Abstract
Purpose: The goal of this study was to define patterns for tumor recurrence on PET following RFA, compare time to imaging recurrence by PET versus CT, evaluate whether pre-treatment tumor uptake predicts recurrence and propose an optimal post-RFA surveillance strategy.
Materials and methods: A retrospective cohort study was performed of biopsy confirmed primary stage I lung cancers treated with RFA. FDG PET and near contemporaneous diagnostic CT imaging pre-ablation, within 30 days post-ablation, and beyond 6 months were independently and retrospectively evaluated for features supportive of recurrence. Time to imaging recurrence by PET (TTR_PET) and by CT (TTR_CT) were determined and compared. FDG avidity of untreated tumors was compared between recurrent and non-recurrent groups.
Results: Thirteen recurrences after 72 RFA treatments were confirmed by diagnostic CT. All recurrences were associated with focally intense and increasing FDG uptake beyond 6 months (sensitivity 100%; specificity 98.5%). Mean TTR_PET was 14 months compared to mean TTR_CT of 17 months (not statistically significant). Normalized SUVmax and total lesions glycolysis of lung cancers that recurred after RFA was 4.0 and 6.0, respectively compared to 2.8 and 5.0, respectively for cancers that did not recur (p = .068).
Conclusion: A pattern of focally intense and increasing FDG PET uptake has high sensitivity and specificity for detecting recurrent lung cancer following RFA. Surveillance after RFA should include a contrast enhanced diagnostic CT at 1 month to diagnose procedural complications, PET at 6 months as a post-treatment metabolic baseline (with diagnostic CT if PET is abnormal) and alternating diagnostic CTs or PET every 6 months for 2 years.
Keywords:
Introduction
The current standard of care for patients with stages I and II non-small cell lung cancer (NSCLC) is surgical resection [Citation1]. However, many patients are not candidates for curative surgical resection as a result of cardiorespiratory comorbidity or insufficient lung function. In these high-risk patients, image-guided radiofrequency ablation (RFA) is an option for treatment, particularly for tumors less than 3.0 cm in diameter [Citation2–4]. During monopolar RFA, an alternating high-frequency current is produced by a radiofrequency generator and destroys targeted tissue by heating cells to over 60 °C to achieve irreversible protein denaturation [Citation5]. The circuit is a closed-loop between the radiofrequency applicator and one or more large grounding pads placed on the patient’s skin.
Computed tomography (CT) is the most widely used imaging modality for assessment following RFA. The RFA treatment zone often increases in size on early follow up imaging (up to 3 months), but then remains stable or decreases in size on subsequent follow up CT imaging [Citation6]. CT features of incomplete ablation and/or recurrence include intratumoral contrast uptake, tumor growth at the periphery of the ablation zone or a 20% increase in longest tumor diameter [Citation7,Citation8].
Superiority of fluorodeoxyglucose positron emission tomography (FDG PET) over CT has been established for staging and surveillance of primary lung cancers [Citation9]. Multiple studies have evaluated the metabolic and morphologic evolution of both primary and metastatic lung tumors following RFA and have shown that FDG PET can reduce false positives for detecting tumor recurrence [Citation10]. While FDG PET combined with diagnostic CT has been suggested to be the most accurate method of post-treatment surveillance, serial FDG PET follow up is not standard of care, and clear guidelines about the appropriateness of FDG PET and FDG PET/CT are not presently available.
We present a large consecutive series of medically inoperable primary lung cancer patients treated with percutaneous RFA. The primary aims of this study were: (1) to define the features on FDG PET that are supportive of tumor recurrence with serial diagnostic CT imaging as a reference standard and (2) to apply those PET criteria to compute a time to imaging recurrence (TTR) by FDG PET as compared to TTR by independent diagnostic CT analysis. Secondary aims were to evaluate the predictive value of pre-treatment tumor avidity on likelihood of recurrence. Our goal was to suggest an optimal surveillance strategy for post-RFA disease surveillance.
Materials and methods
The institutional review board approved this study, and the need for informed consent was waived because of its retrospective design. This study was performed in accordance with the Health Insurance Portability and Accountability Act.
