Abstract
Objective: To retrospectively analyze the adverse effects of high-intensity focused ultrasound (HIFU) in management of benign uterine diseases.
Materials and methods: From 2011 to 2017, 27,053 patients with benign uterine diseases were treated with HIFU in 19 centers in China. Among them, 17,402 patients had uterine fibroids, 8434 had adenomyosis, 876 had caesarean scar pregnancies, and 341 had placenta accreta.
Results: The median age, height, weight, BMI of the patients was 42 years, 158 mm, 56 kg, 22.5 kg/cm2, respectively. After HIFU treatment, 13,170 adverse events were observed. Based on society of interventional radiology classification system, these adverse events were classified as Class A (47.5030%), Class B (0.7947%), Class C (0.3327%), and Class D (0.0518%). The rate of major adverse effects (Class C&D) was 0.3844%. Major adverse effects include skin burn, leg pain, vaginal discharge or bleeding, urinary retention, acute cystitis, intrauterine infection, bowel injury, acute renal failure, deep vein thrombosis, pubic symphysis injury, post-HIFU thrombocytopenia, sciatic nerve injury, and hydronephrosis. In 2011, the annual rate of major adverse effects was 0.9565%; the incidence decreased to 0.2852% in 2017. No significant difference was observed in the rates of major adverse effects between patients with uterine fibroids, adenomyosis and placenta accreta.
Conclusions: Based on the results with low rate of major adverse effects from multiple centers, we concluded that HIFU is safe in treating patients with benign uterine diseases. With development of this technique and more experience on the part of the physicians, the rates of the major adverse effects will be further lowered.
Introduction
High-intensity focused ultrasound (HIFU) is a non-invasive treatment technique using thermal ablation. There are two types of HIFU system available on the market. One is ultrasound-guided HIFU (USgHIFU), while the other type is magnetic resonance imaging guided focused ultrasound surgery (MRgFUS) or MRgHIFU. In 2002, Wang et al. [Citation1] reported their preliminary results of USgHIFU treatment for uterine fibroids. In 2004, MRgFUS was FDA approved for the treatment of uterine fibroids [Citation2]. Over the last ten years, USgHIFU has been widely used in the management of uterine fibroids and adenomyosis in China [Citation3,Citation4]. Currently, this non-invasive technique has also been used in the treatment of cesarean scar pregnancy and placenta accreta [Citation5,Citation6]. Many studies have shown that both USgHIFU and MRgFUS are safe and effective in the treatment of benign uterine diseases [Citation3–7]. However, the results were mainly based on their experience from a single center or with a relatively small number of patients. Recently, Chen et al. [Citation8] retrospectively reviewed 9988 patients with uterine fibroids or adenomyosis treated with USgHIFU in 16 centers between July 2006 and June 2007. Based on the society of interventional radiology (SIR) classification system for complications by outcome, 94.1% of the adverse effects were classified as Class A, 3.4% were classified as Class B, and 1.8% were classified as Class C; only 0.6% were classified as Class D. However, those treatments were performed 10 years ago. With the development of this technique and optimization of treatment protocol, as well as more experience on the part of the physicians, USgHIFU has become a routine treatment choice for patients with uterine benign diseases in many centers in China. Therefore, it is necessary to further evaluate the safety of HIFU treatment for uterine benign diseases.
Materials and methods
Patients
Twenty-seven thousand fifty-three patients with uterine benign diseases who were treated with USgHIFU in Suining Central Hospital of Sichuan, Third Xiangya Hospital of Central South University of China, Chongqing Haifu Hospital, Three Gorges Central Hospital of Chongqing, Fuling Central Hospital of Chongqing, Neijiang Sixth People’s Hospital of Sichuan, Neijiang First People’s Hospital of Sichuan, Zigong Fourth People’s Hospital of Sichuan, First People’s Hospital of Liangshan of Sichuan, Second People’s Hospital of Yibin of Sichuan, Huanggang Central Hospital of Hubei, Xijing Hospital of Fourth Military University, Anhui Cancer Hospital, Zhongshan People’s Hospital of Guangdong, Shanghai First Maternity and Infant Health Hospital, Affiliated Hospital of Zunyi Medical College of Guizhou, Northwest Women and Children Hospital of Shanxi, Fourth Division Hospital of Xinjiang Production and Construction Corps of Xinjiang, and Fifth Hospital of Xinjiang Medical University between January 2011 and December 2017 were retrospectively reviewed.
