Abstract
Aim: The aim of this study was to first assess the feasibility of bipolar radiofrequency ablation in patients with parathyroid adenoma.
Material and methods: Bipolar RFA was performed in 9 patients with primary parathyroid adenoma in one single session. Measured parameters were PTH and calcium serum levels prior to and after bRFA. Furthermore, using an NRS pain scale (1–10), the individual, subjective maximum sensation of pain was documented.
Results: The bRFA resulted in a highly significant (p = .003906) decrease of serum PTH levels (median 67 ng/l) in comparison to those prior to the intervention (median 199 ng/l). Regarding calcium levels, there was no statistical significance (p = .460938), with a decrease of median serum levels comparing pre- and post-bRFA values from 2.82 mmol/l to 2.66 mmol/l. The evaluation of the individual pain sensation during the procedure was assessed by the patients with a median of 5/10 on the NRS scale. In none of the 9 cases complications such as infections, persisting pain or nerve injury occurred.
Conclusion: For the first time, it was possible to depict the successful therapy of parathyroid adenoma by means of bRFA. This work thus proves bRFA to be an effective, safe, applicable and, concerning sensation of pain, very well tolerable thermoablative technique in the treatment of parathyroid adenoma.
Introduction
Primary hyperparathyroidism (pHPT) is a primary disease of the parathyroid, resulting in a pathologically increased production of the parathyroid hormone (PTH). Its epidemiologic incidence is 65/100,000 p.a. in women and 25/100,000 p.a. in men. 85–90% of parathyroid neoplasia are adenomas [Citation1].
Current reference standard in the therapy of primary hyperparathyroidism is based upon the surgical removal of the parathyroid adenoma [Citation2]. According to the current guidelines, drug therapy using calcimimetic medication can be considered in individual cases until surgery can be carried out [Citation2]. Parathyroidectomy is performed under general anaesthesia and followed by hospitalization of several days’ duration. Especially for elderly patients, parathyroidectomy and the required anaesthesia are not without risk [Citation3].
However, considerable minimally invasive alternatives to the aforementioned surgical removal have recently evolved, for example, for those cases where patients either refuse surgical treatment of when it is contraindicated. These are mainly based on the principles of thermoablation. This term describes various non- or minimally invasive thermic procedures in which heat is used to destroy tissue. These include monopolar radiofrequency ablation (mRFA), high intensity focused ultrasound (HIFU), laser-induced thermotherapy (LITT), laser ablation (LA) and microwave ablation (MWA). Most of these have already been used successfully in the treatment of thyroid as well as parathyroid nodes [Citation4–8]. Thermoablative procedures have in fact advanced to being the method of choice for the treatment of benign thyroid nodes, especially when patients have pre-existing conditions that may result in higher post-operative risks [Citation9]. Amongst the thermoablative procedures, the monopolar radiofrequency ablation represents a safe and frequently used method [Citation10]. For this purpose, an electrical current flow is generated in the area around an RFA-probe, which induces an electrical field, thereby causing local heating of the surrounding tissue. In some countries such as China, the USA and Italy, monopolar radiofrequency ablation has already become an officially recommended treatment option for benign thyroid nodes in the respective national guidelines [Citation11–13].
bRFA proves to be a further development of an established method (mRFA), already in use for numerous organs [Citation14–19]. It has already been successfully used as a treatment for thyroid neoplasms [Citation20,Citation21]. The main advantage of bRFA is the improved heat dissipation from the surrounding tissue, thus reducing the risk for collateral damage, mainly that of adjacent nerves, because the energy flow is concentrated on the adenoma only [Citation22]. Furthermore, bRFA showed a better performance in terms of volume reduction as well as superiority respecting feasibility and patient-friendliness [Citation23]. In contrast to bRFA, energy flow in mRFA is not limited to the adenoma but also occurs towards the neutral conductor, resulting in a loss of energy within the adenoma on one hand, as well as posing a considerable risk for patients with cardiac pacemakers [Citation24]. In those cases, bRFA is generally safe to use.
