Abstract
Purpose: We used an impedance-controlled generator with an internally cooled electrode to perform radiofrequency ablation (RFA) in ex vivo bovine livers, with a single injection of either 38.5% sodium chloride (NaCl) or 10% hydrochloric acid (HCl), to determine the relative effects of these two solutions on tissue impedance, temperature and ablation volume.
Materials and methods: We performed 10 ablations each with injections of NaCl (NaCl-RFA), HCl (HCl-RFA) or nothing (RFA-alone), with a power setting of 200 W for 15 minutes. We recorded tissue impedance before and after injection. We logged temperatures obtained from thermocouple probes positioned 5, 10, 15 and 20 mm from the internally cooled RF electrode. After ablation, we measured ablation zone longitudinal and transverse diameters, and we calculated a spherical ratio (SR) for each ablation.
Results: Mean post-injection impedance of 30.3 (standard deviation [SD] 2.5) ohms for HCl was significantly lower than that of 55.4 (SD 3.5) ohms for NaCl (p < .001). Mean maximum temperatures recorded at each respective distance from the RFA electrode were all highest for HCl-RFA and lowest for RFA-alone (p < .001). Mean longitudinal and transverse diameters after HCl-RFA (5.50 [SD 0.25] cm and 5.28 [SD 0.22] cm, respectively) were significantly larger than those after NaCl-RFA (4.24 [SD 0.35] cm and 3.55 [SD 0.43] cm, respectively) and after RFA-alone (3.60 [SD 0.10] cm and 2.70 [SD 0.13] cm, respectively) (p < .001). Mean SR after HCl-RFA (0.93, SD 0.02) was significantly higher than mean SR after NaCl-RFA (0.76, SD 0.06) and RFA-alone (0.72, SD 0.04) (p < .001).
Conclusion: Monopolar, impedance-controlled RFA, with an internally cooled electrode and a single 10% HCl injection may allow larger tumors to be treated, potentially resulting in improved patient outcomes.
Introduction
Radiofrequency ablation (RFA) has become an important treatment for primary hepatocellular carcinoma and focal liver metastases [Citation1,Citation2]. However, one of the major limitations of RFA is that commercially available radiofrequency (RF) systems are unable to consistently create an ablation volume large enough to completely cover liver tumors larger than 5 cm [Citation3,Citation4].
The most commonly utilized systems apply RF energy using either perfusion electrodes, which allow continuous normal saline infusion into tissue through one or more outlets on the electrode surface, or internally cooled electrodes, within which water circulates to cool the tissue next to the electrode and prevent tissue charring. Generators used for RF control the power output based on either the temperature (temperature-controlled) or the impedance (impedance-controlled) of the tissue being treated. In order to create larger ablation volumes, investigators have tried combining elements of these systems in a variety of ways, including with the infusions of different solutions, such as hypertonic sodium chloride (NaCl) and diluted hydrochloric acid (HCl).
Temperature-controlled RF has been performed using perfusion electrodes in ex vivo porcine livers, resulting in significantly larger ablation volumes when HCl perfusion was used during ablation versus when normal saline perfusion was used [Citation5,Citation6]. In contrast, a variety of other studies have looked at the use of impedance-controlled RF systems combined with internally controlled electrodes, some with hypertonic NaCl perfusion, and these have demonstrated not only larger RF ablation volumes, but also increased tissue temperatures and electrical conductivity [Citation7–9]. Although hypertonic saline may refer to any saline solution with a NaCl concentration higher than physiologic (0.9%), the optimal NaCl concentration for increasing ablation volume in internally cooled RF systems appears to be 38.5% NaCl [Citation10].
We hypothesized that RFA with 10% HCl may result in larger ablation volumes than RFA with 38.5% NaCl. In this study, our objective was to use an impedance-controlled RF generator with internally cooled electrodes to perform RFA in ex vivo bovine livers, with a single injection (rather than infusion) of either 38.5% NaCl or 10% HCl, in order to determine the relative effects of these two solutions on tissue impedance, temperature and ablation volume.
Materials and methods
We used 12 freshly harvested bovine livers, each weighing 5.0–6.5 kg, which we acquired from a local slaughterhouse. We employed a 10% HCl solution (Huayi Medical Auxiliary Materials Manufacturing Co., Ltd, Chengdu, China) and a 38.5% NaCl solution, which we prepared using NaCl crystals (1000 g/bottle; Guangzhou Chemical Reagent 2nd Factory, Guangzhou City, China). The solutions were kept at a temperature of 25 °C.
