Abstract
Purpose: Our objective was to determine the safety and ablation size of hydrochloric acid-perfused radiofrequency ablation (HCl-RFA) in liver tissues, prospectively using in vivo rabbit and ex vivo porcine liver models.
Materials and methods: The livers in 30 rabbits were treated in vivo with perfusions of normal saline (controls) and HCl concentrations of 5%, 10%, 15%, and 20%, during RFA at 103 °C and 30 W for 3 min. For each experimental setting, six ablations were created. Safety was assessed by comparing baseline weight and selected laboratory values with those at 2, 7, and 14 days’ post-ablation, and by histopathological analysis. The livers in 25 pigs were treated ex vivo with the same five perfusions during RFA at 103 °C, at both 30 W and 60 W, for 30 min. Ablation diameters and volumes were measured by two examiners.
Results: Rabbit weights and selected laboratory values did not differ significantly from baseline to 7 and 14 days’ post-ablation, liver tissues outside the ablation zones were normal histologically, and adjacent organs showed no macroscopic damage. The mean ablation volumes in the porcine livers treated with HCl-RFA were all larger than those treated with normal saline perfusion during RFA (NS-RFA), at both 30 W and 60 W (p < 0.001). The largest ablation volume and transverse diameter were observed in the porcine livers during 10% HCl-RFA at 60 W, measuring 179.22 (SD = 24.79) cm3 and 6.84 (SD = 0.36) cm, respectively.
Conclusions: Based on our experiments, HCl-RFA in the liver appears to be as safe as NS-RFA while also resulting in larger ablation zones.
Introduction
Radiofrequency ablation (RFA) is considered a potentially curative treatment modality for very early- or early-stage hepatocellular carcinoma (HCC) in international management guidelines [Citation1]. According to a recent review, classical monopolar RFA appears to provide the same long-term results as surgical resection in HCC less than 2–3 cm, with 5-year overall survival rates reaching 76%, but RFA has traditionally been proposed only for hepatic tumours less than 5 cm in size [Citation2]. However, the same review describes reports of patients with HCC between 5 and 10 cm treated with RFA, with complete ablation obtained in 80–87% of cases and 3-year overall survival rates between 70% and 81%.
In spite of these encouraging results and several advances in RFA technology, such as the use of a cool-tip electrode, multipolar RFA, and normal saline perfusion before or during RFA, the risk of incomplete ablation, especially in HCC larger than 5 cm, remains a concern [Citation3–6]. Indeed, percutaneous treatment in general, including RFA, has been associated with a high risk of local (up to 30%) and distant (up to 80%) HCC recurrence at 5 years [Citation2]. Moreover, some preclinical studies have suggested that incomplete neoplastic ablation might actually promote tumour aggressiveness, through an epithelial–mesenchymal transition mechanism [Citation7–9]. Consequently, the overall success of RFA is still hindered by issues of tumour size and of local and distant tumour recurrence, suggesting that new RFA techniques are needed.
To address these issues, some have tried using normal saline perfusion during RFA (NS-RFA), citing evidence that saline has conductivity 3–5 times higher than that of blood and 12–15 times higher than that of soft tissues, allowing an increased amount of energy to be applied [Citation10]. Others have explored the option of hydrochloric acid-perfused radiofrequency ablation (HCl-RFA), perhaps based on previous reports of using HCl successfully as a chemical ablation agent for liver cancer [Citation11]. In one report, 10% HCl-RFA at 30 W for 15 min created a mean ablation zone transverse diameter of 4.49 cm in ex vivo porcine livers, whereas NS-RFA provided a mean transverse diameter of only 2.48 cm (p < 0.001) [Citation12]. In a similar study of 10% HCl-RFA under the same conditions, increasing the ablation duration to 30 min resulted in a mean ablation zone transverse diameter of 5.63 cm [Citation13]. These results suggest that HCl-RFA could potentially provide larger ablation zones than classical RFA or NS-RFA. However, little information exists about the safety of HCl-RFA or the impact on safety and ablation size of using different concentrations of HCl during HCl-RFA.
Our objective was to use an in vivo rabbit liver model to determine the safety of HCl-RFA at different HCl concentrations and relative to NS-RFA. In addition, we sought to use an ex vivo porcine liver model to determine the effect of HCl-RFA on ablation size.
