Abstract
Objective: To evaluate the safety and long-term outcomes of microwave ablation (MWA) combined with transarterial chemoembolization (TACE) in a single stage for the treatment of hepatocellular carcinoma (HCC) with a maximum diameter of 5.0–10.0 cm.
Methods: From January 2013 to December 2016, 84 consecutive HCC patients with cirrhosis from two medical centers who underwent MWA-TACE as a first-line treatment for up to three HCCs with maximum diameters of 5.0–10.0 cm were included. Feasibility, safety and effectiveness were evaluated. Recurrence-free survival (RFS) and overall survival (OS) were analyzed using the Kaplan–Meier method. Cox regression models were used to identify the prognostic factors.
Results: The technique was successfully performed in all the patients. Grade 3 complications consisted of two cases of hemoperitoneum requiring blood transfusions and embolization. The cumulative incidence of local tumor progression was 25.8% at 3 years, with tumor size found to be the only significant predictive factor (p = .007). The cumulative incidence of OS was 81%, 68% and 49% at 1, 2 and 3 years, respectively. According to the Cox proportional hazards model analysis, serum AFP level, Child-Pugh class and tumor number were significant prognostic factors for OS.
Conclusion: MWA-TACE is a safe, feasible and effective therapy for the treatment of 5.0- to 10.0-cm HCC lesions in patients with cirrhosis.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, with a rising incidence in both Eastern and Western countries [Citation1]. Malignant hepatic lesions larger than 5 cm in size are considered large lesions, and >70% of tumors belong to this category [Citation2]. Currently, there has been no universally accepted treatment protocol for such unresectable lesions, especially those associated with multicentricity, large tumor size or poor hepatic function reserve due to pre-existing cirrhosis [Citation3,Citation4]. Liver resection (LR) has been considered the mainstay treatment for solitary large HCC >5 cm in cases unsuitable for liver transplantation [Citation5]. However, the feasibility and long-term effectiveness of LR are affected by liver function, and alternative therapies are needed for patients with compensated cirrhosis and contraindications to surgery [Citation5,Citation6]. Locoregional treatments, such as microwave ablation (MWA) and transcatheter arterial chemoembolization (TACE), are minimally invasive options that can, in combination, achieve the important balance of successful tumor eradication and maximal preservation of liver function.
MWA has recently emerged as a new therapeutic option that provides the capability to perform larger and faster ablations that even exceed the limitations of radiofrequency ablation [Citation7]. Microwave energy has the ability to ablate tissue up to 10 mm around large hepatic blood vessels with larger zones of ablation in areas with high perfusion [Citation8]. The larger ablation zones provided by the microwave technique resulted in its consideration as a curative, rather than palliative, technique for small lesions [Citation9]. TACE slows tumor progression and improves survival by combining the effect of targeted chemotherapy with ischemic necrosis by arterial embolization [Citation10]. TACE is the most commonly used therapy for intermediate-stage HCC in patients with reasonable liver function [Citation11]. Both MWA and TACE have their own limitations; in particular, neither can achieve adequate control of large HCCs [Citation10,Citation12,Citation13]. The combined use of MWA with TACE is appealing [Citation14]. MWA eradicates almost the entire tumor, rendering subsequent TACE more effective because there is greatly reduced use of embolization agents and maximal protection of normal liver tissue [Citation15–17]. Recently, a few studies with finite sample sizes demonstrated the technical success and tumor local control rate of MWA combined with TACE for HCC [Citation16,Citation17]. To the best of our knowledge, there have not yet been multi-center studies with larger sample sizes to evaluate the long-term survival of patients with HCC treated with MWA-TACE. Our study was performed to evaluate the safety and long-term efficacy of MWA with TACE in the treatment of large (5–10 cm) HCC tumors.
Methods
Patients
This investigation was a noncurrent cohort study conducted as a retrospective analysis in two hospital departments (Department of Interventional Oncology, Renji Hospital Affiliated with Shanghai Jiaotong University, Shanghai, China; and Department of Interventional Radiology, Zhongshan Hospital Affiliated with Fudan University, Shanghai, China). From January 2013 to December 2016, 84 patients with HCC who met the eligibility criteria were included.