Equipment
We used a Cool-tip™ RF Ablation Generator (Covidien, CO, USA), which is capable of a maximum power output of 200 W, and a 17-G RF electrode with a 3-cm-long active tip [Citation11]. During ablation, the monopolar electrode was cooled internally by peristaltic perfusion of chilled saline. The generator provided power management by continuously monitoring tissue impedance, automatically adjusting power output to maximize energy delivery. During ablation, impedance levels (in ohms) were displayed on the LCD status window of the generator. We operated the generator in the auto-control mode, so that it generated pulsed RF for 15 min for each ablation [Citation12]. We set power delivery for each ablation procedure at 200 W. We used 20-G thermocouple probes (Vision-China Medical Device R&D Center, Nanjing, China), which were 20 cm long and were coated with a thin layer of epoxy to prevent interference with the RF current [Citation13]. We connected the thermocouple probes to a temperature monitor, and the detector sensitivity, reaction time and measurement range of this monitor were 0.1 °C, 0.1 s and 0 °C to about 200 °C, respectively. We used 15-cm 21-G Chiba needles (Nichiiko Co., Ltd, Toyama, Japan) to inject the solutions into livers.
Protocols
We performed three different ablation protocols: RFA without injection of solution (RFA-alone), RFA preceded by an injection of 2 ml of 38.5% NaCl (NaCl-RFA) and RFA preceded by an injection of 2 ml of 10% HCl (HCl-RFA). Each of these protocols was performed a total of 10 times.
Experimental procedure
For each ablation procedure, we inserted a 21-G Chiba needle and a 17-G RF electrode side by side and in a parallel fashion into the liver, to a depth of at least 7 cm. We positioned the Chiba needle tip at the midpoint of the active tip of the RF electrode. We then inserted four thermocouple probes, parallel to the Chiba needle, RF electrode and to each other. We positioned the thermocouple probes, which were all within the same plane, at 5, 10, 15 and 20 mm from the RF electrode. The insertion depth of the thermocouple probes was the same as that of the Chiba needle. Once positioning was accomplished, 2 ml of the HCl or NaCl solutions was injected through the Chiba needle, and then, the Chiba needle was removed. We recorded the impedance level in the tissue before and after each injection. We then performed each ablation at a power setting of 200 W for 15 min. We logged the temperatures from each thermocouple probe every 30 s during ablation, and we made particular note of the maximum temperature recorded by each of these probes during each ablation.
Ablation zone measurements
After treatment was completed, each ablation zone was identified in the liver by incising along the RF electrode insertion path. Grossly, the ablation zone was defined as the area of grey-white tissue [Citation14]. The longitudinal diameter (LD) of the ablation zone was determined by measuring along the electrode insertion path, and the transverse diameter (TD) of the zone was determined by measuring the maximum diameter that was perpendicular to the longitudinal axis.
We calculated the spherical ratio (SR) for each ablation zone according to the formula: SR = TD/LD [Citation15]. We used the SR to assess the degree to which the shape of the ablation zone was perfectly round or spherical, rather than oval, rectangular or irregular.
Infrared thermography
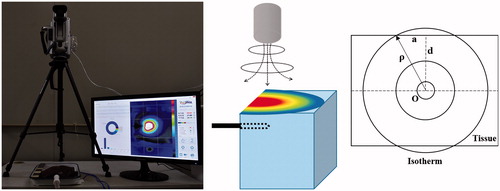
As a separate but related part of our study, we set up an infrared (IR) camera (THERMAL Information Technique, Ltd, Guangzhou, Guangdong, China) to qualitatively determine temperature distribution and ablation zone propagation on the surface of ex vivo bovine livers in real time during RFA (). For this experiment, we placed the IR camera 30 cm above the surface of the liver and an RF electrode parallel to and 1.0 cm below the surface of the liver. Using this setup, we performed ablation once using each of the three RFA protocols (RFA-alone, NaCl-RFA and HCl-RFA), at 200 W for 15 min. We used THERMAL Medical IR Diagnosis System thermography software (THERMAL Information Technique, Ltd, Guangzhou, Guangdong, China) to create temperature–proximity graphs, which allowed us to evaluate the temperatures on the surface of liver and to record the highest temperature identified on the liver surface for each protocol. The resulting graphs displayed distance (mm) from the RFA electrode on the x-axis and surface temperature (°C) on the y-axis.