Patients and follow-up
Electronic medical records were retrospectively queried for a consecutive series of biopsy confirmed primary stage I lung cancer patients who were treated with RFA between January 2004 and December 2014. Inclusion criteria included: (1) tumors measuring at least 1 cm in largest dimension by CT; (2) near contemporaneous FDG PET and CT imaging (defined as a 30 day interval) obtained for standard of care purposes at the following timepoints: pre-treatment, immediate post-RFA (within 30 days) and at 6 months or later; (3) CT imaging follow up of at least 12 months; and (4) clinical follow up of at least 12 months. Exclusion criteria included sites with early recurrence that were retreated and did not have adequate imaging follow-up after the second RFA.
The decision to treat with RFA was determined by a multidisciplinary tumor board consisting of a thoracic radiologist, thoracic oncologist, thoracic surgeon and radiation therapist. All patients had percutaneous transthoracic needle biopsy prior to RFA to document proven lung cancer. Baseline PET and diagnostic CT imaging were available to inform treatment decisions in all cases.
Radiofrequency ablation technique
All RFAs were performed by 2 radiologists with 10 and 25 years of experience in thoracic interventional radiology. The patients were treated using a 17-gauge single and cluster Cool Tip© electrode (Covidien, Burlington, MA). For lesions measuring between 1 and 2 cm in diameter, ablation treatments were performed either using a 17-gauge single or cluster electrode with a 3 cm active tip; electrode choice was determined by the accessibility of the nodule. With lesions greater than 2 cm in largest diameter, ablations were performed using the 17-gauge cluster electrodes with a 3 cm active tip. Prior to the procedure, 2 grounding pads were placed across the patient's thighs when a single electrode was used, and 4 grounding pads were placed across the patient's thighs when the cluster electrode was used for ablation.
RFA was performed whenever possible using conscious sedation. Intravenous administration of Fentanyl, Demerol and Midazolam was titrated to the patient's needs, with the aim of maintaining steady, low level respirations throughout the procedure. Requirements for general anesthesia were determined by the patient’s cardiorespiratory status and associated comorbidities. General anesthesia was also used in larger peripheral lesions where ablation was likely to be more painful. Low-level ventilation and a double lumen tube were used in 3 patients to reduce movement in the lung to be ablated.
The patient was placed in a supine or prone position on a 16 slice GE CT scanner (Milwaukee, Wisconsin). Axial 2.5 mm sections were obtained through the nodule, with gantry angulation if necessary, to find an optimum window.
Following local anesthetic infiltration of the skin and subcutaneous tissues, the 17-gauge electrode was advanced into the nodule with incremental movements until the electrode tip was at least 2 mm beyond the distal edge of the nodule and the active non-insulated 3 cm portion of the electrode spanned the nodule.
A 12 min ablation was initially performed under impedance control. The procedure was considered successful if a thermocouple placed at the electrode tip showed a temperature exceeding 60 °C and a post-ablation CT scan demonstrated ground-glass opacity in the surrounding lung with a circumferential margin of at least 10 mm around the nodule. The initial ablation was supplemented with further 6–12 min ablations after repositioning the electrode in order to achieve an adequate margin of ground-glass opacity surrounding the lesion.
At the end of the procedure, the electrode was removed and the patient immediately rolled onto a stretcher with the puncture site dependent. The patient was monitored in this position for 3 h. A chest radiograph was performed at 1 and 3 h to evaluate for a pneumothorax. All patients were admitted overnight for observation. Most patients received imaging at 6-month intervals, but there was no consensus as to mode or length of imaging follow up.
All patients were followed with CT and/or PET/CT at 1 month. Surveillance CT or FDG PET imaging was determined at the discretion of the referring oncologist and/or oncologic surgeon.
FDG PET imaging
PET imaging was obtained either as a stand alone PET or with diagnostic CT as part of a combined PET/CT exam. Irrespective of whether a diagnostic CT was performed, each PET exam included a low-dose nondiagnostic CT for attenuation correction purposes. The scanning protocol is as described by Wang et al. [Citation11].
Diagnostic CT imaging
Diagnostic chest CT scans were performed either as stand-alone exams or as part of a combined PET/CT exam. Iodinated intravenous contrast was administered at the discretion of the referring physician. Positive oral contrast was administered if the patient was undergoing a contiguous abdominopelvic CT exam. CT scan settings are as follows: 120 kVp, modulated mA, a tube rotation time of 0.5 s per rotation, a variable pitch between 1 and 1.5, depending on patient BMI, and a section thickness of 2.5 mm. Imaging was obtained at maximum inspiration. Chest CT coverage extended from the thoracic inlet to the level of the adrenal glands.
