Abstract
Purpose: To determine the characteristics of ultrasound (US) imaging of completely ablated cases and the effects of duration and clinical experience on accurate microwave ablation (MWA) for the treatment of benign breast tumours.
Methods: With written informed consent and approval of the institutional ethics committee, patients with symptomatic or palpable benign breast tumours (longest diameter, 7–32 mm), to whom MWA (2450 MHz) was performed, were enrolled in this prospective nonrandomised study. US and contrast-enhanced US (CEUS) images were applied for follow-up and analysed.
Results: Forty-seven consecutive patients with 52 completely ablated tumours were enrolled. Of these 52 tumour ablations in US, 16 ablations were defined as concentric type, and 36 were defined as nonconcentric type. Of these 52 ablations, 7 cases were defined as nonaccurate ablation with the largest margin ≥10 mm in US. The nonaccurate ablation rate in the training group (the first consecutive 30 cases, 7/30) was significant higher than that (the last 22 cases, 0/22) in the practiced group (p = 0.016). Of 38 completely ablated cases (9 mm < the longest diameter <20 mm), the average largest margin in >70 s group was significant larger than that in <70 s group (p = 0.019).
Conclusions: Experience was important for accurate MWA in the treatment of benign breast tumour, and at least 30 cases training was recommended. Nevertheless, clinical trials are still required to validate our findings in the future.
Introduction
Breast lumps are the common complaints for women, and biopsy is usually recommended. Most biopsies are benign, and fibroadenoma, cysts and fibrocystic lesions are the most common benign breast tumours [Citation1]. The main management of these benign tumours is serial observation or surgery resection. Most of the benign tumours are managed with serial observation, but several patients will develop anxiety due to local discomfort and worry about cancerous potential [Citation2]. The common methods for tumour resection are conventional surgery, vacuum or endoscopy-assisted minimal invasive surgery [Citation3–5]. However, these therapeutic methods may cause the unnecessary excision of normal tissue and resulting in scar or unsatisfied cosmesis.
Minimally invasive nontumour resection therapies have been applied to stopping growth of benign breast tumours, including microwave ablation (MWA), radiofrequency ablation, cryotherapy and so on [Citation1,Citation2,Citation6–11]. The ideal ablation for benign breast tumours should be only tumour completely ablated without any normal breast tissue ablated, while ideal ablation rarely occurs. MWA has been recognised as an effective therapy for solid tumours [Citation12–16] with several advantages, including large ablation volume and short ablation time [Citation17]. MWA has been attempted to treat benign breast tumours with high complete ablation rate [Citation2,Citation6]. An important principle of MWA for breast benign tumour is to minimise the damage of normal tissue on the basis of complete ablation. To the best of our knowledge, no study has been reported about the damage of normal breast tissue.
In our centre, symptomatic or palpable benign breast tumours were enrolled for MWA from April 2015. In this study, the ultrasound (US) images after MWA were analysed in complete ablation cases, and the ablation margin surrounding the tumour was measured. We defined the accurate MWA as the maximum distance between the tumour and ablation edge <10 mm, and the effects of ablation duration and clinical experience on accurate MWA were analysed.
Patients and methods
Patients for MWA
Patients clinically diagnosed with benign breast tumours in our department were included in our clinical trial from April 2015. Written informed consent was received from every patient. This clinical trial was approved (No. 2013-NT-138) by the institutional ethics committee of The First Affiliated Hospital with Nanjing Medical University. The inclusion and exclusion criterion of this trial were reported in our previous study [Citation6]: (i) less than three tumours in unilateral breast; (ii) US BI-RADS (the Breast Imaging Recording and Data System) score 3 and mammogram BI-RADS 3 if it was given to patients before biopsy; (iii) the tumour smaller than 3 cm in greatest diameter confirmed by using US and contrast-enhanced US (CEUS); (iv) benign breast disease proved by using core biopsy; and (v) Karnofsky performance status >70%. Patient exclusion criteria were as following: (i) patients with medical contraindications to CEUS; (ii) patients who were pregnant or breastfeeding; (iii) patients with evidence of coagulopathy, chronic liver diseases or renal failure; (iv) US BI-RADS score ≥4; and (v) patients with breast implants. MWA was performed to patients diagnosed with benign breast tumours proved by core biopsy.