The eligibility criteria were as follows: (1) largest tumor diameter of 5–10 cm and multiple HCC nodules (i.e., ≤3); (2) less than or equal to three HCC nodules without extrahepatic metastasis or macrovascular invasion; (3) lesions visible on ultrasound (US) with an acceptable and safe path between the lesion and skin as shown on US; (4) Child-Pugh class A or B cirrhosis; (5) an Eastern Cooperative Oncology Group performance status of 0–2; and (6) no previous treatment.
This study was approved by the ethics committee of the two hospitals, and it conformed to the standards of the Declaration of Helsinki. All the patients provided written inform consent to participate in this study.
Diagnosis and definitions
The diagnosis of HCC in patients receiving MWA-TACE was confirmed by biopsy during the MWA procedure or according to the criteria from the EASL and AASLD HCC management guidelines [Citation5,Citation18]. Performance status was assessed at the time of diagnosis by the Eastern Cooperative Oncology Group performance scale. Cirrhosis was confirmed by histological and/or clinical findings (laboratory parameters, US signs) [Citation19]. The Child-Turcotte-Pugh classification was used to define the severity of chronic liver disease [Citation20]. Portal hypertension (PH) was diagnosed in the presence of one or more of the following conditions: esophageal-gastric varices; splenomegaly with a platelet count <100–109/L; actual or pre-existing ascites; and hepatic venous pressure gradient >10 mm Hg [Citation21].
Treatment procedures
US-guided percutaneous MWA
MWA was performed using a single water-cooled microwave system (ECO-100C; Nanjing, Jiangsu, China) under real-time US guidance and a 25-cm cooled-shaft electrode probe (15-gauge) with a 1.5-cm expandable tip. The performing physician aimed to generate a sufficient ablation zone to encompass the visible mass and at least a 5-mm ablation margin. Multiple overlapping ablations were used for the tumors. After administration of analgesia by an anesthesiologist, a 14-Ga antenna was first inserted into the target lesion to reach the deep margin of the tumor. A session was ended if the deep region of the lesion was covered by hyperechoic regions on US. Subsequently, the antenna was gradually withdrawn, and microwave emission was restarted. The procedure was ended when entire portions of the index tumor were covered by hyperechoic regions on US ().
Figure 1. Microwave ablation combined with chemoembolization was performed in a 70-year-old male patient with large HCC. (a) Contrast-enhanced MRI shows a large lesion (8.4 cm* 7.8 cm *6.4 cm) in the right lobe of the liver (arrows). (b) MWA using water-cooled microwave system was performed with cooled-shaft electrode probe (arrows) under real-time ultrasound guidance. (c) After the chemoembolization procedure, selective angiography demonstrated complete devascularization of the targeted lesion (arrows). (d) Contrast-enhanced MRI scan obtained 3 months after combined treatment (arrows).
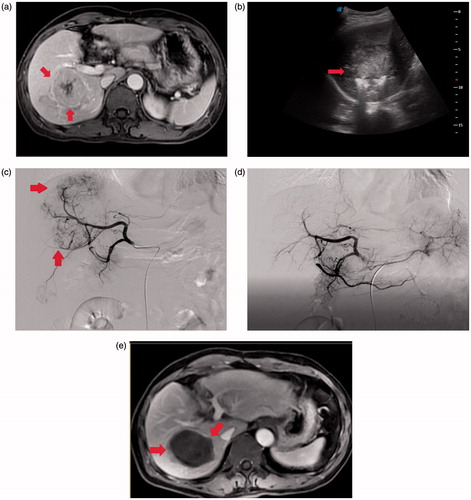
Transarterial chemoembolization procedure
Hepatic angiography was repeated after MWA within 4 weeks to evaluate the ablation response and the vascularity of the residual tumor. A chemotherapeutic emulsion, consisting of 50–150 mg of oxaliplatin and 20–50 mg of epirubicin mixed with 2–10 ml of iodized oil, was slowly injected for chemoembolization under fluoroscopic guidance using a 2.7-F microcatheter until the tumor-feeding arteries were saturated. Then, 350- to 510-μm gelatine sponge particles mixed with contrast medium were injected through the microcatheter to achieve reduced residual blood flow in the tumour-feeding artery (). After embolization, angiography was performed to determine the extent of vascular occlusion and to assess blood flow in other arterial vessels.