Materials and methods
This prospective animal study was approved by the Animal Ethical and Welfare Committee of our Institution (study number IACUC-DB-16-0303).
Equipment
Ablation was performed for both experiments using the single-channel RITA® 1500X Radiofrequency (RF) Generator (AngioDynamics, Inc., Queensbury, NY), which was equipped with computer-driven protocols that automatically adjusted wattage to maintain optimal temperatures during the ablation, with temperature monitoring in real time, and with Set Power and Power Output displays (in watts, W). Ablation energy was applied using a 17-G monopolar UniBlate™ RFA Electrode, which was 15 cm long with an active tip that was able to perform scalable ablations (AngioDynamics, Inc., Queensbury, NY). The active tip of the electrode is made from titanium alloy, which is heat- and corrosion-resistant to acid liquids. This electrode had a built-in thermocouple which provided full temperature feedback and radiofrequency (RF) power control. There are two infusion holes 0.7 cm from the distal end, from which the liquid is perfused into the tissue at a rate of 0.2 ml/min by a peristaltic pump.
While RF energy was being applied, the RITA® IntelliFlow™ Infusion Pump (AngioDynamics, Inc., Queensbury, NY) was used to pump the perfused liquids into the tissues to be ablated through two openings in the tip of the electrode at a constant rate of 0.2 ml/min. Perfusates of different HCl concentrations were created by appropriately diluting 37.5% HCl (Huayi Medical Auxiliary Materials Manufacturing Co., Ltd., Chengdu, China).
In vivo rabbit liver experiment
Thirty New Zealand rabbits (weight range 2.5–3.0 kg) that were purchased from our Laboratory Animal Center were kept in the Animal Laboratory Center and cared for according to standard protocols. The rabbits were divided into five groups of six animals each, based on the perfusate to be used: normal saline (controls) and HCl concentrations of 5%, 10%, 15%, and 20%.
Food was withheld from all rabbits for 24 h before the experiment. General anaesthesia was induced using intravenous 3% pentobarbital sodium (30 mg/kg, Dingguo Biotech Co., Ltd., Guangzhou, China). Each rabbit’s liver was exposed with a 2.5 cm incision below the xiphoid, and a region of the left lateral lobe was ablated with the active-tip electrode set at 1 cm length and the treatment temperature set at 103 °C, using 30 W of power for 3 min, while saline or HCl were perfused. After the ablation was completed, the incision was closed and the rabbits were returned to their cages. All rabbits were euthanized 14 days after ablation with an intravenous injection of pentobarbital sodium, after which the abdomens were explored and the livers were harvested for gross and microscopic evaluation.
The safety of HCl-RFA was assessed in the rabbits by comparing baseline pre-ablation weight and laboratory values with those at 2, 7, and 14 days’ post-ablation. Laboratory tests used included selected liver function tests (plasma alanine aminotransferase [ALT] and aspartate aminotransferase [AST], renal function tests (blood urea nitrogen [BUN] and creatinine [CRE]), blood counts (white blood cell [WBC] and haemoglobin [HGB]) and electrolytes (sodium [Na+] and chloride [Cl−]). Laboratory testing was performed on a Labospect 008 Automatic Analyzer (Hitachi, Ltd., Tokyo, Japan), using standard biochemical analysis kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Ex vivo porcine liver experiment
Twenty-five fresh ex vivo porcine livers (weight range 2.0–3.0 kg) were obtained from a local slaughterhouse. The livers were divided into five groups of five livers each, based on the perfusate to be used: normal saline (controls) and HCl concentrations of 5%, 10%, 15%, and 20%. Each liver was treated with an ablation at 30 W and another separate ablation at 60 W, both for 30 min, resulting in an overall total of 50 ablations. The actual power output was recorded every 2.5 min for each ablation.
Ablation measurements and tissue preparation
For both experiments, each ablation zone was identified by incising along the electrode axis. Grossly, the ablation margin was identified as the boundary between normal-appearing and ablated liver. The longitudinal and transverse diameters of the ablation zone were measured by two researchers, and the average of their measurements was recorded. The volume of the ablation zone was calculated as: V = π × x × y2/6, where x was the longitudinal diameter and y was transverse diameter [Citation3].