FDG PET review
A subspecialty board-certified nuclear radiologist blinded to all diagnostic CT data retrospectively reviewed the FDG PET imaging at all available timepoints. Quantitative metabolic assessment of the primary tumors on baseline FDG PET or post-RFA zone on follow-up RFA zone included measurements of SUVmax and total lesion glycolysis (TLG). SUVmax was measured by drawing a volume of interest (VOI) inclusive of the primary tumor or treated site, as defined by low dose CT obtained for attenuation correction purposes. Methodology for obtaining TLG is as described by Viglianti et al. [Citation12]. In brief, an autocontouring VOI tool using a well-defined threshold of 2.5 was drawn around the site of interest. Since all of our patients had a single site of disease or treatment, the TLG was the product of SUVmean and metabolic volume.
Qualitative assessment involved visual scoring of the magnitude of uptake (none, mild, moderate, intense) at the primary tumor or post-RFA site. Mild uptake was defined as uptake less than mediastinum; moderate uptake was uptake at least as high as mediastinum but not higher than liver; intense uptake was uptake that was at least as high as background liver.
On serial post-treatment FDG PET studies, a change in SUVmax or TLG greater than 30%, after normalization for background liver activity (SUVmax_normalized), was considered true change. To obtain background liver activity, a reference VOI of at least 3 cm3 was drawn in a part of the liver without visible abnormalities on CT. Normalization to liver background was performed to account for variations in biodistribution.
Time to imaging recurrence by PET (TTR_PET) was defined as number of days from RFA treatment to manifestation of findings suspicious by PET independent of CT. Low dose CT findings were used to localize the site of abnormal uptake on PET if concurrent diagnostic CT imaging was not available.
Chest CT review
Two board certified radiologists blinded to patients’ clinical data as well as PET imaging results reviewed CT imaging by consensus. Primary tumors on pre-treatment CT were evaluated for size in two dimensions and composition (ground glass, mixed attenuation or solid) and average density in Hounsfield Units.
Post-RFA zones on post-treatment CT imaging were evaluated for size in two dimensions, change in size compared to prior CT and presence of increased nodularity. Findings confirming recurrence were defined as increased nodularity or enlargement in at least a single dimension of the post-RFA zone by 2 consecutive CTs within the first 6 months or on a single CT beyond the first 6 months. On contrast enhanced CT scans, eccentric enhancement was also considered recurrent disease.
Time to imaging recurrence by CT (TTR_CT) was defined as number of days from RFA treatment to manifestation of findings independently suspicious by CT.
Statistical analysis
Clinicopathologic variables, serial PET and CT imaging timepoints, and recurrence patterns by PET and CT were analyzed. Continuous variables (SUVmax_normalized, TLG, density, size, TTR_PET, and TTR_CT) were compared using a Wilcoxon rank sum test. Categorical variables (CT composition, visual score, uptake pattern) were compared by cross table analysis using the Fisher exact test and χ2 test of linear-to-linear association. A significance level of p < .05 was used.
Follow-up was calculated from time of primary RFA treatment until death or last follow-up. All calculations were performed using SAS 8.0 (SAS Inc., Cary, NC).
Results
Patients and follow up
A total of 89 RFA treatments were performed during the study period. Two treated primary lung cancers were excluded due to early recurrence within 6 months of initial RFA. 15 treated primary lung cancers were excluded due to imaging follow up not meeting inclusion criteria. 72 primary inoperable lung cancers in 70 patients satisfied the inclusion criteria. Mean patient age was 63 (range 45–89). 45 patients were female; 35 patients were male. Patient demographics and RFA treatment techniques are summarized in . RFA was performed with general anesthesia in six patients (9%) and with conscious sedation in 64 patients (91%).
Table 1. Patient demographics and radiofrequency ablation (RFA) technique.
Patients had an average of 4 FDG PET scans (range 4–8). Mean imaging follow up by FDG PET and/or CT was 36 months (range 12–78 months). Mean clinical follow up was 42 months (range 12–82 months). Distribution of FDG PET scans is presented in .
