever. PLoS Pathog 12:e1005577.
- Kluger MJ. (1986). Is fever beneficial? Yale J Biol Med 59:89–95.
- Saper CB, Breder CD. (1994). The neurologic basis of fever. N Engl J Med 330:1880–6.
- Nikaido H. (2009). Multidrug resistance in bacteria. Annu Rev Biochem 78:119–46.
- Friedman ND, Temkin E, Carmeli Y. (2016). The negative impact of antibiotic resistance. Clin Microbiol Infect 22:416–22.
- Patel R. (2005). Biofilms and antimicrobial resistance. Clin Orthop Relat Res 437:41–7.
- Brook I. (2002). Microbiology of polymicrobial abscesses and implications for therapy. J Antimicrob Chemother 50:805–10.
- Singer AJ, Talan DA. (2014). Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med 370:1039–47.
- Lazzarini L, Lipsky BA, Mader JT. (2005). Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int J Infect Dis 9:127–38.
- Hacken NHT, ten Wijkstra PJ, Kerstjens HAM. (2007). Treatment of bronchiectasis in adults. BMJ 335:1089–93.
- Rieck B, Bates D, Zhang K, et al. (2014). Focused ultrasound treatment of abscesses induced by methicillin resistant Staphylococcus aureus: feasibility study in a mouse model. Med Phys 41:063301.
- Pan WY, Huang CC, Lin TT, et al. (2016). Synergistic antibacterial effects of localized heat and oxidative stress caused by hydroxyl radicals mediated by graphene/iron oxide-based nanocomposites. Nanomedicine 12:431–8.
- Chopra R, Shaikh S, Chatzinoff Y, et al. (2017). Employing high-frequency alternating magnetic fields for the non-invasive treatment of prosthetic joint infections. Sci Rep 7:7520.
- O’Toole A, Ricker EB, Nuxoll E. (2015). Thermal mitigation of Pseudomonas aeruginosa biofilms. Biofouling 31:665–75.
- Zou G-Y, Shen H, Jiang Y, et al. (2009). Synergistic effect of a novel focal hyperthermia on the efficacy of rifampin in staphylococcal experimental foreign-body infection. J Int Med Res 37:1115–26.
- Meeker DG, Jenkins SV, Miller EK, et al. (2016). Synergistic photothermal and antibiotic killing of biofilm-associated Staphylococcus aureus using targeted antibiotic-loaded gold nanoconstructs. ACS Infect Dis 2:241–50.
- Stuart Hogg. Essential microbiology. West Sussex, England: John Wiley & Sons Ltd; 2005. Chapter 5, Microbial growth; p. 96–7.
- Murray PR, Rosenthal KS, Phaller MA. (2016). Medical microbiology. 8th ed. Philadelphia (USA): Elselvier Inc. Chapter 17, Antibacterial agents; p. 162–169.
- Schreckenberger PC, Daneshvar MI, Hollis DG. (2009). Acinetobacter, Achromobacter, Chryseobacterium, Moraxella and other nonfermentative Gram-negative rods. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Ankara: Atlas Kitapcilik, 770–802.
- Blondel-Hill E, Henry DA, Speert DP. (2009). Pseuodomonas. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Ankara: Atlas Kitapcilik, 734–48.
- LiPuma JJ, Currie BJ, Lum GD, Vandamme PAR. (2009). Burkholderia, Stenotrophomonas, Ralstonia, Cupriavidus, Pandoraea, Brevundimonas, Comamonas, Delftia and Acidovorax. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Ankara: Atlas Kitapcilik, 74969.
- Conville PS, Witebsky FG. (2009). Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Ankara: Atlas Kitapcilik, 5155–42.
- Lanyi B. (1987). Classical and rapid identification methods for medically important bacteria. In: Colwell RR, Grigorova R, editors. Methods in microbiology, Volume 19. London: Academic Press, 1–67.
- Manero A, Blanch AR. (1999). Identification of Enterococcus spp. with a biochemical key. Appl Environ Microbiol 65:4425–30.
- Noguerola I, Blanch AR. (2008). Identification of Vibrio spp. with a set of dichotomous keys. J Appl Microbiol 105:175–85.
- On SL, Holmes B, Sackin MJ. (1996). A probability matrix for the identification of campylobacters, helicobacters and allied taxa. J Appl Bacteriol 81:425–32.
- Holmes B, Dawson CA, Pinning CA. (1986). A revised probability matrix for the identification of gram-negative, aerobic, rod-shaped, fermentative bacteria. J Gen Microbiol 132:3113–35.
- Murray PR, Rosenthal KS, Phaller MA. (2016). Medical microbiology. 8th ed. Philadelphia (USA): Elselvier Inc. Chapter 25, Enterobacteriaceae; p. 251–264.
- Murray PR, Rosenthal KS, Phaller MA. (2016). Medical microbiology. 8th ed. Philadelphia (USA): Elselvier Inc. Chapter 27, Pseudomonas and related bacteria; p. 272–279.
- Holmes B, Pinning CA, Dawson CA. (1986). A probability matrix for the identification of gram-negative, aerobic, non-fermentative bacteria that grow on nutrient agar. J Gen Microbiol 132:1827–42.
- Murray PR, Rosenthal KS, Phaller MA. (2016). Medical microbiology. 8th ed. Philadelphia (USA): Elselvier Inc. Chapter 29, Miscellaneous Gram negative rods; p. 287–300.