Subsequently, in the in vivo rabbit experiment, representative tissue samples containing each ablation zone surrounded by normal-appearing liver were harvested, sectioned, and stained for histopathological examination, in order to determine the extent of damage and necrosis within the zone and to look for any evidence of damage outside the zone.
Statistical methods
For the in vivo experiment, pre- and post-ablation mean body weight, selected laboratory values, and ablation zone diameters and volumes for each group were compared using the one-way analysis of variance (ANOVA). For the ex vivo experiment, mean ablation zone diameters and volumes and actual power output for each group were compared using the multivariate analysis of variance (MANOVA). Adjustments for variability among different groups and time points were made using the Bonferroni test. Data were analysed using the SPSS 15.0 statistical software programme (SPSS Inc., Chicago, IL) with an alpha of 0.05.
Results
In vivo rabbit liver experiment
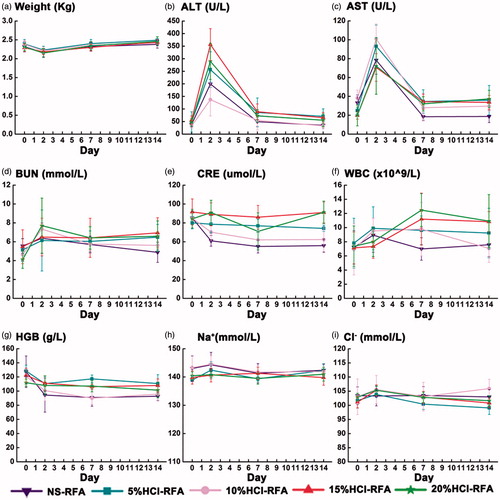
Each group of rabbits experienced transient mean weight loss and liver function test elevation when evaluated 2 days after the procedure, but these parameters had both returned to baseline levels for all groups when the rabbits were evaluated 7 days after the procedure (). In addition, neither the weight changes nor the liver function changes differed significantly among the five groups (p > 0.05). Renal function test, blood count, and electrolyte values remained normal and also did not vary significantly between the groups at all test time points (p > 0.05).
Figure 1. Trends in weight and laboratory values among 30 rabbits before and 2, 7, and 14 days after hydrochloric acid- or saline-perfused radiofrequency liver ablation (RFA). (a) In all groups, mean body weight declined 2 days after ablation and recovered 7 days after ablation; (b-c) In all groups, mean plasma alanine aminotransferase (ALT) and aspartate transaminase (AST) concentrations were elevated 2 days after ablation and returned to baseline 7 days after ablation; mean concentrations did not differ significantly between hydrochloric acid-perfused and saline-perfused control groups; (d–i) In all groups, concentrations of blood urea nitrogen (BUN), creatinine (CRE), white blood cell (WBC) counts, haemoglobin (HGB), sodium (Na+), and chloride (Cl−) were normal throughout the study.
The rabbits appeared to tolerate the procedures well, and no rabbits died. There was no visible damage to adjacent organs. Gross examination revealed that the rabbit liver ablation zones were all well demarcated and surrounded by normal liver tissue, shaped approximately spherically, and partitioned into three distinct sub-zones (). The mean ablation volume of the 20% HCl group (7.39 cm3; SD = 1.00) was significantly larger than the mean ablation volumes of the 15% HCl (4.86 cm3; SD = 1.54), 10% HCl (4.94 cm3; SD = 0.77), 5% HCl (4.29 cm3; SD = 1.18), and saline control (1.96 cm3; SD = 0.63) groups (p < 0.05) (). Similarly, the mean ablation volume of the control group was significantly smaller than that of all four HCl groups (p < 0.001).
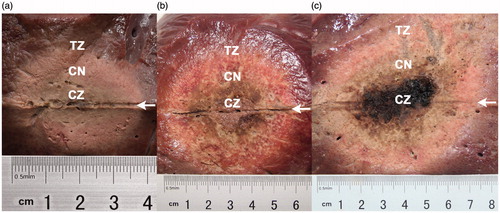
Figure 2. Macroscopic features of the ablation zones in vivo rabbit livers, 14 days after radiofrequency ablation (RFA) at 103 °C and 30 W for 3 min, by perfusion group. (a) Normal saline control, (b) 5% HCl, (c) 10% HCl, (d) 15% HCl, and (e) 20% HCl. Based on gross and microscopic morphology, three ablation sub-zones were identified from the line of electrode insertion outward as the black and moist sediment central zone (CZ), the grey coagulation necrosis zone (CN), and the well-demarcated transition zone (TZ).