Follow-up and further treatment
Four weeks after the first treatment using MWA-TACE, dynamic enhanced computed tomography or magnetic resonance imaging was performed to assess the extent of the treated areas. Thereafter, the patients were followed up once every 3 months for the first 2 years. At each follow-up visit, contrast-enhanced CT/magnetic resonance imaging and blood tests, including serum liver function tests and alpha-fetoprotein, were conducted. Chest radiography was performed once every 6 months. Bone scintigraphy was performed when clinically indicated. Follow-up started from the day of MWA-TACE, and it ended at patient death, last visit or liver transplantation. Local tumor progression (LTP) and intrahepatic distant recurrence (IDR) were evaluated at 3-month intervals [Citation22]. The definitions of local tumor treatment response used were based on the modified Response Evaluation Criteria in Solid Tumors, included complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). Major and minor complications were assessed in accordance with the Society of Interventional Radiology guidelines [Citation23]. Transaminitis requiring medication was defined by an increase in serum transaminase to more than three times the upper limit of normal values.
Statistical analysis
Continuous variables are expressed as the means ± standard deviations or as medians, and categorical data are represented as frequencies and percentages. The cumulative recurrence rates for each type of disease (i.e., LTP, IDR and extrahepatic metastases) and survival rate were estimated using the Kaplan–Meier method. Univariate and multivariate analyses were performed to determine the prognostic factors, including clinical and biological parameters for LTP, OS and RFS. All the variables with p values <.05 in the univariate analyses were included in the multivariate analysis with Cox proportional hazards models. A p value <.05 was considered to indicate significant difference. All statistical analyses were conducted with SPSS software, version 18 (SPSS, Chicago, IL)
Results
Patients
From March 2013 to July 2017, 164 HCC patients underwent MWA-TACE. The median follow-up duration was 36.7 months. Of these patients, 84 (51.2%; mean age, 59.5 years old ±13.7 [standard deviation]; age range, 30–83 years; 70 men, 14 women) met the inclusion criteria and were enrolled in the study. Patient backgrounds and tumor characteristics are presented in . These 84 patients had a total of 114 tumors; 64 patients had one tumor, 10 patients had two tumors, and 10 patients had three tumors. The mean maximum tumor diameter was 6.6 ± 1.5 cm (range, 5–10 cm). Fifty-eight tumors (69%) were 7.0 cm or smaller, and the other 26 tumors (31%) were larger.
Table 1. Baseline characteristics of the overall study population (n = 84).
Feasibility and safety of MWA
The technical success rate for MW ablation/chemoembolization procedures was 100% in all the patients. The mean ablation time was 18.9 min, ranging from 5 to 67 min, and the mean total power used was 70.1 W, ranging from 60 to 100 W. The mean number of ablation zones per patient was eight (range, 2–16). The mean dose of lipiodol used per patient was 6 ml (2–12 ml).
No procedure-related deaths occurred. Major complications occurred in seven patients (7.1%). Grade 3 hemoperitoneum requiring blood transfusions and embolization occurred in one patient (1.2%), and Grade 2 symptomatic pleural effusion requiring percutaneous drainage occurred in two patients (2.4%). Grade 2 transaminitis requiring medical therapy and prolonged hospitalization occurred in three patients (3.6%). The following minor complications were identified in 34 patients (39%): postembolization syndrome, including mild-to-moderate pain, fever, nausea and vomiting requiring supportive treatment, was detected in 30 patients (36%).Alanine aminotransferase (202.7 ± 138.6 U/L) levels were transiently increased on the third day after combination therapy compared with baseline levels (43.8 ± 34.5 U/L). The respective aminotransferase levels decreased to 39.2 ± 28.6 U/L at 1 month after treatment. Asymptomatic pleural effusion was detected in four patients (3%), and it spontaneously resolved within a few days.
Local tumor progression
During the median follow-up period of 13 months (range, 1–31 months), LTP was found in 20 patients (23.8%). The cumulative incidence of LTP was estimated as 14.7%, 22.2% and 25.8% at 1, 2 and 3 years, respectively. The estimated mean LTP-free survival was 32.7 months (95% confidence interval [CI]: 28.4, 36.5). According to the univariate analysis, tumor size (p = .012) and INR (p = .027) were significant predictive factors for developing LTP. However, tumor size (p = .007) was the only significant predictive factor for developing LTP according to the multivariate analysis. Among the 20 patients with LTP, 10 were treated successfully with repeat MWA. Surgical resection was performed in one patient, and TACE therapy was performed in one patient.