Figure 1. Ex vivo bovine radiofrequency ablation experimental setup for infrared thermography. (A) Infrared camera placed 30 cm above liver, which is in tray with ablation electrode in position; temperature–proximity image is pictured on monitor; (B) animation with infrared camera above block of liver tissue, ablation electrode in position and temperature–proximity illustration on top of tissue block; and (C) coronal plane diagram of tissue demonstrates cross-section of RFA electrode (O), distance from electrode to margin of ablated zone on liver surface (P), radius of ablated zone on liver surface (a) and vertical distance from electrode to liver surface (d), which was set at 1.0 cm. Isotherm line identifies points having the same temperature, which occurred along the margin of the ablation zone.
Statistical methods
We calculated means and standard deviations (SD) for pre- and post-injection impedance, maximum temperature in each thermocouple probe, longitudinal and transverse ablation zone diameters, and spherical ratio for each of the three protocol groups. For each variable, we compared the means for each of three protocol groups with analysis of variance, using the least significant difference test to control for multiple comparisons. We initially defined statistical significance at the 5% level (p < .05). However, to control for the familywise error in the setting of multiple comparisons, we used the Bonferroni correction, which involved dividing the initial alpha by the number of comparisons (between the results of the three protocols), Thus, we defined statistical significance for individual comparisons at the 1.7% level (p < .017). We used SPSS software (version 15.0, SPSS Inc., Chicago, IL, USA) for statistical analyses.
Results
The mean post-injection impedance was substantially lower than the mean pre-injection impedance for both NaCl (55.4 ohms vs. 74.3 ohms) and HCl (30.3 ohms vs. 75.7 ohms) (). More importantly, the mean post-injection impedance of 30.3 (SD 2.5) ohms for HCl was significantly lower than the mean post-injection impedance of 55.4 (SD 3.5) ohms for NaCl (p < .001).
Table 1. Mean impedance and maximum temperatures of ablation zones in ex vivo bovine livers during radiofrequency ablation alone, with sodium chloride injection, and with hydrochloric acid injection, using a monopolar, internally cooled electrode at 200 W for 15 min.
The mean maximum temperatures recorded by the thermocouple probes at each respective distance from the RFA electrode differed significantly among the three ablation groups (p < .001) (). Mean maximum temperatures recorded by each thermocouple probe at each respective distance from the RFA electrode were all highest for HCl-RFA and lowest for RFA-alone (p < .001). Mean maximum temperatures recorded by the thermocouple probes at distances of 5 mm and 10 mm from the RF electrode in the HCl-RFA group were the only ones reaching higher than 100 °C, at 101.9 °C (SD 2.3) and 101.2 °C (SD 2.1), respectively. Mean maximum temperatures greater than 60 °C, at which point coagulation necrosis has been shown to occur [Citation16], were recorded by all four thermocouple probes (i.e., out to 20 mm) in the HCl-RFA group, but only by the two closest probes (i.e., out to 10 mm) in the NaCl-RFA group and the single closest probe (i.e., out to 5 mm) in the RFA-alone group.
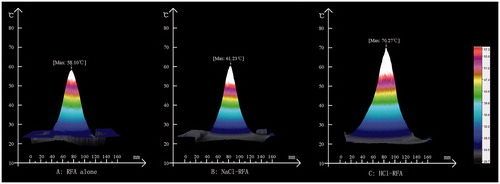
In our separate but related experiment, the maximum liver surface temperatures (at 1 cm from the electrode) measured by the IR camera were 58.1 °C, 61.2 °C and 70.3 °C, in the RFA-alone, NaCl-RFA and HCl-RFA groups, respectively (). Temperature distributions were symmetrical around both sides of the RFA electrode for all three protocols.
Figure 2. Infrared camera temperature–proximity graphs of ex vivo bovine liver surface, with radiofrequency electrode placed 1.0 cm below surface, during radiofrequency ablation (RFA)-alone, with 38.5% sodium chloride (NaCl) injection, and with 10% hydrochloric acid (HCl) injection, at 200 W for 15 min. Graphs display distance (mm) from the electrode on the x-axis and temperature (°C) on the y-axis. Temperature distributions were symmetrical around both sides of the electrode in all three protocols, with maximum temperatures at the liver surface of: (A) 58.1 °C for RFA-alone, (B) 61.2 °C for NaCl-RFA and (C) 70.3 °C for HCl-RFA.