US-guided MWA and follow-up
US and contrast-enhanced US (CEUS) were applied to evaluate the lesions before MWA by one radiologist with 15 years of experience in breast US. After evaluation of three dimensions of the lesion, the longest diameter was identified. Pre-operative biopsy and MWA were performed by one doctor with 6 years of experience in breast intervention under local anaesthesia. Similar to previous studies [Citation6,Citation18–20], the microwave irradiation frequency of the system (Nanjing Yigao Microwave Electric Institute, Nanjing, China) was 2450 MHz, and output power 40 W was selected in this study. The antenna was placed into the tumour along the longest diameter. The antenna was connected to the microwave generator through a flexible coaxial cable, and the two cooling-water tubes were connected. After testing the cold-water cycling system, the MWA procedure was started to cover the entire tumour. Each tumour was ablated with one antenna. MWA was stopped when the tumour disappeared completely with US. After MWA treatment, the antenna was removed. Patients were monitored for any complications induced by the interventions.
US and CEUS were applied for follow-up at 1 week, 1 month, 3 months and 6 months after MWA. US and/or CEUS were also performed at other time points when the patients asked for. The US unit applied for follow-up was reported in the previous study [Citation6]. According to previous studies [Citation21,Citation22], complete ablation was defined as no enhancement of the tumour on CEUS images after MWA.
US imaging analysis of enrolled cases
Cases enrolled in the present study met the following criteria: (i) the tumour completely ablated assessed by using CEUS; (ii) the follow-up time was more than 3 months after MWA; (iii) the US imaging of first follow-up after MWA clear enough for analysis; and (iv) treated with MWA from April 2015 to January 2017.
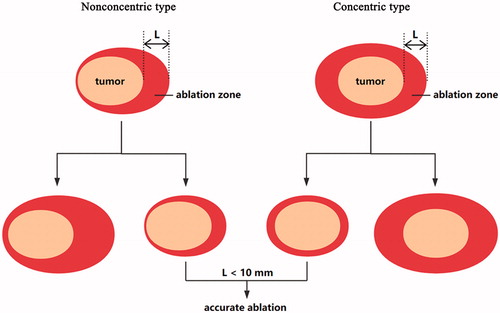
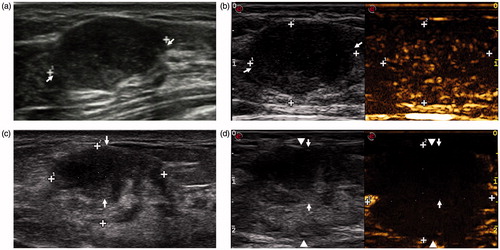
The US and CEUS imaging of first follow-up after MWA was enrolled for analysis in the present study. According to the location of the tumour in the ablation zone, two types of ablation () were defined: concentric type and nonconcentric type. The ablated margin around the tumour was measured in US, and the largest margin of every case was obtained (). When the largest margin was <10 mm, the case was defined as accurate ablation.
Statistical analysis
Median, percentiles, range, mean and standard deviation (SD) were analysed for each continuous variable. Fisher’s exact test was used for univariate analyses. One-way analysis of variance (ANOVA) was used to identify the difference of largest margin among different groups. All statistical analyses were performed using STATA version 11.0 (Stata Corp, College Station, TX), and all p values were two-tailed with 5% significance levels.
Results
Basic characteristics
In all, 47 consecutive patients with 52 completely ablated tumours were enrolled in the present study. Of these 47 patients, the median age was 35 years, with a range of 15–50 years. Forty-two patients had a single tumour in unilateral breast ablated, three patients had two tumours in unilateral breast, and two patients had a single tumour in bilateral breasts. Of these 52 tumours, the median longest diameter was 15 mm assessed by using US, with a range of 7–32 mm. The mean duration of MWA was 80.65 ± 30.48 s, with a range of 40–150 s. Prescheduled MWA was successfully performed to all patients. No skin or chest wall damage was observed.