Intrahepatic distant recurrence and extrahepatic metastases
Sixty-eight of the 84 patients (82.9%) had IDR. The treatment modalities used for the initial IDR in these patients were as follows: repeat radiofrequency ablation/MWA in 35 patients; TACE in 30 patients; and conservative management in three patients. The cumulative incidence of IDR was estimated as 85.3% at 3 years.
Extrahepatic metastases developed in 18 of the 84 patients (21.7%) after MWA-TACE treatment, and the location of the initial extrahepatic metastases was as follows: lung (n = 10), bone (n = 4), lymph nodes (n = 5), adrenal gland (n = 3) and brain (n = 3). Among these patients, 5 were treated with systemic chemotherapy, 14 with radiation therapy, and 3 with conservative management. The cumulative incidence of extrahepatic metastases was estimated as 25.8% at 3 years.
Recurrence and survival
According to the modified Response Evaluation Criteria in Solid Tumors, CR and PR were observed in 57 (67.9%) and 24 (28.6%) patients, respectively. The objective response rate (i.e., CR plus PR) was 96.4%. SD and PD were observed in one and two patients, respectively. The estimated 1-, 2- and 3-year RFS rates were 31.0%, 15.0% and 11.0%, respectively (). Estimates of the mean and median RFS were 12.4 months (95% CI: 10.4, 14.5) and 10.0 months (95% CI: 7.8, 12.2), respectively. The factors associated with RFS are summarized in .
Figure 2. (a) Graph shows Kaplan–Meier recurrence-free survival curves estimation for 84 patients who underwent MWA-TACE as the first-line treatment for 5.0–10.0 cm HCC. (b) Graph shows Kaplan–Meier overall survival estimation for 84 patients who underwent MWA-TACE as the first-line treatment for 5.0–10.0 cm HCC.
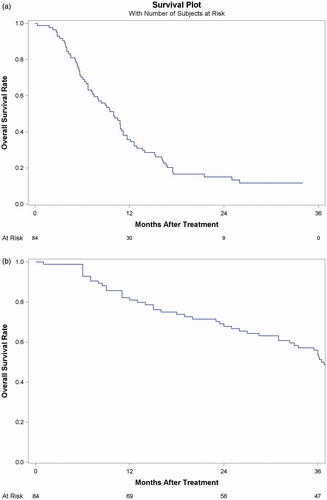
Table 2. Cox survival analysis of the predictors for recurrence-free survival after successful MWA-TACE treatment.
Thirty-nine patients (46.4%) died during the follow-up period. Of the 39 deceased patients, 33 died of cancer progression, and six (15%) died of cirrhosis-related complications. Among the thirty-three patients who died of cancer progression, 15 had LTP. The causes were progression of existing intrahepatic metastases in 13 patients and extrahepatic metastases in five patients. The estimated overall 1-, 2- and 3-year survival rates after MWA-TACE were 81.0%, 68.0%, and 49.0%, respectively (). The factors used to predict overall survival (OS) are summarized in .
Table 3. Cox survival analysis of the predictors for overall survival after successful MWA-TACE treatment.
Discussion
Patients with large tumors have worse prognoses than do those with small tumors due to existing vascular invasion, satellite nodules and underlying liver disease [Citation5]. For patients with compensated cirrhosis and a single HCC ≥5 cm who are found to be unsuitable for LR, the best therapeutic approach remains a matter of debate. HCC with lesions exceeding 5 cm poses a very challenging problem. Several studies have mentioned the rare possibility of achieving complete ablation/chemoembolization of large lesions [Citation4]. Thus, different percutaneous or intravascular techniques have been used and combined. A common combination approach is TACE followed by RFA because TACE can reduce the cooling effect of hepatic blood flow and increase the necrotizing effect of RFA [Citation7]. A study from Italy has found that RFA plus TACE is an effective treatment option in patients with compensated cirrhosis and solitary HCC < = 5 cm unsuitable for resection [Citation24]. Peng ZW et al. [Citation25] compared RFA with or without TACE in the treatment of HCC <7 cm. They concluded that TACE-RFA was superior to RFA alone in improving survival for patients with HCC <7 cm. Takuma et al [Citation26] retrospectively compared the outcome of combined TACE and RFA with that of surgical resection in patients with HCC <3 cm. Their results showed that TACE-RFA may be a viable alternative treatment for early-stage HCC when resection is not feasible. With a limited scope of ablation, this treatment is more likely to be used for small lesions. A second option is to combine TACE with MWA, which has the theoretical advantages of achieving higher intratumoral temperatures, establishing larger ablation areas and reducing susceptibility to the heat-sink effect [Citation16,Citation17].