Table 2. Distribution of FDG PET scans at various timepoints following RFA.
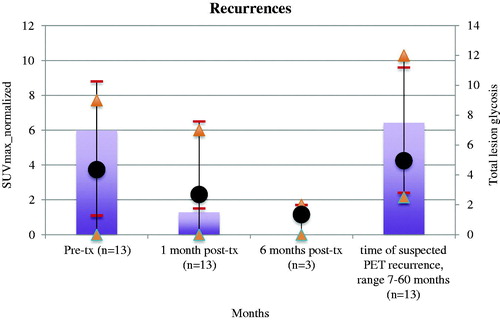
There were 13 local recurrences in 12 patients confirmed by serial CT as the reference standard. Recurrences occurred on average 14 months following RFA (range, 7 months-60 months, median 12 months). Twelve of 13 local recurrences occurred within 2 years. No histopathologic sampling was performed for any of the presumed recurrences.
Imaging analysis
Baseline scans
At baseline CT, 69 primary lung cancers presented as solid nodules; 3 lung cancers presented as ground glass or mixed attenuation nodules. Mean size was 1.5 cm in largest dimension (range 1.0–2.9 cm). At baseline FDG PET, the distribution of visual scores was as follows: 44 intense, 21 moderate, 5 mild and 2 none. Mean SUVmax_normalized was 3.3 (range, background to 10.2). Mean TLG was 5.2 (range, 0 to 10.2). FDG PET scanning at baseline did not show any regional or distant metastatic disease that would upstage the patients.
Non-recurrence post RFA
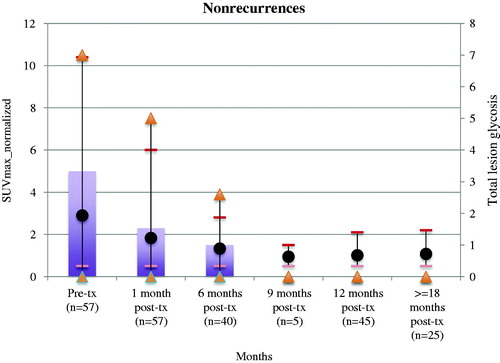
Among the 59 cancers without suspected recurrence by post-treatment CTs, 56 post-ablation zones demonstrated high (moderate or intense) but non-focal FDG uptake in the post-RFA zone 1 month after treatment followed by serial decline in magnitude on follow up FDG PET scans. Among the 56 post-ablation zones with this pattern of evolution, 23 (41%) had uptake higher than that of primary tumor and 25 (45%) demonstrated at least moderate uptake on FDG PET imaging beyond 6 months (range 12–48 months). 8 (14%) post-RFA zones continued to demonstrate non-focal uptake higher than that of primary tumor at scans beyond 6 months. The evolution of uptake magnitude over time aggregated across non-recurrent ablation sites is shown in .
Figure 1. Mean SUVmax_normalized with maximum and minimum range bars (black dots, short lines) and total lesion glycolysis (bars, triangles) of lung cancers at baseline and subsequent timepoints at radiofrequency ablation zones (in months post-treatment) among non-recurrent sites. *Please note that two patients, both with intense and increasing focal uptake at last available PET follow up but no findings on CT confirming recurrence are considered non-recurrent by previously established methodology, and are not included in figure.
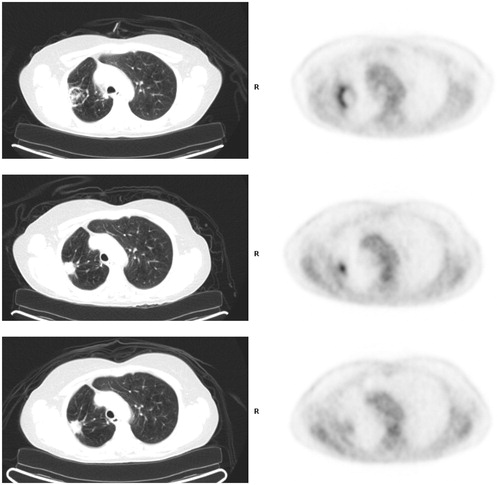
One patient who was treated with a cluster electrode device demonstrated intense uptake peripherally on 1 month post-RFA treatment imaging with a focal site of intense uptake centrally at 6 month FDG PET imaging. Follow-up PET imaging at 13 months showed low level uptake at the treatment site, confirming benignity ().