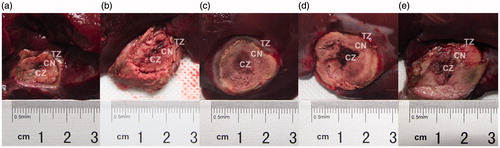
Table 1. Dimensions of ablation zones created by hydrochloric acid- or saline-perfused radiofrequency ablation in 30 in vivo rabbit livers and 25 ex vivo porcine livers.
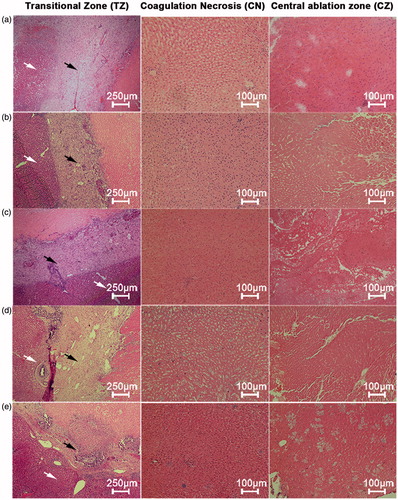
The three sub-zones identified grossly in the rabbit livers corresponded to three distinct microscopic zones. Based on microscopic morphology, we designated these three zones from the line of electrode insertion outward as the central zone (CZ), the coagulation necrosis zone (CN), and the transition zone (TZ) (). Grossly, the CZ in the rabbit livers treated with HCl-RFA and NS-RFA each appeared black; the CZ after HCl-RFA was wider and appeared moist sediment. Microscopically the CZ in the livers treated with HCl-RFA demonstrated disrupted cytoarchitecture with necrosis and fragments of hepatocytes, whereas the cytoarchitecture of the CZ in the livers treated with NS-RFA was intact (). In the livers treated with either NS-RFA or HCl-RFA, grossly the CN was a region of grey tissue that surrounded the black tissue of the CZ; microscopically the CN demonstrated necrosis, maintenance of the normal structures as “ghost cells”, and small infiltrates of inflammatory cells. In addition, grossly the TZ was well demarcated from normal liver tissue, and microscopically it consisted primarily of fibrous tissue and an infiltrate of inflammatory cells.
Figure 3. Microscopic appearance of the three ablation sub-zones in vivo rabbit livers, 14 days after radiofrequency ablation (RFA) at 103 °C and 30 W for 3 min, by perfusion group. (a) Normal saline control, (b) 5% HCl, (c) 10% HCl, (d) 15% HCl, and (e) 20% HCl. Transition zone (TZ): fibrous tissue and infiltrates of inflammatory cells (black arrow), adjacent to normal liver parenchyma (white arrow) (hematoxylin–eosin, original magnification 40×); coagulation necrosis zone (CN): necrosis, maintenance of normal structures as “ghost cells”, and small infiltrates of inflammatory cells (hematoxylin–eosin, original magnification 100×); central zone (CZ): hepatic lobules and cytoarchitecture preserved in saline-RFA control group but damaged in all HCl-RFA groups with necrosis and fragments of hepatocytes (hematoxylin–eosin, original magnification 100×).
Ex vivo porcine liver experiment
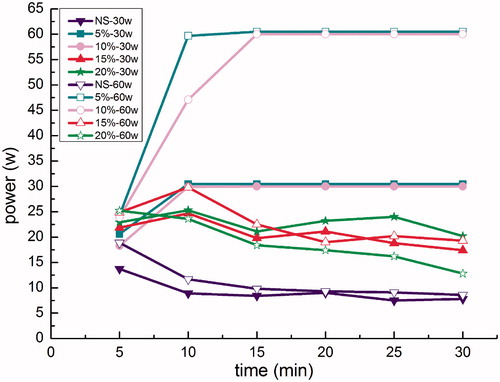
When the 5% HCl and 10% HCl perfusates were used for porcine liver RFA, the average power outputs reached the set powers of both 30 W and 60 W and remained stable throughout the procedure (). However, when the 15% HCl and 20% HCl perfusates were used at both the 30 W and 60 W set powers, the average power outputs stabilised for both at no higher than 25 W, which was significantly lower than these respective set powers (p < 0.001). Furthermore, the average power output was lowest in the NS-RFA control group, for both the 30 W and 60 W set powers, stabilising at about 10 W.