With an adequate follow-up duration, our study demonstrated that MWA-TACE is a safe and effective treatment that can reinforce anticancer effects in large HCCs (5.0–10.0 cm maximum diameter). First, although several studies have reported that the occlusion of hepatic arterial flow by embolization can reduce the cooling effect of hepatic blood flow during thermal coagulation, MWA has the ability to create larger ablation zones more rapidly and a relative insensitivity to the heat-sink effect compared with RF ablation [Citation7,Citation8]. At the same time, a new method consisting of double-needle multipoint ablation not only improves the ablation efficiency but also expands the ablation boundary [Citation9]. Second, a large number of embolization agents are required in the treatment of large HCC, which has a great influence on the surrounding normal liver tissue and increases the risk of liver damage. MWA can significantly reduce the iodized oil dose and protect the surrounding normal liver tissue at doses less than those previously reported for chemoembolization alone [Citation27,Citation28]. Third, tumors located near the major hepatic vessels are not easy to ablate completely, increasing the possibility of local recurrence and metastasis. TACE after MWA can further eliminate these local residual lesions [Citation29]. Fourth, TACE after MWA controls micro-lesions, which contribute to recurrence after treatment [Citation30].
The mortality and major complication rates after liver MWA for large HCCs have been reported as 0–0.2% and 7.5–21.4%, respectively [Citation15,Citation31]. The thermal ablative treatment for large HCC was well tolerated in the current study, with a mortality rate of 0% and a morbidity rate of 7%. Our results were lower than those reported in the medical literature. The low complication rate was due to the precautions adopted based on advanced knowledge of possible risk conditions. Severe MWA-related complications, such as hemorrhage, should be identified and treated promptly. In the present study, hemorrhage was detected in two patients after MWA, and all of them were managed promptly with blood transfusions and embolization. Therefore, MWA-TACE could be considered a safe treatment modality for large HCC lesions.
The LTP rate after MWA-TACE reportedly depends on tumor size. Abdelaziz et al. [Citation32] conducted a single-center study to evaluate MWA in the treatment of large HCCs in 26 patients with 5–7 cm HCC lesions. They assessed the effect on LTP and concluded that LTP occurred with 5 treated lesions (19.2%) during the follow-up period. A study from China evaluated the local efficacy of MWA for treating large unresectable HCCs and found local recurrence in 17 (20.7%) patients during the follow-up period [Citation33,Citation34]. The 1-, 2- and 3-year LTP rates were 14.6%, 19.5%, and 20.7%, respectively. However, given that all of the lesions treated in our study were 5.0 cm or larger, the LTP rate in our study is similar to that after single local treatments.
In our study, the 1-, 2- and 3-year OS rates were 81%, 68% and 49%, respectively. This rate was better than the rate of ∼20–40% reported in other studies at 3 years after a single local treatment [Citation32,Citation35]. Jin et al. [Citation32] evaluated TACE as a first-line therapy for 123 HCC patients with a large solitary HCC of BCLC stage A. The results showed that cumulative OS rates in the TACE group were 68.5% and 45% at 1 and 3 years, respectively. Similarly, an Egyptian cohort study conducted by Abdelaziz et al. [Citation32] evaluated the efficacy of percutaneous MWA versus TACE for large tumors (5–7 cm). The results showed that the OS rates at 12 and 18 months were 78.2% and 68.4%, respectively, in the MWA group and 52.4% and 28.6% in the TACE group. Furthermore, these results were almost identical to those after surgical resection, which was widely used to treat large liver tumors [Citation1–4]. Chang et al. [Citation36] assessed long-term survival after LR for large HCCs with a large sample size. Patients without compensated cirrhosis were divided into four groups, which included a large tumor group (5.0–10.0 cm). Their results revealed that OS rates for patients with large HCCs were 82.1% and 63.1% at 1 and 3 years, respectively.