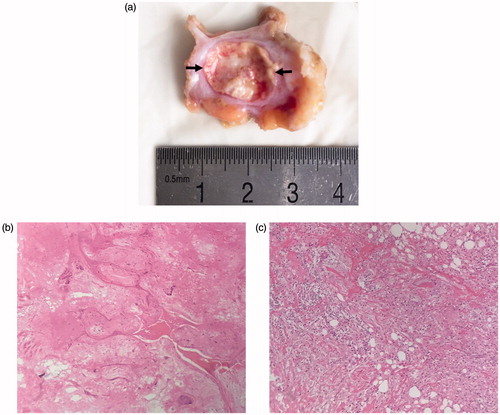
During follow-up, no recurrence was observed in all enrolled cases, and the volume of the ablated tumour decreased gradually. Of these 47 patients, one patient was still nervous about the palpable lesion after MWA, and lumpectomy was performed to this patient about 11 months after ablation. The macroscopic appearance showed the ablation zone clearly (). The HE staining showed the fibroadenoma was replaced by the stroma, and seldom nucleus was observed in ablated area (). Complete ablation in CEUS was further confirmed by HE staining in this case.
Figure 2. Macroscopic appearance of excised specimen after surgery (a) and HE staining (100×) of ablative area (b) and surrounding tissue (c) in 42-year-old woman. (a) The ablated area (arrow) is easily identified. (b) The ablated tumour is replaced by the stroma, and seldom nucleus is observed. (c) The surrounding tissue of the ablated tumour shows inflammatory cells infiltration.
US imaging subtypes of MWA
Of these 52 tumour ablations, 16 ablations were defined as concentric type (), and 36 were defined as nonconcentric type (). The average largest margin of these 52 cases was 6.87 ± 3.08 mm, with a range of 0–17.4 mm. Of these 52 ablations, 7 cases were defined as nonaccurate ablation when the largest margin ≥10 mm, and other 45 cases were defined as accurate ablation. Of these seven nonaccurate ablation cases, four were nonconcentric type, and other three were concentric type.
Figure 3. Conventional US and CEUS images in a 38-year-old woman before and 3 days after MWA. (a) Conventional US shows a tumour with a clear margin before MWA. (b) CEUS shows heterogeneous enhancement before MWA. (c) The tumour (arrow) shrank after ablation, but it is still clear in US. (d) The tumour (arrow) locates in the centre of the ablation zone (arrow-head) in CEUS. This case is defined as concentric type.
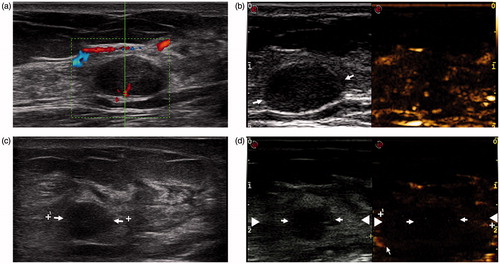
Figure 4. Nonconcentric type of ablation in a 26-year-old woman. (a) A clear tumour (arrow) in US is observed before MWA. (b) Enhancement of the tumour is observed in CEUS before MWA. (c) The shrank tumour (arrow) is still clear in US Week 1 after ablation. (d) The tumour (arrow) does not locate in the centre of the ablation zone (arrow-head) in CEUS.
Clinical experience in accurate MWA
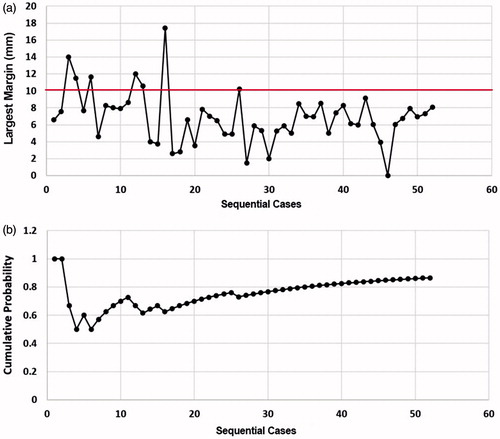
All these 52 consecutive cases were divided into two groups: the training group (the first consecutive 30 cases) and the practiced group (the last 22 cases).In the training group, 10 cases were defined as concentric type; another 6 concentric type were in the practiced group. No significant difference of ablation types was observed (p = 0.765). Moreover, no significant difference of the average largest margin between the two groups (7.17 ± 3.69 mm vs. 6.45 ± 1.96 mm) was observed (p = 0.412). However, the nonaccurate ablation rate in the training group (7/30) was significant higher than that (0/22) in the practiced group (p = 0.016).
As shown in , a wide variation of the largest margin was observed in the training group. The largest margins in the practiced group were almost all between 4 and 10 mm, and MWA seemed to be steady in the practiced group. All seven nonaccurate ablations were reported in the training group, and the cumulative probability of accurate ablation was <80% (). In the practiced group, the cumulative probability steadily increased ().