This study aims at demonstrating the tolerable, successful and safe treatment of parathyroid adenoma using bRFA. In the following, we shall present our findings using the example of 9 patients which were treated using bRFA.
Material and methods
Nine patients with primary hyperparathyroidism underwent bRFA therapy. The patients were aged between 47 and 75 years (median age 60 years). Seven of them were female. The following inclusion criteria were applied: All patients were diagnosed with parathyroid adenoma and had either refused surgical treatment or it was contraindicated for various reasons. All patients consented to the collection of data and its analysis. Excluded were patients with indications of malignancy as well as those patients below the age of eighteen years.
Coagulation-values (blood count, INR, aPTT) were routinely determined prior to treatment. Four out of nine patients were on anticoagulant medication. Three had to discontinue taking ASS 100, in accordance with guidelines, seven days prior to the intervention [Citation25]. For the remaining patient, treatment with marcumar was switched to heparin (bridging), also according to current guidelines [Citation25].
Sonographic imaging of the adenoma using LogIQ5 (GE Healthcare), was followed by the percutaneous insertion of the bipolar coagulation electrode (CelonProSurge micro, either cooled 15 gauge electrodes, active tip 20–40 mm, or un-cooled 18 gauge electrodes, active tip 9–15 mm; Olympus Winter & Ibe GmbH, Hamburg). The utilised high frequency ablator (CelonLab POWER, Olympus) was used with an output of 9 to 40 W and a frequency of 470 ± 10 kHz, if needed in combination with a cooling device (Celon Aquaflow III, Olympus). The local anaesthetic applied was Mepivacain 10 mg/ml s.c. (Mepivacainhydrochloride 1%; AstraZeneca, Wedel, Germany). Sedatives were deliberately not used.
Contrast-enhanced ultrasound was not used because of the already excellent visibility of all relevant structures (compare ). The emerging bubbles around the electrode allow for a very good monitoring of the processes within the treated tissue (see ). Furthermore, the contrast agent associated risks for the patients could thus be eliminated.
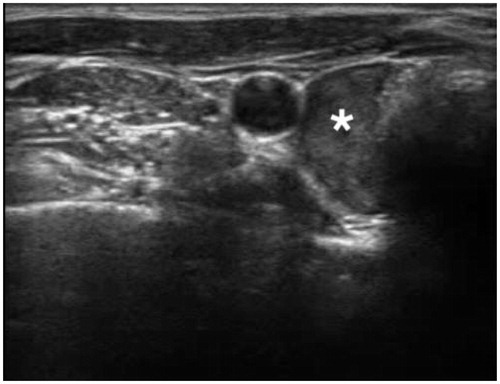
Figure 2. Percutaneous bRFA-probe placed in close proximity to the parathyroid adenoma. (* = Adenoma).
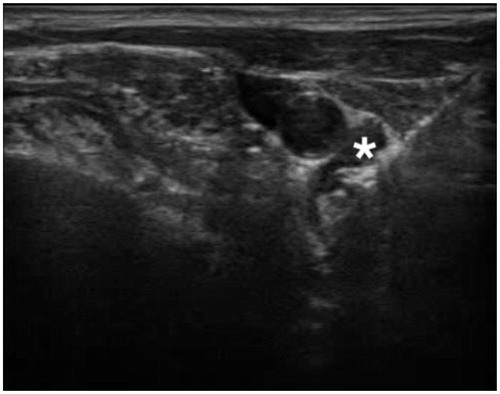
Figure 3. Characteristic ‘bubbles’ around the bRFA-probe indicating a thermal process. (* = Adenoma).