This study had some limitations. First, because it was a retrospective study, there is a potential risk of selection bias. The majority of the patients had one lesion, and almost half of the patients had a tumor ≤7 cm. Second, the study design was a single-arm noncomparative study. Third, the treatment interval between MWA and TACE was heterogeneous. The intervals of treatment for all the patients were <4 weeks in our study. Recently, Feng et al. reported that a period of 3–5 weeks is the optimal time interval between RFA and TACE for HCC patients with Child-Pugh class A or B cirrhosis. Thus, we believe that the time interval was optimized for the evaluation of LTP, as well as prognosis and safety after combined therapy.
Conclusion
In conclusion, US-guided percutaneous MWA followed by TACE appeared to be safe and effective and may be a treatment option in the future, especially for unresectable large HCC lesions with compensated liver cirrhosis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- IARC. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013 [cited 2014 Oct 21]. Available at: http://globocan.iarc.fr
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917.
- Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458.
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524.
- European Association for the Study of the Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Shindoh J, D Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer. 2012;1:159–167.
- Poggi G, Montagna B, DI Cesare P, et al. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res. 2013;33:1221–1227.
- Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087–1092.
- Brace CL. Dual-slot antennas for microwave tissue heating: parametric design analysis and experimental validation. Med Phys. 2011;38:4232–4240.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442.
- Llovet JM, Bru ´ C, Bruix J, et al. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338.
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469.
- Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma or 4 cm. Gastroenterology. 2004;127:1714–1723.
- Sturm JW, Keese M, et al. Multimodal treatment of hepatocellular carcinoma (HCC). Onkologie. 2004;27:294–303.
- Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914–1923.
- Takaki H, Yamakado K, Uraki J, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol. 2009;20:217–224.
- Si ZM, Wang GZ, Qian S, et al. Combination therapies in the management of large (≥5 cm) hepatocellular carcinoma: microwave ablation immediately followed by transarterial chemoembolization. J Vasc Interv Radiol. 2016;27:1577–1583.
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022.
- Hsu CY, Lee YH, Hsia CY, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013;57:112–119.
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871.
- Ishizawa T, Hasegawa K, Aoki T. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–739.
- Cardella JF, Kundu S, Miller DL, Society of Interventional Radiology, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20(7 Suppl):S189–S191.
- Saviano A, Iezzi R, Giuliante F, et al. Liver resection versus radiofrequency ablation plus transcatheter arterial chemoembolization in cirrhotic patients with solitary large hepatocellular carcinoma. J Vasc Interv Radiol. 2017;28:1512–1519.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432.
- Takuma Y, Takabatake H, Morimoto Y, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013;269:927–937.
- Luo J, Peng ZW, Guo RP, et al. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259:286–295.
- Yoon HM, Kim JH, Kim EJ, et al. Modified cisplatin-based transcatheter arterial chemoembolization for large hepatocellular carcinoma: multivariate analysis of predictive factors for tumor response and survival in a 163-patient cohort. J Vasc Interv Radiol. 2013;24:1639–1646.
- Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis?. J Vasc Interv Radiol. 1998;9:101–111.
- Higuchi T, Kikuchi M, Okazaki M, et al. Hepatocellular carcinoma after transcatheter hepatic arterial embolization: a histopathologic study of 84 resected cases. Cancer. 1994;73:2259–2267.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology. 2009;251:933–940.
- Abdelaziz AO, Nabeel MM, Elbaz TM, et al. Microwave ablation versus transarterial chemoembolization in large hepatocellular carcinoma: prospective analysis. Scand J Gastroenterol. 2015;50:479–484.
- Xu Y, Shen Q, Wang N, et al. Percutaneous microwave ablation of 5-6 cm unresectable hepatocellular carcinoma: local efficacy and long-term outcomes. Int J Hyperthermia. 2016;247–254.
- Ahmed M, Solbiati L, Brace CL, International Working Group on Image-guided Tumor Ablation, and Interventional Oncology Sans Frontières Expert Panel, and Technology Assessment Committee of the Society of Interventional Radiology, and Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Imageguided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273:241–260.
- Jin YJ, Lee JW, Choi YJ, et al. Surgery versus transarterial chemoembolization for solitary large hepatocellular carcinoma of BCLC stage A. J Gastrointest Surg. 2014;18:555–561.
- Chang YJ, Chung KP, Chang YJ, et al. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg. 2016;103:1513–1520.
