Abstract
Purpose: Acute kidney injury (AKI), especially oliguric AKI, is a recognized complication following microwave ablation (MWA) of large liver tumors. This study evaluated the clinical features, mechanisms, risk factors and prevention strategies for oliguric AKI after MWA of large liver tumors.
Methods: From March 2011 to May 2015, 441 patients with liver tumors ≧5 cm received MWA in our hospital. The clinical features, prevention strategies, further mechanisms and possible risk factors for oliguric AKI after MWA were analyzed.
Results: One hundred four (23.6%) patients had AKI after MWA; 11 (10.6%) patients had oliguric AKI, and 93 (89.4%) patients had nonoliguric AKI. All patients with nonoliguric AKI recovered without any special treatments. The eleven patients with oliguric AKI received appropriate treatments and had completely normal renal function three months later. Using double needles for ablation was a risk factor for nonoliguric AKI, while high preoperative levels of red blood cells (RBC), hemoglobin (HGB) and albumin (Alb) were risk factors for oliguric AKI. The decrease levels of hemoglobin were significantly high in oliguric AKI patients (p < .05). Patients with oliguric AKI had abnormally high postoperative transaminase and renal function indicators. Compared to postoperative prevention, intraoperative prevention significantly lowered the occurrence of oliguric AKI (0% vs. 3.7%, p = .018) and shortened the hospital stay.
Conclusions: Patients who underwent MWA for large liver tumors are prone to develop oliguric AKI. Implementation of intraoperative strategies during MWA can effectively prevent the occurrence of this severe complication.
Introduction
Local thermal therapy, represented by radiofrequency ablation (RFA) and microwave ablation (MWA), has become the preferred therapy for liver tumor patients who are unsuitable for surgery due to its safety and effectiveness [Citation1,Citation2]. A large number of retrospective and prospective studies have confirmed that radiofrequency ablation has similar effectiveness as radical surgery for safe areas of small hepatocellular carcinoma [Citation3–7]. According to the Barcelona Clinic Liver Cancer (BCLC) staging classification and treatment recommendations, ablation treatment is currently limited to single liver tumors with diameters less than 3 cm [Citation5,Citation8], while the ‘Expert consensus’ of Chinese scholars limits it to liver tumors with diameters less than 5 cm [Citation9]. In recent years, with the improvement of ablation devices and image-guided techniques, scholars have begun to explore the application of thermal ablation for large liver tumors with diameters greater than 5 cm and have achieved good safety and efficacy [Citation10–12].
Nevertheless, with more and more patients with large liver tumors having undergone thermal ablation, acute kidney injury (AKI), especially oliguric AKI, has gained increasing attention from surgeons as a recognized complication of ablation. Keltner et al. [Citation13] reported one case of renal failure after laparoscopic thermal ablation of liver tumors; Rodriguez et al. [Citation14] reported two cases of postoperative renal failure in 41 cases of thermal ablation of liver tumor; Ong et al. [Citation15] reported a 1.7% renal failure rate for ablation of liver tumor without previous renal failure. However, few systematic studies have explored the mechanisms, risk factors and prevention strategies for renal failure after ablation of the liver tumor.
This study explores possible mechanisms and risk factors for oliguric AKI caused by thermal ablation and attempts to establish a set of valuable prevention strategies for the safe and effective application of thermal ablation to large liver tumors.
Materials and methods
Patients
From July 2010 to May 2015, 441 cases of patients with large liver tumors (≧5 cm in diameter) received MWA at our medical center. All patients were confirmed by at least two of following test results: 1. two or more imaging techniques; 2. serum tumor markers (AFP); and 3. biopsy. The basic characteristics of all the patients are displayed in .
Table 1. Baseline characteristics of patients.
Patients met the following inclusion criteria: 1. age between 20 and 90 years old; 2. at least one lesion with a diameter of 5–10 cm; 3. for multiple tumors, the cumulative volume of one session of ablation was less than 1/4 of the total volume of the liver; 4. liver function was Child-Pugh A/B; 5. no significant liver or kidney failure (the results of serology examination were within normal limits) or other serious concomitant diseases; 6. refused surgical resection; and 7. had not received MWA treatment previously. This study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by our hospital ethics committee, and all patients had signed the preoperative informed consent for treatment.
Preoperative preparation, surgical instruments and ablation procedure
Preoperative preparation
MWA strategies for liver tumors, including patient selection, treatment preferences (simple percutaneous, percutaneous plus laparoscopy, percutaneous plus laparotomy, percutaneous plus hydrodissection), number of electrodes used and method of anesthesia, were cooperatively developed by three doctors with 10 years, 5 years and 4 years of percutaneous ablation experience. General anesthesia was used for all patients, and either the MyLab Twice or the HM1498XS1 with a 3.5-MHz probe was chosen as the ultrasound scanner. MWA was performed using a 2450-MHz MTC-3C microwave generator and a 25-cm-long, 15-G cooled-shaft electrode probe (Vision Medical, Nanjing, China) with a 1.5-cm-long expandable tip.