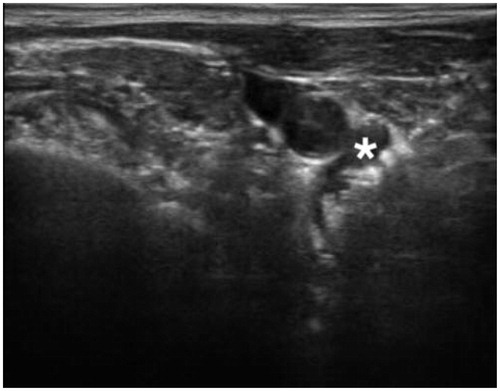
Figure 4. Noticeable reduction in the size of the parathyroid adenoma after bRFA (in comparison to ). (* = Adenoma).
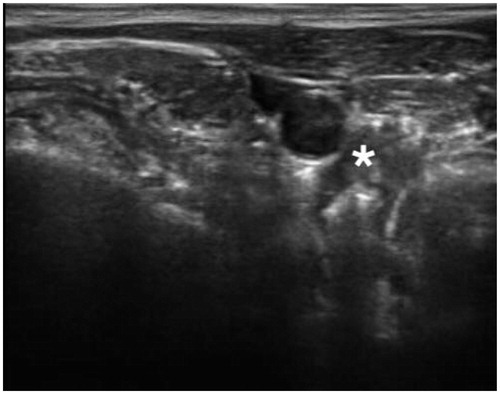
Measured parameters were the PTH and Calcium laboratory values prior to and after the bRFA. Furthermore, patients were asked to rate their personal experience of pain during the treatment, and the maximum was documented using a NRS pain scale (0–10, 0 = no pain, 10 = maximum pain).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all patients for being included in the study.
Statistical analysis
In this study, statistical significance was assessed using the biometric-statistics analysis software ‘BIAS’ (software version 10.04, epsilon Verlag, 1989–2013 Hochheim, Darmstadt, Germany), utilising the Willcoxon matched pairs test. p Values below .01 were rated as statistically significant.
Bipolar radiofrequency ablation procedure
After patient information about the planned treatment, the inclusion and exclusion criteria were reconfirmed. The patients lay supine, their cervical spine brought into hyperextension using a positioning roll placed under the thoracic spine. The thorough disinfection was followed by an orienting sonographic investigation, the objective of which is to give the practitioner an overview of the size and location of the adenoma. In case of patients with more than one, this also helps to determine which one will be treated first. Moreover, it is crucial in determining the size of the probe that will be used. Next, the area of the intended puncture site is anaesthetised using Mepivacain 10 mg/ml s.c. As soon as the applied anaesthetic has kicked in, a small (<1 cm) skin incision is carried out. Ultrasound-guided, the parathyroid adenoma is punctured with the bRFA probe through the aforementioned skin incision. Depending on the size and location of the parathyroid adenoma, the position of the probe may have to be adjusted slightly to ensure an ideal position. Then, energy is applied using the connected high frequency ablator. For all patients in our study, an ablation energy of 100 Watt was chosen. Under continuous ultrasound control, the resulting heat development could be followed as a so-called bubble phenomenon within the parathyroid adenoma, serving as an additional position control. Sedatives were not required. This allowed a continuous communication with the patients, enabling an optimization of the absorbed dose as well as the monitoring of the functioning of the recurrent laryngeal nerve. The individually experienced pain severity was assessed using a NRS pain scale (0–10), documenting the maximum as indicated by the patients. As soon as a satisfactory volume reduction of the adenoma can be detected sonographically, bRFA is completed. The ablation energy is down-regulated and the probes removed from the tissue. Subsequently, the incisions are covered with a sterile patch and the patients are asked to remain in hospital until the required post-ablative serum values have been determined. Given that no complications have occurred, patients are then discharged from hospital. A check-up for the purpose of controlling serum values and volume ratios is advised 3 months following bRFA.