Figure 4. Radiofrequency ablation (RFA) power output curves in ex vivo porcine livers at power settings of 30 W and 60 W, by perfusion group. Average power output during ablation in the saline-perfusion control groups were approximately 10 W at both 30 W and 60 W settings. Average power output during ablation with 5% HCl and 10% HCl perfusions attained the set powers at both 30 W and 60 W and remained stable throughout the procedure. Average power output during ablation with 15% HCl perfusion only reached approximately 20 W for both 30 W and 60 W power settings, and average power output during ablation with 20% HCl perfusion only reached 25 W and 15 W at settings of 30 W and 60 W, respectively.
Grossly, the ablation zones were shaped spherically in the livers treated with HCl-RFA, but they were more ellipsoid in those treated with NS-RFA (). The CZ in the NS-RFA group consisted of a narrow area of carbonised tissue along the electrode path, whereas in the HCl-RFA groups the CZ consisted of a wider moist sediment area along the electrode path, similar to that seen in the rabbit livers.
Figure 5. Longitudinal macroscopic features of the ablation zones in ex vivo porcine livers after radiofrequency ablation (RFA), by perfusion group. (a) Ablation zone after saline-perfused RFA at 103 °C, 30 W, and 30 min, ellipsoid-shaped, measuring 3.8 cm ×2.1 cm; (b) ablation zone after 10% HCl-perfused RFA at 103 °C, 30 W, and 30 min, spherical-shaped, measuring 6.8 cm ×5.5 cm; (c) Ablation zone after 10% HCl-perfused RFA at 103 °C, 60 W, and 30 min, spherical-shaped, measuring 7.3 cm ×6.8 cm. With the saline perfusate (a), the longitudinal electrode path (arrow) was surrounded by a narrow, black carbonised belt, whereas with the HCl perfusates (b, c), the electrode paths (arrows) were surrounded by wider belts that appeared moist sediment.
The mean ablation volumes in the livers treated with HCl-RFA were all larger than those treated with NS-RFA, at both 30 and 60 W (p < 0.001) (; ). At 30 W, the mean ablation volume of the 5% HCl-RFA group was 93.97 (SD = 15.09) cm3, significantly smaller than the mean ablation volumes of the 15% (126.03 cm3; SD = 17.44; p = 0.003), and 20% (117.36 cm3; SD = 15.39; p = 0.02) HCl-RFA groups. The largest mean ablation volume was 179.22 (SD = 24.79) cm3 in the 10% HCl-RFA group treated at 60 W, and it was significantly larger than those of the NS-RFA and other HCl-RFA groups, using either 30 W or 60 W (p < 0.001); the mean ablation volumes in the other three HCl-RFA groups did not differ significantly from each other.
Figure 6. Mean ablation zone volumes in 25 ex vivo porcine livers after saline- and hydrochloric acid-perfused radiofrequency ablation (RFA), by perfusion group and power setting (30 W or 60 W). Mean ablation volume after RFA with 10% HCl perfusion at 103 °C, 60 W, and 30 min was significantly larger than after RFA with both other power settings as well as with both saline and other HCl perfusions (p < 0.001). Mean ablation volume after RFA with saline perfusion (control) was significantly smaller than it was for RFA with the four HCl perfusions at both 30 W and 60 W (p < 0.001).
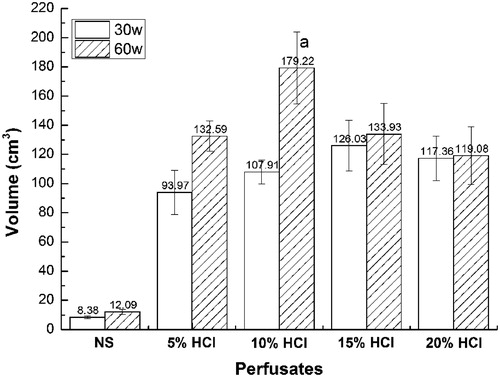
Although all mean ablation volumes using 60 W were larger than those using 30 W, the most substantial differences between 60 W and 30 W were seen in the 5% (132.59 cm3 vs. 93.97 cm3, p = 0.002) and 10% (179.22 cm3 vs. 107.91 cm3, p <0.001) HCl-RFA groups, respectively (). Moreover, the mean ablation transverse diameters for HCl-RFA and 60 W ranged from 5.96 cm to 6.84 cm (vs. 2.34 for NS-RFA), whereas those for HCl-RFA and 30 W ranged from 5.48 cm to 5.88 cm (vs. 2.06 cm for NS-RFA) ().