Figure 2. FDG PET pitfall. (Top) FDG PET/CT 1 month post-radiofrequency ablation with electrodes shows expected ring of intense uptake along periphery of treated right upper lobe cancer and inflammatory soft tissue by CT. (Middle) FDG PET/CT 6 months post-RFA showed focal intense uptake centrally within the treatment zone that is modestly higher in magnitude compared to immediate prior PET. There is contracting soft tissue by CT. (Bottom) FDG 13 months post-RFA shows non-focal moderate uptake (similar to mediastinal background) at treatment site. Soft tissue at treatment site is stable by CT.
Two patients demonstrated new focal intense uptake at their RFA ablation zone that was markedly increased compared to the immediate prior surveillance FDG PET scan (12 and 60 months post-RFA) and prospectively called suspicious for recurrence; same day diagnostic CT exams did not show any change in post-RFA treatment site. Recurrence could not be confirmed in the absence of follow up CT imaging. One patient has 12 month clinical follow up beyond the last PET scan and is now deceased, though cause of death is not documented. The second patient continues to be living but is on palliative care for debilitating multilevel compression fractures.
Local recurrence post RFA
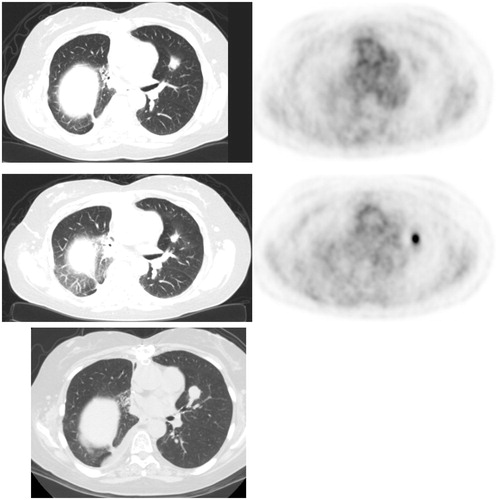
Among 13 CT-confirmed recurrences, all FDG PET scans showed focally intense uptake at the site of recurrence that was increased in magnitude compared to the immediate prior FDG PET exam (). The evolution of uptake intensity over time aggregated across recurrent sites is shown in .
Figure 3. Lung cancer recurrence. (Top) FDG PET/CT 1 month post-RFA shows low level uptake at treatment site in the left upper lobe. (Middle) FDG PET 7 months post-RFA shows focal intense uptake at ablation zone (increased compared to immediate post-RFA FDG PET), suspicious for recurrence. Concurrent diagnostic CT showed no evidence for recurrence, and possibly even slightly contracted soft tissue. (Bottom). Follow up diagnostic CT 10 months post-RFA showed enlargement of soft tissue at ablation zone, confirming recurrence.
FDG avidity of recurrent and nonrecurrent treatment sites at baseline
At baseline, the mean SUVmax_normalized and TLG of primary lung cancers that did not recur after RFA was 2.8 and 5.0, respectively, while mean SUV of lung cancers that did recur was 4.0 and 6.0, respectively. This result was not statistically significant.
Time to recurrence analysis
Using intense focal FDG uptake that was higher than the immediate prior PET as the criteria for recurrence by FDG PET, the TTR_PET was 14 months (range, 7–60 months). The TTR_CT was 17 months. This difference was not shown to be statistically significant.
Discussion
To our knowledge, this is one of the largest reported series exclusive to primary lung cancers treated with RFA with serial FDG PET and CT follow up. Prior studies on the role of FDG-PET in post-RFA surveillance investigated the expected post-treatment evolution at the RFA zone [Citation13–15]. Expected post-RFA findings may consist of little to no uptake on post-treatment FDG PET imaging or prominent but non-focal increased uptake in the ablation zone that may continue to increase over the initial 6 month period but regresses in the 6–12 month period. A ring of peripheral activity and central photopenia is a characteristic post-treatment pattern associated with RFA by cluster electrodes [Citation15]. As a result of the variable pattern of post-RFA FDG PET findings, high uptake within the first 6 months is generally considered post-inflammatory and not specific for tumor recurrence [Citation16,Citation17]. Our data support this observation and also demonstrate high and rising uptake at a subset of non-recurrent ablation sites. For this reason, early recurrences, defined as those occurring within 6 months of RFA, were excluded from our study.