Results
The bRFA could be carried out without any complications in all nine cases. There were no signs of infection or other forms of delayed wound healing, nor did any of the patients show evidence of a compromising of the recurrent laryngeal nerve such as dyspnea or hoarseness. Edema or symptomatic hypocalcaemia were also not reported. All nine patients were able to communicate during the treatment. The procedure was performed under outpatient conditions in all cases.
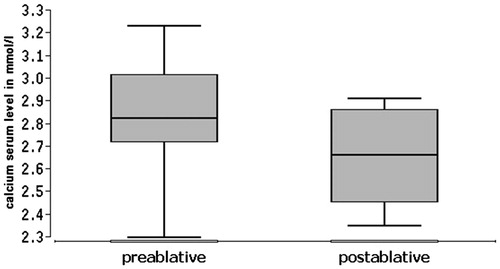
The analysis of the laboratory parameters revealed a median reduction of the serum calcium concentration from 2.82 mmol/l to 2.66 mmol/l (norm: 2.09–2.54 mmol/l) between pre- and post-bRFA values (). This corresponds with a reduction of 5.7% during the first 4–6 h post ablative. Overall, the comparison of pre- and post-bRFA Ca values was not statistically significant (p = .460938, i.e., p > .05). Before bRFA, calcium levels were above the norm value (>2.65 mmol/l) in eight patients. Three of those were within the normal range (2.2–2.65 mmol/l) after the procedure. One patient was already within the normal range from the very beginning, and remained within it post-ablative.
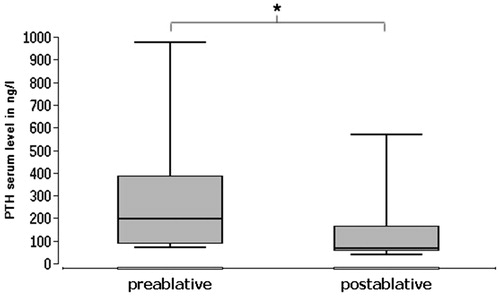
Regarding the PTH (), we could also establish a considerable reduction between the pre- and post-bRFA serum levels from in median 199 ng/l to 67 ng/l (norm: 15–65 ng/l). This is equivalent to a reduction of 66.3%, which is highly statistically significant (p = .003906, i.e., p < .01). All nine patients showed PTH levels above the normal range (>72 ng/l) before bRFA. Post-ablative, in five of them the PTH serum level had decreased to a normal level (12–72 ng/l).
Figure 6. PTH serum levels in preablative and postablative comparison. Highly significant reduction of PTH serum levels after bRFA (p < .01).
A volume reduction of the adenoma could be observed in all patients, a complete disappearance, however, not.
The individual sensation of pain was assessed by all patients as absolutely tolerable during the entire intervention. The average given value on the NRS pain scale was 4.6/10, ranging from 3 to 6.
Discussion
The main question this study sought to answer is whether the bRFA allows a successful, safe and mostly painless treatment of parathyroid adenoma.
After performing bRFA, we were able to establish an average reduction of the Ca serum levels of 5.7% compared to the one before the treatment. Accordingly, the comparison of pre- and post- bRFA PTH levels showed an average decline of 66.3% and was highly statistically significant (p < .01). The maximum pain level as evaluated using an NRS pain scale was 4.6/10, thus in a tolerable range. There were no complications during or after the treatment whatsoever. These findings confirm our assumption that bRFA can be regarded as a successful, mostly painless and safe treatment method for parathyroid adenoma.
The presented findings indicate that bRFA can be seen as a possible alternative therapeutic approach in the treatment of patients with parathyroid adenoma. In our cohort, all patients showed a statistically highly significant (p < .01) decline of the PTH serum level within the first 4–6 h after the treatment. Regarding the Ca serum levels, we found a reduction in 5 of our patients within the first 4–6 h after treatment, which corresponds to a reduction of 5.7%. This was, however, not statistically significant (p > .05). Nevertheless, the obvious tendencies, especially with regard to the substantial decrease of PTH levels, allow the conclusion, that the treatment was successful. However, it has to be emphasised that these parameters are only snapshots, so a follow-up period with further both clinical and laboratory examinations is crucial in order to prove the definitive, long-term therapeutic success.