The mean longitudinal and transverse diameters after HCl-RFA (5.50 [SD 0.25] cm and 5.28 [SD 0.22] cm, respectively) were significantly larger than those after NaCl-RFA (4.24 [SD 0.35] cm and 3.55 [SD 0.43] cm, respectively) and after RFA-alone (3.60 [SD 0.10] cm and 2.70 [SD 0.13] cm, respectively) (p < .001) (). The mean SR after HCl-RFA (0.93, SD 0.02) was significantly higher than the mean SR after NaCl-RFA (0.76, SD 0.06) and RFA-alone (0.72, SD 0.04) (p < .001). The mean SRs after NaCl-RFA and RFA-alone were not significantly different (p = .45).
Table 2. Ablation zone diameters and spherical ratios in ex vivo bovine livers after radiofrequency ablation alone, with sodium chloride injection and with hydrochloric acid injection, using a monopolar, internally cooled electrode at 200 W for 15 min.
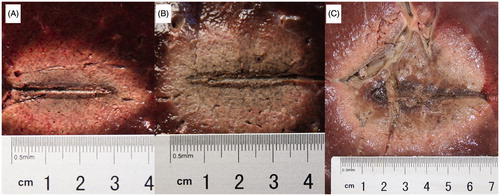
On examination of the gross tissue specimens, the ablation zones in the RFA-alone and NaCl-RFA groups were oval, with thin, dark brown carbonization bands distributed along the electrode tracts (). In contrast, the ablation zones in the HCl-RFA group were more spherical, with an expanded central region that appeared to be ‘silt-like’ (similar to moist sediment).
Figure 3. Ablation zones and diameter measurements after radiofrequency ablation (RFA) in ex vivo bovine livers, using a monopolar, internally cooled electrode at 200 W for 15 min. (A) RFA without injection (3.80 cm longitudinal ×2.70 cm transverse diameters); (B) RFA with 38.5% sodium chloride (NaCl) injection (4.10 cm longitudinal ×3.20 cm transverse diameters); (C) RFA with 10% hydrochloric acid (HCl) injection (5.80 cm longitudinal ×5.50 cm transverse diameters). The ablation zones from RFA-alone and NaCl-RFA oval, with thin, dark brown carbonization bands distributed along the electrode tracts. In contrast, ablation zones from HCl-RFA were more spherical, with expanded central ‘silt-like’ (similar to moist sediment) regions.
Discussion
The RFA can reliably induce tissue thermal coagulation necrosis and has become the non-surgical treatment of choice for hepatocellular carcinoma and focal metastatic liver tumors [Citation17,Citation18]. However, the therapeutic efficacy of RFA declines as the size of hepatic tumors increases. Because of that, a variety of adjuvant RFA techniques have been employed in an effort to create larger ablation zones, including the use of multiprobe arrays, expandable electrodes, bipolar energy, internally cooled electrodes and perfusion electrodes with hypertonic NaCl or diluted HCl [Citation16].
In our study, we demonstrated that the longitudinal and transverse diameters of the ablation zones obtained by injecting 38.5% NaCl as part of RFA in the liver were significantly larger than those obtained with RFA alone. We also showed that tissue impedance before 38.5% NaCl injection was substantially higher than after 38.5% NaCl injection. Furthermore, we demonstrated that during RFA, the maximum tissue temperatures observed at all distances 5 through 20 mm from the RFA electrode were significantly higher with RFA and 38.5% NaCl injection than with RFA alone. Others have shown similar results, though with low-flow (0.1 ml/min) infusion of hypertonic saline at a distance of 2 mm from the RFA electrode, and have suggested that their results stemmed from hypertonic NaCl reducing tissue carbonization, increasing tissue conductivity and reducing tissue impedance [Citation19].
We had reason to believe that the results of RFA with 10% HCl would be better than those of RFA with 38.5% NaCl, based on the previously published studies [Citation5,Citation6], and on findings that HCl is, in and of itself, an effective chemical ablation agent [Citation20]. Indeed, we did find that HCl-RFA created significantly larger liver ablation diameters than did NaCl-RFA.