Long ablation duration contributed to large margin
Ablation duration was based on the appearance of the tumour in US. To explore the role of the duration in MWA, the relationship between the largest margin and MWA duration was determined in 38 completely ablated cases with the longest diameter >9 mm and <20 mm. According to the ablation duration, these 38 cases were divided into two groups: <70 s group and >70 s group. There was no significant difference of the average longest diameters between the two group (13.46 ± 2.42 mm vs. 15.43 ± 3.46 mm, p = 0.079). However, the average largest margin in >70 s group was significant larger than that in <70 s group (8.28 ± 2.94 mm vs. 5.64 ± 2.63 mm, p = 0.019). These results suggested that about 1 min MWA was suitable for complete ablation of tumours with the longest diameter >9 mm and <20 mm, and MWA duration based on the appearance of the tumour in US may be not very accurate for the treatment of benign breast tumour.
Discussion
MWA has been confirmed to be an effective minimally invasive therapy for the treatment of benign breast tumours, with little adverse effects [Citation2,Citation6]. To our experiences and previous study, US monitoring during MWA procedure is not accurate for complete ablation [Citation6,Citation18,Citation20,Citation23]. The effect of MWA has been assessed by using CEUS after ablation. In our prospective study, only one case was not completely ablated [Citation6], and the characteristics of images after MWA have not been reported. In the present study, two types of ablation were defined: concentric type and nonconcentric type (). Nonaccurate ablation (the largest margin ≥10 mm) was observed in seven cases, and all these seven cases were observed in the training group. Furthermore, a wider variation of the largest margin was observed in the training group than that in the practice group (). All the results suggested that clinical experience was important for accurate ablation. At least 30 consecutive cases training of MWA may be necessary for accurate ablation of benign breast tumours.
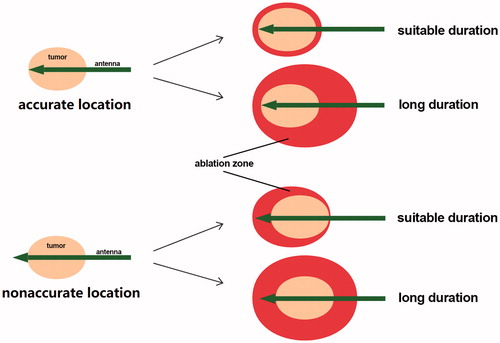
In our opinion, the location of antenna and ablation duration is the two main factors for accurate ablation (). For complete ablation, four major cases may occur. However, the direction of the antenna was not recorded in our study, and the role of the location in nonaccurate ablation cannot be analysed in this study. For complete ablation, MWA with long duration may be applied in clinical practice. In our study, seven cases of nonaccurate ablation were observed. Three concentric nonaccurate ablation may be caused by both nonaccurate location of the antenna and long duration, and other four may be contributed to nonaccurate location of the antenna and/or long duration. Then, the relationship between the largest margin and MWA duration was determined in 38 completely ablated cases with the longest diameter >9 mm and <20 mm. Our results suggested that long duration may contribute to large margin, and US monitoring during ablation is not accurate.
Figure 6. The role of the antenna location and the ablation duration in accurate MWA of benign breast tumours. For complete ablation, four major cases may occur. Both accurate location of the antenna and suitable duration contribute to ideal MWA in the treatment of benign breast tumours.
Training is important for accurate MWA in the treatment of benign breast tumours. In this study, all the ablations were performed by one doctor with 6 years of experience in breast intervention. High complete ablation rate may be contributed to our experience in breast intervention [Citation6,Citation20]. For doctors without experience in MWA, more cases training may be necessary, not 30 consecutive cases training in our study. Different from MWA in the treatment of breast cancer [Citation24,Citation25], ideal MWA of benign breast tumour should be only tumour ablated without any margin. Trainings of MWA in the treatment of benign breast tumour and breast cancer are different ().
Several limitations still existed in this study. First, only one case was not completely ablated. The failure factors cannot be analysed, which may be very important for beginners. Second, the follow-up data was partly reported in our previous study, and the volume of the ablated tumour decreased significantly during the first 6 months. About 12 months after MWA, the ablated tumour disappeared completely in most cases (data not shown). Because this study focussed on the accurate MWA of benign breast tumour, the long-term outcomes have not been reported. Future studies are still needed to determine the long-term outcomes. Third, most enrolled tumours were diagnosed with fibroadenomas, and others were diagnosed with other solid benign breast disease. The difference between them was not analysed. Fourth, although our results were encouraging, they should be confirmed in other centres.