The diagnoses of uterine fibroids, adenomyosis, cesarean scar pregnancy, and placenta accreta were made through clinical evaluation, ultrasound and MRI. The inclusion criteria were as follows: (1) the diagnosis was confirmed; (2) patients agreed to have pre-HIFU and post-HIFU MRI evaluation; (3) patients could communicate with physicians during the procedure of HIFU treatment. Exclusion criteria: (1) the lesion could not be visualized; (2) patients undergoing pregnancy or lactation; (3) patients with suspected or confirmed uterine malignant diseases. Among these patients, 17,402 had uterine fibroids, 8434 had adenomyosis, 876 had cesarean scar pregnancy, and 341 had placenta accreta. In the patients with uterine fibroids, 12,342 had a solitary fibroid and 5060 had multiple fibroids. In patients with adenomyosis, 7020 had focal adenomyosis and 1414 cases had diffuse adenomyosis ().
Table 1. Baseline characteristics of patients with uterine benign diseases treated with HIFU.
Pre-HIFU preparation
The pre-HIFU preparation protocols used for patients with uterine fibroids, adenomyosis, cesarean scar pregnancy, and placenta accreta are similar. The protocol has been described previously [Citation4,Citation5]. Briefly, all patients were required to have bowel preparation before HIFU treatment as follows: the patients were suggested to ingest a semi-liquid diet on day 1 and liquid food on day 2, and had to fast 12 h. In addition, an enema was also performed before the procedure of HIFU. In the morning of the treatment day, the skin from the umbilicus level to the upper margin of the pubic symphysis must be carefully shaved, degreased and degassed before HIFU. A urinary catheter was inserted to control the bladder volume through injection of normal saline.
USgHIFU treatment
USgHIFU treatment for all these patients with uterine benign diseases was performed under intravenous conscious sedation. JC or JC200 models of focused ultrasound tumor therapeutic system (Chongqing Haifu Medical Technology, Co., Ltd., Chongqing, China) were used in the treatment of uterine fibroids, adenomyosis, cesarean scar pregnancy, and placenta accreta. The transducer of JC or JC model has a diameter of 20 cm, a focal length of 15 cm and a frequency of 1 MHz. Real-time monitoring was performed using My-Lab70 type B ultrasonic equipment (product of the Esaote Group, Italy). The patients were placed in a prone position on the HIFU table, with the anterior abdominal wall in contact with degassed water. A degassed water balloon was placed between the abdominal wall and the transducer to help compress and push the bowel away from the acoustic pathway. The sagittal view of the ultrasound scanning mode was selected, and the treatment plan was made by dividing the lesions of uterine fibroids, adenomyosis, or placenta accreta into different slices with a thickness of 5 mm each; while the lesions of cesarean scar pregnancy was divided into slices with a thickness of 3 mm each. During the whole course of HIFU treatment, real-time ultrasonography was used to determine the location of the target area and to monitor the response to HIFU. The ablation procedure began from the innermost slice. During the procedure, the ultrasound images with the target and their grey-scale values were compared each other to identify any coagulation necrosis by observing the lesions. HIFU ablation was terminated when any sign of blood flow disappeared or a grey-scale change in the target tissue was observed on the color Doppler ultrasound. The adverse effects were recorded as they occurred during and after the procedure until one month after HIFU treatment.
Adverse effects classification
The severity of adverse effects were evaluated according to the SIR classification system for complications by outcome: (1) Class A: no therapy, no consequence; (2) Class B: nominal therapy, no consequence; (3) Class C: require therapy, minor hospitalization (<48 h); (4) Class D: required major therapy, unplanned increase in level of care, prolonged hospitalization (>48 h); (5) Class E: permanent adverse sequelae; (6) Class F: death. In this system, Class A and B were considered to be minor complications; Class C, Class D, Class E and Class F were considered to be major complications [Citation9].
Statistical analysis
SPSS 21.0 statistical analysis software was used for descriptive analysis. Normally distributed data were depicted as mean ± standard deviation; skewed distributed data were depicted as median and interquartile range (IQR). The χ2 test was used for statistical comparisons of the incidence of adverse events. A p values below .05 was considered to indicate a significant difference.
Results
Baseline characteristics of patients
The median age of the patients was 42 (IQR: 37–45) years, the median height was 158 (IQR: 155–160) cm, the median weight was 56 (51–61) kg, and the median BMI was 22.5 (IQR: 20.7–24.6) kg/cm2 ().