These ablation size results may be in part attributable to the capability of RFA with 10% HCl to create higher temperatures within liver tissue than with 38.5% NaCl. We showed that at a range of 5 mm to 20 mm from the RF electrode, maximum tissue temperatures during RFA were all significantly higher in the tissue injected with HCl than in the tissue injected with NaCl. Furthermore, we were able to confirm these direct temperature probe HCl versus NaCl differences in a separate but related experiment, in which we used real-time IR thermography to analyze temperatures during RFA, and once again found that maximum temperatures for HCl-RFA were higher than those for NaCl-RFA. The higher temperatures seen with HCl may be the result of the fact that its boiling point differs from that of H2O. Normal liver tissues are rich in H2O, which has a boiling point of 100 °C, and so temperatures within these tissues do not reach or surpass 100 °C [Citation14]. However, we found that liver tissue within 10 mm of the electrode during HCl-RFA exhibited maximum temperatures of 101.2 °C and higher, which were feasible because the boiling point of 10% HCl is 103 °C. It was not clear why the maximum temperatures identified by IR at the liver surface, 1 cm from the RF electrode, were not quite as high as those identified directly with the temperature probes at 1 cm from the RF electrode. One possible explanation is that because the IR experiment was done so close to the liver surface, the energy provided to the internal ablation zone was not retained to the degree that it was in the main experiment, in which the surrounding tissue may have provided an insulation or enhancement effect.
The ablation size results may also be in part attributable to the differences in conductivity and impedance created by the two solutions. Based on previous work, it has been reported that the conductivity of 10% HCl is 7 × 106 μS/cm, more than three times higher than that of 38.5% NaCl (2 × 106 μS/cm) [Citation21]. Not surprisingly, given the reciprocal relationship between conductivity and impedance, we were able to demonstrate a similar difference within the liver tissue itself; tissue impedance was significantly lower after injection of 10% HCl than it was after injection of 38.5% NaCl.
Ultimately, the results of our study suggest that when comparing 10% HCl and 38.5% NaCl for use during liver RFA, ablation volumes are likely to be larger when HCl is injected rather than when 38.5% NaCl or no solution is injected. In addition, they suggest that the higher tissue temperatures, increased chemical conductivity, reduced tissue impedance, higher boiling point and direct chemical effect associated with HCl injection may work synergistically to expand the size of the ablation zones achieved during liver RFA.
On gross examination of the bovine livers treated with HCl-RFA, we identified an expanded ‘silt-like’ zone along the RF electrode tract, a finding that has been previously described [Citation5]. Conversely, in the livers treated with NaCl-RFA or RFA-alone, the only finding was what appeared to be thin, dark bands of carbonization along the electrode tract. Thus, the appearance of this ‘silt-like’ zone seems to be directly related to the injection of HCl. HCl is known to produce cytotoxic effects in local liver tissue, including protein denaturation and the dissolution of basement membranes [Citation20]. Because of its ability to directly damage local tissues, once it is injected, HCl may initially disseminate in the form of a small spherical zone, creating in essence a ‘virtual spherical active electrode’. In other words, the process of injecting HCl may create a high level of electrical conductivity in the region around the active electrode, reducing the RF current density at the electrode–tissue interface and effectively extending the effective or active surface of the electrode. As a result of these dynamics, tissue temperature and coagulation necrosis would be increased, and ablation zones would be more spherical. This would fit with our finding that the SR was significantly higher for HCl-RFA than for either NaCl-RFA or RFA-alone, and at a mean of 0.93, it was a strong indicator of the degree to which the shape of the ablation zone was close to perfectly spherical when HCl injection was used.
Strengths and limitations
A strength of this study is that it describes the use of an internally cooled electrode in combination with impedance-controlled RFA, and it reports results using this technique along with single injections of 10% HCl and 38.5% NaCl (rather than continuous perfusion) into the target tissue. Another strength is our confirmation of larger ablation zones with HCl-RFA, and our suggestion of a potential synergistic effect that occurs with HCl injection due to higher temperatures, increased conductivity, reduced impedance, higher boiling point and direct chemical ablation. Finally, it also provides descriptions of a ‘silt-like’ zone and a possible ‘virtual spherical active electrode’ effect that result from HCl-RFA.
As for limitations, first, because of the difficulty in finding livers thicker than 5 cm in living animals, such as rabbits and pigs, we used ex vivo bovine livers for this experiment. However, the qualities of this tissue (and surrounding structures) may differ from those of live animal or human liver tumor tissue, so it is possible that our results will not transfer exactly to use of RFA in these tissues. Nevertheless, it seems likely that the relative differences in results that we identified between the use of 10% HCl and 38.5% NaCl in RFA can be instructive for those using these solutions in RFA of live tumor tissue in the future. Second, although some clinical studies have reported that HCl can be safely injected into human livers as a chemical ablation agent without substantial side effects [Citation20], the safety of RFA with HCl injection has yet to be confirmed in live animal experiments. Finally, we did not evaluate different concentrations and doses of HCl in these experiments, and the optimal ablation duration and power settings for HCl-RFA remained to be determined.