- Wayne LG, Good RC, Krichevsky MI, et al. (1991). Fourth report of the cooperative, open-ended study of slowly growing mycobacteria by the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol 41:463–72.
- Hennekinne JA, Kerouanton A, Brisabois A, et al. (2003). Discrimination of Staphylococcus aureus biotypes by pulsed-field gel electrophoresis of DNA macro-restriction fragments. J Appl Microbiol 94:321–9.
- Song CW. (1984). Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res 44(10 Suppl):4721–30.
- Sekins KM, Lehmann JF, Esselman P, et al. (1984). Local muscle blood flow and temperature responses to 915 MHz diathermy as simultaneously measured and numerically predicted. Arch Phys Med Rehabil 65:1–7.
- Wiper DJ, McNiven DR. (1976). The effect of microwave therapy upon muscle blood flow in man. Br J Sports Med 10:19–21.
- Pittman RN. (2011). Regulation of tissue oxygenation. San Rafael (CA): Morgan & Claypool Life Sciences. Chapter 4, Oxygen Transport; https://www.ncbi.nlm.nih.gov/books/NBK54103/
- Stuart Hogg. Essential microbiology. West Sussex, England: John Wiley & Sons Ltd; 2005. Chapter 5, Microbial growth; p. 99–100.
- Horsman MR. (2006). Tissue physiology and the response to heat. Int J Hyperthermia 22:197–203.
- Giaouris E, Chorianopoulos N, Nychas GJ. (2005). Effect of temperature, pH, and water activity on biofilm formation by Salmonella enterica enteritidis PT4 on stainless steel surfaces as indicated by the bead vortexing method and conductance measurements. J Food Prot 68:2149–54.
- Ramli NS, Eng Guan C, Nathan S, et al. (2012). The effect of environmental conditions on biofilm formation of Burkholderia pseudomallei clinical isolates. PLoS One 7:e44104. doi: 10.1371/journal.pone.0044104
- Park H, Park HJ, Kim JA, et al. (2011). Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J Microbiol Methods 84:41–5.
- Fang C-H, Tsai P-I, Huang S-W, et al. (2017). Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect Dis 17:516.
- Kappel M, Stadeager C, Tvede N, et al. (1991). Effects of in vivo hyperthermia on natural killer cell activity, in vitro proliferative responses and blood mononuclear cell subpopulations. Clin Exp Immunol 84:175–80.
- Shen RN, Lu L, Young P, et al. (1994). Influence of elevated temperature on natural killer cell activity, lymphokine-activated killer cell activity and lectin-dependent cytotoxicity of human umbilical cord blood and adult blood cells. Int J Radiat Oncol Biol Phys 29:821–6.
- Baronzio G, Gramaglia A, Fiorentini G. (2006). Hyperthermia and immunity. A brief overview. In Vivo 20:689–95.
- Foti M, Granucci F, Pelizzola M, et al. (2006). Dendritic cells in pathogen recognition and induction of immune responses: a functional genomics approach. J Leukoc Biol 79:913–16.
- Abbas AK, Lichtman AHH, Pillai S. (2015). Celluler and molecular immunology. 8th ed. Philadelphia: Elsevier, Saunders. Properties and overview of immune responses; p. 1–12.
- Knippertz I, Stein MF, Dörrie J, et al. (2011). Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia 27:591–603.
- Liso A, Castellani S, Massenzio F, et al. (2017). Human monocyte-derived dendritic cells exposed to hyperthermia show a distinct gene expression profile and selective upregulation of IGFBP6. Oncotarget 37:60826–40.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. (2003). Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 19:267–94.
- Schooneveldt G, Bakker A, Balidemaj E, et al. (2016). Thermal dosimetry for bladder hyperthermia treatment. An overview. Int J Hyperthermia 32:417–33.
- van Rhoon GC, Samaras T, Yarmolenko PS, et al. (2013). CEM43 °C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol 23:2215–27.
- Clinical and Laboratory Standards Institute (CLSI). (2012). Performance standards for Antimicrobial susceptibility testings; Twenty-second informational supplement. Pennsylvania: Clinical and Laboratory Standards Institute.
- Høiby N, Bjarnsholt T, Givskov M, et al. (2010). Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–32.
- Villa-Rojas R, Zhu M-J, Narayan CP, et al. (2017). Biofilm forming Salmonella strains exhibit enhanced thermal resistance in wheat flour. Food Control 73:689–95.
- Huo W, Li GH, Qi RQ, et al. (2013). Clinical and immunologic results of local hyperthermia at 44 °C for extensive genital warts in patients with diabetes mellitus. Int J Hyperthermia 29:17–20.
- Kim MH, Yamayoshi I, Mathew S, et al. (2013). Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann Biomed Eng 41:598–609.
- Chen C, Chen L, Yi Y, et al. (2016). Killing of Staphylococcus aureus via magnetic hyperthermia mediated by magnetotactic bacteria. Appl Environ Microbiol 82:2219–26.
- CLSI. (2017). Reference method for broth dilution antifungal susceptibility testing of yeasts. 4th ed. LSI standard M27. Wayne (PA): Clinical and Laboratory Standards Institute.
- Alastruey-Izquierdo A, Melhem MS, Bonfietti LX, et al. (2015). Susceptibility test for fungi: clinical and laboratorial correlations in medical mycology. Rev Inst Med Trop S Paulo 57:57–64.