Due to the fact that there were no complications during our treatments, it can be assumed that bRFA can be regarded as a safe therapeutic approach. Here, the expertise of the clinician carrying out the bRFA, who had previously carried out numerous bRFA for the treatment of thyroid neoplasms, is to be emphasized. Of course, the small number of cases in this study will not suffice to conclusively determine the safety of the investigated treatment procedure. Thus, further studies with higher case numbers are needed. Ultimately, each case has to be rated individually in order to decide on the best minimally-invasive treatment method for each patient. Concerning effectiveness and safety, bRFA and LA can be regarded as equal [Citation26,Citation27]. However, studies have shown a slight superiority of LA in terms of effectiveness in the treatment of larger nodes, whilst being inferior compared to bRFA concerning therapy duration [Citation8,Citation28].
The individual pain levels on the NRS pain scale as given by the patients, indicate temporary light to moderate pain, reaching a peak rather than being perceived constantly. It should be noted that all patients received local anaesthesia, its onset of effect being waited for. After careful evaluation of the risk-benefit ratio, no sedatives were applied. Of course, it has to be noted that pain is a rather individual, subjective sensation. Thus, one cannot generally state that this treatment method is perceived as merely moderately painful. More studies concerning pain sensation during bRFA treatment of parathyroid adenoma with larger case numbers are needed.
mRFA, which was initially established as a treatment option in the field of hepatic surgery, has since been successfully used treating thyroid nodes as well, with around 7000 interventions every year [Citation10]. Our findings confirm the assumption that bRFA can be considered as viable alternative for the treatment on parathyroid adenoma. One of the essential aspects here is, that the further development of bRFA that has taken place over the years, enables an enhanced deduction of the produced heat from the body [Citation20]. This allows for improved control, as well as risk and pain minimization [Citation3]. Our findings confirm this, as there were no complications during any of the treatments and all patients assessed the pain level as tolerable at all times.
There is, however, one limitation to the feasibility of the bRFA: Patients can only be treated using this alternative approach when there is no evidence of malignancy, as this would represent an absolute contraindication and were thus exclusion criteria of the present study, because it would result in a high risk of spreading tumor cells. Of course, there is no perfectly safe way to rule out malignancy, but by evaluation of certain laboratory parameters and clinical examination the probability can be reduced to an acceptable level. Hyperparathyroid carcinoma can be predicted by means of increased iPTH levels, severe hypercalcaemia (>14 mg/dl) and a tumor size of over 3 cm. Combined with an incidence of hyperparathyroid carcinoma of <1% of all patients with primary hyperparathyroidism, the occurrence of this can be stated as relatively rare [Citation29–31].
This study confirms the successful, safe and mostly painless practicability of bRFA in patients with parathyroid adenoma, thus establishing it as a credible alternative therapeutic approach. Our aim is not to question the parathyroidectomy as being the reference standard in the treatment of parathyroid adenoma, we merely want to show alternative choices, especially when surgical intervention is contraindicated or rejected by the patient. The advantages of bRFA are especially that no sedatives are needed, the low complication rate, the outpatient implementation, as well as the only small scar and tolerable sensation of pain during the intervention.
Conclusion
In conclusion, bRFA proves to be a further development of an established method, already in use for numerous organs. In this case it has been successfully used in the treatment of hyperparathyroid adenoma. Concerning the therapy of primary hyperparathyroidism, it is likely that bRFA may represent a way of reducing the risks while increasing patients’ comfort. This assumption must, however, take into account the fact that the present study was only carried out within a very small cohort. In the future, further investigations, especially with higher numbers of patients, are required in order to verify the promising findings of the present study.
Ethical approval
All authors adhere to follow the standard ethical principles.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Herold G. Mitarbeiter. Innere Medizin. Köln: Dr. med. G. Herold; 2016.