Conclusions
Liver RFA with 10% HCl injection results in significantly larger ablation zones than with 38.5% NaCl injection, likely because of the synergistic effects of higher tissue temperatures, increased chemical conductivity, reduced tissue impedance, higher boiling point and direct chemical ablation associated with the use of HCl in this setting. If this can be translated to the treatment of human hepatic tumors, it suggests that monopolar, impedance-controlled RFA, with an internally cooled electrode and a single HCl injection, may allow larger tumors to be treated, potentially resulting in improved patient outcomes.
Ethical standards
This study did not require institutional review board approval.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–1033.
- Bale R, Schullian P, Schmuth M, et al. Stereotactic radiofrequency ablation for metastatic melanoma to the liver. Cardiovasc Intervent Radiol. 2016;39:1128–1135.
- Mulier S, Ni Y, Miao Y, et al. Size and geometry of hepatic radiofrequency lesions. Eur J Surg Oncol. 2003;29:867–878.
- Poulou LS, Botsa E, Thanou I, et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. WJH. 2015;7:1054–1063.
- Jiang XY, Gu YK, Huang JH, et al. Ex vivo liver experiment of hydrochloric acid-infused and saline-infused monopolar radiofrequency ablation: better outcomes in temperature, energy, and coagulation. Cardiovasc Intervent Radiol. 2016;39:600–605.
- Luo RG, Fao F, Huang JH, et al. Diluted hydrochloric acid generates larger radiofrequency ablation lesions in excised porcine livers. Diagn Intervent Radiol. 2013;19:145–149.
- Chen MH, Wei Y, Yan K, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Intervent Radiol. 2006;17:671–683.
- Solazzo SA, Liu Z, Lobo SM, et al. Radiofrequency ablation: importance of background tissue electrical conductivity-an agar phantom and computer modeling study. Radiology. 2005;236:495–502.
- Lee JM, Han JK, Chang JM, et al. Radiofrequency ablation of the porcine liver in vivo: increased coagulation with an internally cooled perfusion electrode. Acad Radiol. 2006;13:343–352.
- Qadri AM, Chia NJY, Ooi EH. Effects of saline volume on lesion formation during saline-infused radiofrequency ablation. Appl Math Model. 2017;43:360–371.
- Trujillo M, Alba J, Berjano E. Relationship between roll-off occurrence and spatial distribution of dehydrated tissue during RF ablation with cooled electrodes. Int J Hyperthermia. 2012;28:62–68.
- Lobo SM, Afzal KS, Ahmed M, et al. Radiofrequency ablation: modeling the enhanced temperature response to adjuvant NaCl pretreatment. Radiology. 2004;230:175–182.
- Li X, Zhang L, Fan W, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J Hyperthermia. 2011;27:240–248.
- Burdio F, Navarro A, Berjano EJ, et al. Radiofrequency hepatic ablation with internally cooled electrodes and hybrid applicators with distant saline infusion using an in vivo porcine model. Eur J Surg Oncol. 2008;34:822–830.
- Ibitoye AZ, Nwoye EO, Aweda AM, et al. Microwave ablation of ex vivo bovine tissues using a dual slot antenna with a floating metallic sleeve. Int J Hyperthermia. 2016;32:923–930.
- Ni Y, Mulier S, Miao Y, et al. A review of the general aspects of radiofrequency ablation. Abdom Imaging. 2005;30:381–400.
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590.
- Yang HJ, Lee JH, Lee DH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014;271:909–918.
- Burdio F, Tobajas P, Quesada-Diez R, et al. Distant infusion of saline may enlarge coagulation volume during radiofrequency ablation of liver tissue using cool-tip electrodes without impairing predictability. AJR. 2011;196:W837–W843.
- Weijian F, Zan L, Suhong H, et al. Destructive effect of percutaneous hydrochloric acid injection therapy for liver cancer—a preliminary experimental and clinical study. Gan to Kagaku Ryoho. 2006;33:1852–1856.
- Kameyama N. [Denkikagaku no Riron Oyobi Ouyou Part I] (Theory and Application of Electrochemistry Part I). Tokyo, Japan: Maruzen Co., Ltd., 1963:31.