Discussion
Using rabbit models, we observed that liver RFA using HCl concentrations up to 20% provided a favourable safety profile under the conditions studied, based on monitoring of weight and selected laboratory values and on histopathological analysis. Using both rabbit and porcine models, we found that liver ablation zone volumes after RFA with a variety of HCl concentrations were all larger than those after NS-RFA under the same conditions. In the porcine model, we determined that the largest liver ablation zone volume occurred with RFA using a perfusion of 10% HCl at 103 °C, 60 W, and 30 min.
In the rabbit model, which we used primarily to assess safety, we identified no substantial persistent complications or injuries to surrounding organs during or after RFA. However, other published reports of ultrasound-guided RFA in vivo rabbit livers which used internally cooled electrodes, pre-ablation hypertonic saline or acetic acid perfusions, and 30 W power for 3 min did report significant complications, including perihepatic haematoma, chemical peritonitis, and thermal injury to the stomach [Citation14–16]. There are several possible reasons why we did not observe similar complications. First, we inserted the probe into the liver under direct vision through an incision, rather than percutaneously with ultrasound guidance. Second, we perfused HCl during the RFA procedure, rather than beforehand, possibly allowing the HCl to be localised primarily in the ablation zone and reducing the risk of harm to adjacent liver as well as other tissues or organs. It is possible that the pre-ablation infusion of acetic acid used in other studies may have caused some of the complications listed above. Finally, we used only 0.6 ml of perfusate (0.2 ml/min for 3 min) during our in vivo rabbit study, which is smaller than the 1 ml volume used in other studies.
In monitoring laboratory values to assess safety, in both the HCl and saline (control) perfusion RFA groups of the rabbit model, we observed only a transient elevation in selected liver function values, and these returned to baseline levels within 7 days after RFA. We suspect that this was because hepatocytes were injured during ablation, consistent with microscopic findings 14 days after RFA. Transient disruptions in liver function have also been reported after microwave ablation, cryoablation, and transarterial embolisation of areas within the liver [Citation17,Citation18]. The fact that the liver function abnormalities were seen in both HCl and saline perfusion groups, and that they were reversible in all groups, suggests that HCl-RFA may be no more toxic to the liver than NS-RFA.
In addition, we found that selected renal function (BUN and CRE) and electrolyte (Na+ and Cl−) values did not change after HCl-RFA in the rabbit model. One possible reason that we did not see changes in these values is that if H+ and Cl− ions had diffused into the blood, the buffer system in the blood may have neutralised these ions, helping to maintain acid–base balance and electrolyte homeostasis [Citation19]. Another possible reason is that during HCl-RFA, both H+ and Cl− ions may have diffused into only the CZ, and not into surrounding tissues or systemically. We identified necrosis, fragmented hepatocytes, and a complete loss of cytoarchitecture (which corresponded to the moist sediment appearance grossly) in the CZ of those livers perfused with HCl (but not those perfused with NS), indicating that HCl, which is known dehydrate cells, coagulate protein, and destroy the integrity of the cell membranes, may have made it only into the CZ [Citation9].
In the in vivo rabbit model, we observed that the largest ablation zone was achieved using 20% HCl-RFA. In RFA, the more ions that are oscillating, the more heat that will be produced [Citation20]. Consequently, it follows that because the 20% HCl perfusate contained the largest number of ions, it produced the most heat and thus the largest ablation zone. Because the power settings and ablation duration were substantially lower in the in vivo rabbit experiment than in the ex vivo porcine experiment, we were interested to see if the higher powers and longer ablation durations used in the porcine experiment would yield similar results under otherwise similar conditions. Indeed, we found that the largest ablation zones in the porcine experiment at the same power setting (30 W) also occurred in those livers receiving 15% HCl and 20% HCl.