- Deutsche Gesellschaft fu¨r Kinderendokrinologie und –diabetologie (DGKED) e.V. S1-Leitlinie – Prima¨rer Hyperparathyreoidismus. 03/2016. German.
- Hamdy NA. Parathyroid gland: is parathyroidectomy safe and beneficial in the elderly? Nat Rev Endocrinol. 2009;5:422–423.
- Xu SY, Wang Y, Xie Q, et al. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. Singapore Med J. 2013;54:e137–e140.
- Korkusuz H, Sennert M, Fehre N, et al. Localized thyroid tissue ablation by high intensity focused ultrasound: volume reduction, effects on thyroid function and immune response. Rofo. 2015;187:1011–1015.
- Heck K, Happel C, Grunwald F, et al. Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia. 2015;31:560–567.
- Korkusuz H, Fehre N, Sennert M, et al. Volume reduction of benign thyroid nodules 3 months after a single treatment with high-intensity focused ultrasound (HIFU). J Ther Ultrasound. 2015;3:4.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. 2016;15:1–5.
- Mauri G, Sconfienza LM. Percutaneous ablation holds the potential to substitute for surgery as first choice treatment for symptomatic benign thyroid nodules. Int J Hyperthermia. 2016;22:1–2.
- Fuller CW, Nguyen SA, Lohia S, et al. Radiofrequency ablation for treatment of benign thyroid nodules: systematic review. Laryngoscope. 2014;124:346–353.
- Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. 2015;18:423–430.
- Hossein Gharib JRG, Mack Harrell R. American Associtation of Clinical Endocrinologists, American Collage of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical pratctice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract. 2016;22:1–60.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36:1321–1325.
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121:3491–3498.
- Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation using a multiple-electrode switching system for lung tumors with 2.0-5.0-cm maximum diameter: phase II clinical study. Radiology. 2015;277:895–902.
- Chinnaratha MA, Chuang MY, Fraser RJ, et al. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294–301.
- Mazzoccoli G, Tarquini R, Valoriani A, et al. Management strategies for hepatocellular carcinoma: old certainties and new realities. Clin Exp Med. 2016;16:243–256.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194:1137–1142.
- Deandrea M, Sung JY, Limone P, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid. 2015;25:890–896.
- Kohlhase KD, Korkusuz Y, Groner D, et al. Bipolar radiofrequency ablation of benign thyroid nodules using a multiple overlapping shot technique in a 3-month follow-up. Int J Hyperthermia. 2016;32:511–516.
- Korkusuz Y, Erbelding C, Kohlhase K, et al. Bipolar radiofrequency ablation of benign symptomatic thyroid nodules: initial experience. Rofo. 2016;188:671–675.
- Bruners P, Lipka J, Gunther RW, et al. Bipolar radiofrequency ablation: is the shape of the coagulation volume different in comparison to monopolar RF-ablation using variable active tip lengths? Minim Invasive Ther Allied Technol. 2008;17:267–274.
- Korkusuz Y, Mader A, Groner D, et al. Comparison of Mono- and Bipolar Radiofrequency Ablation in Benign Thyroid Disease. World J Surg. 2017;41:2530–2537.
- Tong NY, Ru HJ, Ling HY, et al. Extracardiac radiofrequency ablation interferes with pacemaker function but does not damage the device. Anesthesiology. 2004;100:1041.
- Hoffmeister HM, Bode C, Darius H, et al. Unterbrechung antithrombotischer Behandlung (Bridging) bei kardialen Erkrankungen. Kardiologie. 2010;4:365–374.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39:1023–1030.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:911–919.
- Bae JH, Choi HJ, Lee Y, et al. Preoperative predictive factors for parathyroid carcinoma in patients with primary hyperparathyroidism. J Korean Med Sci. 2012;27:890–895.
- Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485–493.
- Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol. 2012;13:11–23.