Peri-HIFU evaluation
As shown in , the median sonication power used for treating uterine fibroids, adenomyosis, cesarean scar pregnancy and placenta accreta was 399 or 400 W. The median treatment time for uterine fibroids, adenomyosis, caesarean scar pregnancy and placenta accreta was 72, 70, 53, and 72 min, respectively. The median sonication time for uterine fibroids, adenomyosis, cesarean scar pregnancy and placenta accreta was 607, 580, 400, and 701 s, respectively. The total energy used for uterine fibroids, adenomyosis, cesarean scar pregnancy and placenta accreta was 240, 222, 160, and 280 kJ, respectively. A median NPV ratio of 85% was achieved in uterine fibroids; a ratio of 72% was observed in the lesions of patients with adenomyosis. All patients with cesarean scar pregnancy or placenta accreta had successful suction curettage under hysteroscopic or ultrasound guidance after HIFU.
Table 2. Treatment results of HIFU for patients with uterine benign diseases.
Adverse effects
Following the SIR classification, a total of 12,851 (47.5030% of) adverse events were classified as Class A, 215 (0.7945% of) events were classified as Class B, 90 (0.3327% of) events were classified as Class C, and 14 (0.0518% of) events were classified as Class D. No any adverse effect of Class E or F occurred in this study. Among the Class A adverse effects, 8263 (30.5437% of) events were lower abdominal pain, 2833 (10.4720% of) events were mild sacrococcygeal pain, 1270 (4.6945% of) events were abnormal vaginal discharge, 188 (0.6949% of) events were lower limb parenthesis, 108 (0.3992% of) events were nausea and vomiting, 86 (0.3179% of) events were erythema on skin, 79 patients had fever with temperature lower than 38.0 °C, and 24 patients reported temporary hematuria. All these adverse effects subsided within three days after HIFU without any treatment. Among the Class B adverse effects, 113 (0.4177% of) patients reported lower abdominal pain, 33 (0.1220% of) patients had sacrococcygeal pain, 27 (0.0998% of) patients had vaginal bleeding or abnormal discharge, 20 (0.0739% of) patients had skin blisters, and 3 (0.0111% of) patients had fever with temperature higher than 38 °C. Among the Class C adverse effects, 38 (0.1405%) of them were skin burns, 17 (0.0628% of) patients reported leg pain, 13 (0.0481% of) patients had vaginal bleeding, 8 (0.0296%) patients had urinary retention, 7 (0.0259% of) patients had hyperpyrexia, 4 (0.0148% of) patients had acute cystitis, and 3 (0.0111% of) patients had intrauterine infection. Among the Class D adverse effects, 4 (0.0148% of) events were bowel injury, 4 (0.0148%) were acute renal failure, 2 (0.0074% of) patients had deep vein thrombosis, pubic symphysis injury occurred in one patient, one patient had post-treatment thrombocytopenia, one patient had sciatic nerve injury, and one patient had hydronephrosis ().
Table 3. Society of interventional radiology classification of adverse effects after HIFU treatment.
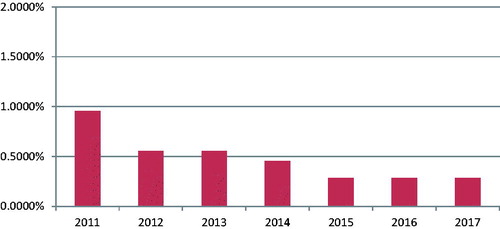
We further compared the annual incidence of major complications from 2011 to 2017. In 2011, the incidence of major complications was 0.9565%; the annual rates of major complications decreased since then. It was 0.5559% in 2012, 0.5539% in 2013, and 0.4561% in 2014. From 2015 to 2017, the annual incidence of major adverse effects further decreased; they were 0.2841%, 0.2835%, and 0.2852%, respectively (). In this period of seven years, the total number of major complications (Class C&D) was 104 and the overall incidence was 0.3844%.
Figure 1. Annual rate of major adverse effects of patients with benign uterine diseases treated with HIFU.
In this study, we found that the HIFU related major complications (Class C&D) were only present in patients with uterine fibroids, adenomyosis or placenta accreta. showed the incidence rates of major complications in patients with uterine fibroids, adenomyosis and placenta accreta. In patients with uterine fibroids, the major complications that occurred after HIFU include skin burns in 26 patients (0.1494%), leg pain in 10 patients (0.0575%), urinary retention in seven patients (0.0402%), vaginal bleeding in six patients (0.0345%), hyperpyrexia in five patients (0.0287%), renal failure in four patients (0.0230%), acute cystitis in three patients (0.0172%), bowel injury in three patients (0.0172%), intrauterine infection in two patients (0.0115%), deep vein thrombosis in two patients (0.0115%), hydronephrosis in one patient (0.0057%) and one patient had thrombocytopenia. In patients with adenomyosis, we also found skin burns (0.1304%), leg pain (0.0830%), urinary retention (0.0119%), vaginal bleeding (0.0830%), hyperpyrexia (0.0119%), acute cystitis (0.0119%), bowel injury (0.0119%), intrauterine infection (0.0119%), sciatic nerve injury (0.0119%); but no other major complications occurred. In 341 patients with placenta accreta, only one (0.2933%) patient had skin burns, and one (0.2933%) had high fever after HIFU treatment. We also compared the incidence rates of the major complications among the patients with uterine fibroids, adenomyosis and placenta accreta; no significant difference was found (p > .05).