In the porcine model, we noted that the ablation zones created with HCl-RFA were significantly larger than those created by NS-RFA, a result which has been confirmed by others [Citation12,Citation13]. Several studies have also reported that the volume of the ablation zone tended to increase as the power output during RFA increased and that the power output was higher when RFA was done with HCl rather than saline [Citation21,Citation22]. Also, others have shown that the conductivity of HCl runs about 3–6 times higher than that of normal saline at the same concentrations [Citation23], providing an explanation for why the ablation zone in HCl-RFA tends to be larger than in NS-RFA. Also, it is possible that during HCl-RFA, the moist sediment CZ, with its microscopic mixture of hepatocyte fragments and HCl, might further increase the tissue conductivity and electrothermal conversion, resulting in even additional enlargement of the ablation volume.
In the porcine model, we also found that the power outputs when using 5% HCl and 10% HCl perfusions with RFA reached the set powers at both 30 W and 60 W (the highest power output that could be achieved during each respective ablation), remained stable throughout the procedures, and were higher than the power outputs reached when using 15% HCl and 20% HCl perfusions. The explanation for these findings starts with the fact that the power output of the RF generator we used was controlled by the temperature of the sensor in the electrode tip during RFA [Citation24]. Thus, when the temperature of the sensor reached the set temperature (103 °C in all experiments), the power output automatically decreased. Moreover, given that heating rate is proportional to conductivity (
) [Citation25], and that, as noted above, the conductivity for 15% and 20% HCl is higher than that for 5% and 10% HCl, it follows that the heating rate for 15% and 20% HCl would be higher. This can be calculated using the following formula:
where
is the heating rate; gradV is the voltage gradient, V·m−1; σ is the conductivity, S·m−1; ρ is the tissue density, kg·m−3; and Cp is the specific heat, J·kg−1·K−1. Based on the formula, the average power output needed to maintain the same working temperature (103 °C) during RFA with 5% HCl and 10% HCl should be higher than that needed with 15% HCl and 20% HCl. This is why the power output during RFA with 5% HCl and 10% HCl could reach the set power and remain higher than that with during RFA with 15% HCl and 20% HCl [Citation25].
Furthermore, we observed that although the average power output during RFA did not differ with either 5% HCl or 10% HCl perfusions, the resulting ablation zone volume when using 10% HCl was significantly larger than it was when using 5% HCl. Once again, this might be explained by the fact that 10% HCl has more ions than 5% HCl, and the more ions that are oscillating, the more heat that is produced, resulting in a larger ablation volume when using 10% HCl.
In contrast to our findings in the rabbit models, the largest ablation zone volumes at the 30 W power setting occurred in those livers receiving RFA with 15% HCl or 20% HCl in the porcine model, when using the higher 60 W power setting, the largest ablation volumes occurred instead in those receiving 10% HCl and the smallest (apart from the saline control) in those receiving 20% HCl. This may involve a complicated mechanism. Similar to the situation with 30 W, the average power output in RFA with 60 W was highest when using 5% HCl and 10% HCl. On the other hand, the number of oscillating ions when using 5% HCl and 10% HCl should have been lower. It may be that when 10% HCl was used, the impact of the relatively higher power output outweighed that of the lower conductivity, resulting in an overall higher amount of energy deposition and ablation volumes with 10% HCl [Citation26].
Strengths and limitations of the study
Strengths of the study included the use of two different animals, the application of both in vivo and ex vivo models, the determination of effect on ablation size using the larger porcine model as used by others [Citation12,Citation13], and the evaluation of safety using a living rabbit model as also used by others [Citation14,Citation16].
The study also had several limitations. The liver lobes of the rabbit were quite small and thin, and so ablation procedures were limited to 30 W and 3 min, which may have been an inadequate power and duration to fully evaluate the safety of RFA with HCl perfusions. Also, we conducted experiments using normal ex vivo porcine livers, and the impedance and conductivity of this tissue may differ from those of in vivo tumour tissue. Additional experimentation using HCl-RFA using a live porcine liver tumour model, higher ablation powers, and longer ablation durations would be helpful in further assessing the mechanism, safety, and efficacy of HCl perfusion during liver RFA.
Conclusions
Based on our experiments, HCl-RFA in the liver appears to be as safe as NS-RFA while also resulting in larger ablation zones.
Disclosure statement
There are no any actual or potential conflicts of interest exist.