45% (25/69) of treated tumors presumed to be non-recurrent by serial CT follow up demonstrated at least moderate uptake (similar or higher than mediastinal background) on post-RFA FDG PET imaging, even beyond 6 months, likely due to reactive changes. However, the pattern of uptake is always non-focal, supporting benignity. In contradistinction, all CT confirmed recurrences were associated with focally intense and increasing FDG uptake beyond 6 months (sensitivity 100%), a pattern characteristic for recurrent tumor (specificity 98.5%). This pattern, however, is not pathognomonic; in a right upper lobe lung cancer treated with a cluster electrode, serial post-RFA imaging showed intense peripheral activity at the post-RFA site paralleling CT inflammatory changes (). A PET study performed approximately 7 months post-RFA showed persistent focal intense uptake centrally, modestly higher in magnitude compared to the immediate prior PET, but associated with retracting soft tissue by CT. A follow up PET at 13 months showed resolution of both focality and intensity of FDG uptake abnormality and regressing soft tissue by CT at the treatment site, consistent with evolving post-treatment changes, not recurrence.
If we apply the above criteria of focally intense FDG uptake prospectively to all post-RFA FDG PET exams, the sensitivity for detecting recurrences is 100% and specificity is 98.5%. Mean time to recurrence (TTR_PET) as demonstrated by PET alone was slightly shorter (mean 14 months) compared to mean time to recurrence (TTR_CT) as evident by diagnostic CT alone (mean 17 months), though this difference was not shown to be statistically significant. Interestingly, the TTR calculated using CT reports issued as part of standard of care (rather than independent retrospective CT analyses), was essentially identical to TTR_PET (14 months). This observation suggests that the original diagnostic CT impression was influenced by the PET findings and that any PET abnormalities likely increase confidence in detecting CT abnormalities. Our sample of recurrences is too small to draw any meaningful conclusions about whether consistent use of contrast enhancement on diagnostic CTs would have shortened the TTR_CT, however, there were no instances where a recurrence was inapparent on a non-contrast CT and subsequently detected on a contrast enhanced CT.
In all instances where recurrences were confirmed, both the SUVmax_normalized and TLG more than doubled compared to the preceding timepoint, well exceeding the 30% threshold supportive of progression required by PET response criteria in solid tumors (PERCIST), albeit for SUV peak [Citation18]. Conversely, among non-recurrences, there was a decline or stability in SUVmax_normalized and TLG at all imaging timepoints outside the 6-month window. If we were to determine recurrence outside the 6-month window using PERCIST criteria, that is an increase in SUVmax_normalized or TLG of atleast 30%, we could achieve an identical sensitivity (100%) and superior specificity (100%) as qualitative methods. These analyses suggest quantitation could be used reliably for disease monitoring and assessment of metabolic tumor burden. Of note, the liver uptake in our patient population varied little across timepoints (less than 20%), likely due to presence of limited disease burden. An additional caveat is that PERCIST criteria appears not to be appropriate for assessment of early response to RFA treatment due to the incidence of inflammatory uptake at the RFA site.
A number of studies have suggested that pre-treatment FDG avidity of primary and metastatic lung tumors can predict recurrence free survival [Citation16]. In our cohort of stage I lung cancers, the FDG avidity and metabolic burden of primary tumors that recurred was higher than those that did not, however this difference was not statistically significant.
Our cohort has a rate of local recurrence (13/72, 18%) that is similar to other published series [Citation19]. A limitation of our study is that we used an imaging gold standard (diagnostic chest CT) for post-treatment recurrence without tissue sampling. This method precludes detection of slow microscopic recurrence and therefore biases our results toward higher FDG PET sensitivity. Two cases of focal intense uptake without suspicious findings by CT could not be considered recurrences by our established criteria, and would have benefitted from definitive characterization by tissue sampling. While our inclusion criteria stipulated FDG PET imaging at specific timepoints to align our follow up intervals, it is unclear if more frequent FDG PET scanning could further improve timely detection of recurrence.