Table 4. Comparison in incidence of major complications after HIFU treatment among patients with uterine fibroids, adenomyosis and placenta accreta.
Discussion
The present study showed that USgHIFU is an effective treatment for benign uterine diseases. In this study, a median NPV ratio of 85% was achieved in uterine fibroids, 72% in adenomyosis. The NPV ratios are similar to the previous studies [Citation4,Citation10–13]. Patients with cesarean scar pregnancy or placenta accreta had suction curettage under hysteroscopic or ultrasound guidance after HIFU; the procedure was successful. Although we found a total of 13,170 adverse effects in 27,053 patients, 12,851 out of 13,170 (97.58%) were classified as Class A, which did not need any treatment; 215 of 13,170 (1.63%) were Class B, and just needed nominal treatment. The major adverse effects (Class C&D) were only seen in 104 patients, which account for 0.79% (104/13,170). The rate of major adverse effects was lower than that of other treatment modalities [Citation14–17]. showed that the common minor adverse effects observed in this study were mild lower abdominal pain, sacrococcyygeal pain, and abnormal vaginal discharge. These minor adverse effects may be explained by the inflammation caused by the thermal effect of HIFU; the symptoms subsided in three days. Other minor adverse effects include lower limb paresthesia, nausea and vomiting, skin blisters, fever, hematuria were not frequent seen. These less frequently seen minor adverse effects occurred either because of the medication used for conscious sedation or inflammation; some of them caught a cold or urethral catheterization injury. In this study, the overall incidence rate of major adverse effects (Class C&D) was 0.3842%; it was similar to previous studies [Citation3,Citation8]. Among the major adverse effects, skin burn was the most frequent seen complication. The rate was 0.1405% (38/27053). Xiong et al. [Citation18] reported that patients with prior surgical scars have a higher risk of skin burns with increased severity in comparison with patients without prior surgical abdominal scars because the scar tissue had less blood supply, and had skin sensory loss. In this study, we found all 38 patients who had deep second degree or third degree skin burns had prior surgical abdominal scars. The burned tissue was removed through surgical resection. Leg pain longer than two weeks was reported from 17 (0.0628%) patients after HIFU treatment. The pain may be explained by the sacral nerve irritation because the patients had the symptom relieved from one to three months after taking NSAIDs. Another patient had the leg pain lasted for 12 months, we considered that patient had sciatic nerve injury. We observed 13 (0.0481%) patients with had abnormal vaginal discharge and bleeding. This complication was seen in patients with submocosal fibroids and adenomyosis whose endometrium was damaged during treatment. Although the volume was small, it lasted for longer than two weeks. These patients underwent hysteroscopy to clear the necrotic tissue and the abnormal discharge and bleeding subsided. Urinary retention occurred in 8 patients. We further reviewed these cases and found that all of them had a retroverted uterus. During HIFU treatment, the bladder was excessively filled with normal saline. These patients recovered after indwelling urinary catheters for three days. We also had seven (0.0259%) patients with a high fever, four patients with acute cystitis, and three with intrauterine infection. Antibiotics were prescribed for these patients and temperature returned to normal after three days. In this study, we encountered bowel injury in four (0.0148%) patients. This complication is rare but severe. Among them, three patients had uterine fibroids and one had adenomyosis. We reviewed these cases and found that the bowel was not completely pushed away from the acoustic pathway and the lesions were over treated. The bowel perforation occurred between 4 and 12 days after HIFU and all patents underwent a surgery. We also observed two patients had deep vein thrombosis after HIFU. Two patients were give treatment with medications and returned to normal. One of the patients reported an increased fibrin degradation product (FDP) value (5.61 μg/ml) before HIFU; therefore, it is important to evaluate the blood coagulation function of patients before HIFU treatment. In this study, one patient complained of pain on pubic symphysis after HIFU treatment for a cervical uterine fibroid. The MRI showed signal changes of pubis symphysis. Another patient with a large cervical uterine fibroid had hydronephrosis in the left kidney after HIFU because the left ureter was compressed by the treated fibroid. The size of fibroid was 10 cm in diameter. The size was even larger after HIFU because of edema. Therefore, cervical