Additional information
Funding
References
- Bruix J, Reig M, Sherman M. (2016). Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 150:835–53
- Nault JC, Sutter O, Nahon P, et al. (2017). Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. [Epub ahead of print]. DOI:10.1016/j.jhep.2017.10.004
- Kim YS, Lim HK, Rhim H, et al. (2013). Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 58:89–97.
- Lee DH, Lee JM, Lee JY, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–9.
- Liu C, Liang P, Liu F, et al. (2011). MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperther 27:654–62.
- Xu Y, Shen Q, Wang N, et al. (2016). Percutaneous microwave ablation of 5–6 cm unresectable hepatocellular carcinoma: local efficacy and long-term outcomes. Int J Hyperther 33:247–54.
- Yoshida S, Kornek M, Ikenaga N, et al. (2013). Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology 58:1667–80.
- Zhang N, Wang L, Chai ZT, et al. (2014). Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating beta-catenin signaling. PLoS One 9:e115949.
- Dong S, Kong J, Kong F, et al. (2013). Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Transl Med 11:273.
- Kettenbach J, Kostler W, Rucklinger E, et al. (2003). Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. Am J Roentgenol 180:1537–45.
- Weijian F, Zan L, Suhong H, et al. (2006). Destructive effect of percutaneous hydrochloric acid injection therapy for liver cancer – a preliminary experimental and clinical study. Gan to Kagaku Ryoho 33:1852–6.
- Luo RG, Fao F, Huang JH, et al. (2013). Diluted hydrochloric acid generates larger radiofrequency ablation lesions in excised porcine livers. Diagn Interv Radiol 19:145–9.
- Jiang XY, Gu YK, Huang JH, et al. (2016). Ex vivo liver experiment of hydrochloric acid-infused and saline-infused monopolar radiofrequency ablation: better outcomes in temperature, energy, and coagulation. Cardiovasc Intervent Radiol 39:600–5.
- Lee JM, Kim YK, Lee YH, et al. (2003). Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in vivo study in a rabbit liver model. Korean J Radiol 4:27–34.
- Lee JM, Kim YK, Kim SW, et al. (2004). Combined radiofrequency ablation and acetic acid hypertonic saline solution instillation: an in vivo study of rabbit liver. Korean J Radiol 5:31–8.
- Lee JM, Lee YH, Kim YK, et al. (2004). Combined treatment of radiofrequency ablation and acetic acid injection: an in vivo feasibility study in rabbit liver. Eur Radiol 14:1303–10.
- Sun AX, Cheng ZL, Wu PP, et al. (2015). Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol 21:2997–3004.
- Kim HJ, Shin JH, Kim TH, et al. (2009). Efficacy of transarterial embolization with arsenic trioxide oil emulsion in a rabbit VX2 liver tumor model. J Vasc Interv Radiol 20:1365–70.
- Gomez H, Kellum JA. (2015). Understanding acid base disorders. Crit Care Clin 31:849–60. Epub 2015/09/28.
- Ni Y, Mulier S, Miao Y, et al. (2005). A review of the general aspects of radiofrequency ablation. Abdom Imaging 30:381–400.
- Cho JH, Lee KH, Kim JM, et al. (2017). Safety and effectiveness of endobiliary radiofrequency ablation according to the different power and target temperature in a swine model. J Gastroenterol Hepatol. 32:521–6.
- Jiang Y, Possebon R, Mulier S, et al. (2016). A methodology for constraining power in finite element modeling of radiofrequency ablation. Int J Numer Method Biomed Eng. 33. DOI:10.1002/cnm.2834
- Kameyama N. (1963). [Denkikagaku no Riron Oyobi Ouyou Part I] (Theory and application of electrochemistry: part I). Tokyo, Japan: Maruzen Pub, 31.
- Berber EFN, Siperstein AE. (2000). Initial clinical evaluation of the RITA 5-centimeter radiofrequency thermal ablation catheter in the treatment of liver tumors. Cancer J 6:S319–S29.
- de Alwis A, Halden K, Fryer P.J. (1989). Shape and conductivity effects in the ohmic heating of food. Chem Eng Res Des 67–9.
- Aube C, Schmidt D, Brieger J, et al. (2007). Influence of NaCl concentrations on coagulation, temperature, and electrical conductivity using a perfusion radiofrequency ablation system: an ex vivo experimental study. Cardiovasc Intervent Radiol 30:92–7.