In conclusion, experience was important for accurate MWA in the treatment of benign breast tumour, and at least 30 cases training was recommended. Nevertheless, clinical trials are still required to validate our findings in the future.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Littrup PJ, Freeman-Gibb L, Andea A, et al. (2005). Cryotherapy for breast fibroadenomas. Radiology 234:63–72.
- Yu J, Chen BH, Zhang J, et al. (2017). Ultrasound guided percutaneous microwave ablation of benign breast lesions. Oncotarget 8:79376–86.
- Greenberg R, Skornick Y, Kaplan O. (1998). Management of breast fibroadenomas [Review]. J Gen Intern Med 13:640–5.
- Lai HW, Lin HY, Chen SL, et al. (2017). Endoscopy-assisted surgery for the management of benign breast tumors: technique, learning curve, and patient-reported outcome from preliminary 323 procedures. World J Surg Oncol 15:19.
- Yom CK, Moon BI, Choe KJ, et al. (2009). Long-term results after excision of breast mass using a vacuum-assisted biopsy device. ANZ J Surg 79:794–8.
- Zhou W, Wang R, Liu X, et al. (2016). Ultrasound-guided microwave ablation: a promising tool in management of benign breast tumours. Int J Hyperthermia 33:263–270.
- Dowlatshahi K, Wadhwani S, Alvarado R, et al. (2010). Interstitial laser therapy of breast fibroadenomas with 6 and 8 year follow-up. Breast J 16:73–6.
- Hynynen K, Pomeroy O, Smith DN, et al. (2001). MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 219:176–85.
- Kaufman CS, Bachman B, Littrup PJ, et al. (2002). Office-based ultrasound-guided cryoablation of breast fibroadenomas. Am J Surg 184:394–400.
- Parker SH, Klaus AJ, McWey PJ, et al. (2001). Sonographically guided directional vacuum-assisted breast biopsy using a handheld device. AJR Am J Roentgenol 177:405–8.
- Teh HS, Tan SM. (2010). Radiofrequency ablation – a new approach to percutaneous eradication of benign breast lumps. Breast J 16:334–6.
- Liang P, Dong B, Yu X, et al. (2005). Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 235:299–307.
- Morikawa S, Naka S, Murakami K, et al. (2009). Preliminary clinical experiences of a motorized manipulator for magnetic resonance image-guided microwave coagulation therapy of liver tumors. Am J Surg 198:340–7.
- Wang Y, Liang P, Yu X, et al. (2009). Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia 25:455–61.
- Yu J, Liang P, Yu XL, et al. (2012). US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology 263:900–8.
- Yu J, Liang P, Yu XL, et al. (2014). US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology 270:880–7.
- Simon CJ, Dupuy DE, Mayo-Smith WW. (2005). Microwave ablation: principles and applications. Radiographics 25:S69–S83.
- Zhou W, Jiang Y, Chen L, et al. (2014). Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur J Radiol 83:1771–7.
- Zhou W, Liang M, Pan H, et al. (2013). Comparison of ablation zones among different tissues using 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine models. PLoS One 8:e71873.
- Zhou W, Zha X, Liu X, et al. (2012). US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology 263:364–73.
- Wu H, Wilkins LR, Ziats NP, et al. (2014). Real-time monitoring of radiofrequency ablation and postablation assessment: accuracy of contrast-enhanced US in experimental rat liver model. Radiology 270:107–16.
- Zhang W, Li JM, He W, et al. (2017). Ultrasound-guided percutaneous microwave ablation for benign breast lesions: evaluated by contrast-enhanced ultrasound combined with magnetic resonance imaging. J Thorac Dis 9:4767–73.
- Fornage BD, Hunt KK. (2015). Image-guided percutaneous ablation of small breast cancer: which technique is leading the pack? Technol Cancer Res Treat 14:209–11.
- Fornage BD, Sneige N, Ross MI, et al. (2004). Small (< or =2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology 231:215–24.
- Burak WE Jr., Agnese DM, Povoski SP, et al. (2003). Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer 98:1369–76.