uterine fibroids are not recommended for HIFU treatment. Over the last seven years, two centers reported four patients with uterine fibroids with acute renal function failure after HIFU. Three of them had the renal function returned to the normal level after treatment with medications. One patient was treated with medication and had dialysis, but the blood creatine level remained around 200 μmol/L, higher than the normal level. Causes of acute renal failure in these four patients remain unclear. Of these four patients, three of them had high blood pressure and two of them had anemia. During HIFU, SonoVue, a contrast ultrasound agent, was used to perform contrast-enhanced ultrasound to evaluate the treatment efficacy. After HIFU treatment, antibiotics and NSAID were administered to the patients. These factors may all contribute to acute renal function failure. Therefore, if the patients have hypertension or anemia, we do not recommend performing contrast-enhanced ultrasound during HIFU or give NSAIDs after HIFU. Last year, one patient presented exhaustion after HIFU; the lab test showed platelet count decreasing. Later, chest X-ray showed pleural effusion in right chest. We are not clear if this complication is related to HIFU. Our results also demonstrated that HIFU can be safely used to treat different benign uterine diseases because no significant difference was observed in the rates of major adverse effects between patients with uterine fibroids and adenomyosis (). Also, with the development of this technique and with more experience on the part of the physicians, the major adverse effects will be further decreased. shows the downward trend.
Conclusions
Based on our large sample size of patients from multiple centers, we concluded that HIFU is safe and effective in treating benign uterine diseases. The major adverse effects after HIFU include skin burn, leg pain, vaginal discharge or bleeding, urinary retention, acute cystitis, intrauterine infection, bowel injury, acute renal failure, deep vein thrombosis, pubic symphysis injury, post-HIFU thrombocytopenia, sciatic nerve injury, and hydronephrosis. The rates of major adverse effects were low. With the development of this technique and with more experience on the part of the physicians, the major adverse effects will be further lowered.
Acknowledgments
We are grateful to Wesley Zhang for helping us edit and revise this paper.
Disclosure statement
No potential conflict of interest was reported by the authors. Lian Zhang is a senior consultant to Chongqing Haifu.
Additional information
Funding
References
- Wang W, Liu WY, Zhou JM, et al. High intensity focused ultrasound treatment for symptomatic uterine fibroids: preliminary results. Zhonghua Chao Sheng Ying Xiang Xue Za Zhi. 2002;11:161–163.
- Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–287.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31:280–284.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem. 2015;27:677–681.
- Zhu X, Deng X, Wan Y, et al. High intensity focused ultrasound combined with suction curettage for the treatment of cesareanscar pregnancy. Medicine (Baltimore). 2015;94:e854.
- Ye M, Yin Z, Xue M, et al. High-intensity focused ultrasound combined with hysteroscopic resection for the treatment of placenta accreta. BJOG. 2017;124 Suppl 3:71–77.
- Quinn SD, Vedelago J, Gedroyc W, et al. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstetr Gynecol Reprod Biol. 2014;182:247–251.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Intervent Radiol. 2009;20:S377–SS90.
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73:396–403.
- Gong C, Setzen R, Liu Z, et al. High intensity focused ultrasound treatment of adenomyosis: the relationship between the features of magnetic resonance imaging on T2 weighted images and the therapeutic efficacy. Eur J Radiol. 2017;89:117–122.
- Liu Z, Gong C, Liu Y, et al. Establishment of a scoring system for predicting the difficulty level of high-intensity focussed ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2018;34:77–86.
- Zhang X, Li K, Xie B, et al. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124:207–211.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485–493.
- Salehi M, Jalilian N, Salehi A, et al. Clinical efficacy and complications of uterine artery embolization in symptomatic uterine fibroids. Glob J Health Sci. 2015;8:245–250.
- Popovic M, Puchner S, Berzaczy D, et al. Uterine artery embolization for the treatment of adenomyosis: a review. J Vasc Interv Radiol. 2011;22:901–909.
- Zhu X, Deng X, Xiao S, et al. A comparison of high-intensity focused ultrasound and uterine artery embolisation for the management of caesarean scar pregnancy. Int J Hyperthermia. 2016;32:144–150.
- Xiong Y, Yue Y, Shui L, et al. Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia. 2015;31:777–783.