A comparison of how the independent PET and CT findings altered management would have been interesting, but difficult to resolve retrospectively due to the near contemporaneous timing of the exams. A utility of a prospective evaluation is likely also obviated by the common practice to order a complementary scan as a problem solving or confirmation tool if a CT or PET scan shows a potentially worrisome finding.
In conclusion, FDG PET alone is highly sensitive for detecting recurrences after RFA ablation of primary lung cancers. In an era when health care cost and cumulative radiation exposure are concerns, high sensitivity by FDG PET allows FDG PET alone (with low dose CT for localization and attenuation correction only) with intercurrent diagnostic CTs to supplant combined serial FDG PET and diagnostic CT scans as an effective follow-up strategy. A proposed follow-up algorithm is pre-treatment PET/CT to confirm local disease, contrast Chest CT at 1 month post-RFA to exclude postprocedural complications and a PET at 6 months to serve as a new metabolic baseline. A near contemporaneous CT is only required if the PET is abnormal. Surveillance by alternating stand-alone PET and CTs should be performed every 6 months for 2 years, as the majority of recurrences occur within the first 2 years.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):234S–242S.
- Chan VO, McDermott S, Malone DE, et al. Percutaneous radiofrequency ablation of lung tumors: evaluation of the literature using evidence-based techniques. J Thorac Imaging. 2011;26:18–26.
- Gomez FM, Palussiere J, Santos E, et al. Radiofrequency thermocoagulation of lung tumours. Where we are, where we are headed. Clin Transl Oncol. 2009;11:28–34.
- Lanuti M, Sharma A, Willers H, et al. Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence. Ann Thorac Surg. 2012;93:921–927. [discussion 927–988].
- Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129–147.
- Steinke K, King J, Glenn D, et al. Radiologic appearance and complications of percutaneous computed tomography-guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr. 2003;27:750–757.
- Abtin FG, Eradat J, Gutierrez AJ, et al. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics. 2012;32:947–969.
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621–628.
- Kang S, Luo R, Liao W, et al. Single group study to evaluate the feasibility and complications of radiofrequency ablation and usefulness of post treatment position emission tomography in lung tumours. World J Surg Oncol. 2004;2:30.
- Singnurkar A, Solomon SB, Gonen M, et al. 18F-FDG PET/CT for the prediction and detection of local recurrence after radiofrequency ablation of malignant lung lesions. J Nucl Med. 2010;51:1833–1840.
- Wang Y, Brett CW, Muse V, et al. Potential pitfall in the assessment of lung cancer with FDG-PET/CT: Talc pleurodesis causes intrathoracic nodal FDG avidity. Lung Cancer Intl. 2013;2013:683582.
- Viglianti BL, Wale D, Wong KK, et al. Effects of tumor burden on reference tissue standardized imaging uptake for PET imaging: modification of PERCIST criteria. Radiology. Forthcoming 2018.
- Alafate A, Shinya T, Okumura Y, et al. The Maximum standardized uptake value is more reliable than size measurement in early follow-up to evaluate potential pulmonary malignancies following radiofrequency ablation. Acta Med Okayama. 2013;67:105–112.
- Purandare NC, Rangarajan V, Shah SA, et al. Therapeutic response to radiofrequency ablation of neoplastic lesions: FDG PET/CT findings. Radiographics. 2011;31:201–213.
- Sharma A, Lanuti M, He W, et al. Increase in fluorodeoxyglucose positron emission tomography activity following complete radiofrequency ablation of lung tumors. J Comput Assist Tomogr. 2013;37:9–14.
- Bonichon F, Palussiere J, Godbert Y, et al. Diagnostic accuracy of 18F-FDG PET/CT for assessing response to radiofrequency ablation treatment in lung metastases: a multicentre prospective study. Eur J Nucl Med Mol Imaging. 2013;40:1817–1827.
- Rasmussen F, Madsen HH. Imaging follow-up of RF ablation of lung tumours. Cancer imaging: the official publication of the International. Cancer Imaging Soc. 2011;11 Spec No A:S123–S128.
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 (Suppl 1):122S–150S.
- Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol. 2012;81:395–399.