Ablation procedure
Simple ultrasound-guided percutaneous microwave ablation
Under ultrasound guidance, one or two electrodes were percutaneously placed at the distal edge of the tumor. For tumors with a smaller diameter (generally less than 6 cm) and clear boundary, multi-needle-track, multiplane and multipoint ablation could be adopted using a single electrode. For tumors with a larger diameter (greater than 6 cm) and unclear boundary, double electrodes were used for parallel needle puncture. The distance between the two electrodes was less than 3 cm, and they were used for multi-needle-track, multiplane and multipoint ablation. The power was set as 80–100 W; the distal margin of the lesion was ablated first, and the electrodes were then withdrawn by 1–1.5 cm for multipoint ablations until the tumors were completely covered by the gasification zone. The ablation was completed 0.5 cm adjacent to the liver to ensure a tumor-free margin. After this ablation procedure, another one or two needle tracts were ablated in the same or adjacent inter-costal space, if necessary. Generally, for a giant hepatic hemangioma, the number of needle-tracts in a plane, the ablation sites in a single needle-tract and the number of ablation planes were determined according to the tumor transverse diameter (X-axis), longitudinal axis (Y-axis) and vertical axis (Z-axis), respectively.
Ultrasound-guided percutaneous-assisted microwave ablation plus other techniques
For tumors that are adjacent or directly attached to the gastrointestinal tract, gall bladder, diaphragm or other surrounding organs, ultrasound-guided percutaneous-assisted MWA with laparoscopic treatment was used to prevent thermal damage to surrounding organs. Ultrasound-guided percutaneous ablation was used for tumor portions far from the critical organs (usually ablated until approximately 1 cm from the nearby organs); pneumoperitoneum was then created to separate hollow organs or diaphragms that were adjacent or attached to the tumors; an electrode was inserted into the tumor under laparoscopic ultrasound guidance or direct vision for ablation.
For tumors located in high-risk ablation sites but not suitable for laparoscopic ablation (patient had abdominal surgery previously and severe abdominal adhesions), laparotomy combined with ultrasound-guided percutaneous ablation or ultrasound-guided hydrodissection was selected for assisting percutaneous ablation. The ablation steps were similar to those of percutaneous ablation.
Assessment of AKI and preventive measures
All patients underwent preoperative catheter insertion with 24-h urine volume monitoring. The blood test, liver and kidney function test and electrolyte test were done before and on the first day of ablation. Postoperative AKI was defined as follows: an absolute increase in serum creatinine was more than or equal to 26.4 µmol/L (0.3 mg/dL), or a percentage increase in serum creatinine was more than or equal to 50% within 48 h [Citation16]. 1) Postoperative oliguric AKI was defined as: less than 400 ml of 24-h urine output after ablation; 2) Nonoliguric AKI was defined as more than or equal to 400 ml of 24-h urine output after ablation.
The two measures for preventing oliguric AKI were as follows: 1) From 2010 July to September 2013, 274 (62.1%) patients underwent preoperative catheterization, where an intravenous injection of 20 mg of furosemide and an intravenous infusion of 125 ml of sodium bicarbonate solution were administered after ablation. 2) After October 2013, a total of 167 (37.9%) patients underwent preoperative catheterization, where an intravenous injection of 20 mg of furosemide and an intravenous infusion of 125 ml of sodium bicarbonate solution were administered during ablation (when half of the tumor was ablated). After ablation, changes in indicators such as urine volume, liver and kidney function, and electrolytes were monitored closely.
Statistical analysis
Data were analyzed using the SPSS software, version 19 (SPSS In., Chicago, IL). Continuous and categorical variables were expressed as the means and percentages. For statistical analysis, categorical variables were compared using the Chi-square test, while continuous variables were compared using an ANOVA test for more than two groups and an independent t-test for two groups. Missing data were not considered in the analysis. A p value of <0.05 was considered statistically significant.
Results
Occurrence and treatment of AKI after MWA
One hundred four (23.6%) of a total of 441 patients had AKI after MWA, of whom 93 (89.4%) had nonoliguric AKI and 11 (10.6%) had oliguric AKI. Patients with nonoliguric AKI had no clinical symptoms, and their renal functions recovered within one week after ablation without any special treatments.
Among the patients with oliguric AKI, ten had significant gastrointestinal reactions and edema of the limbs, eyelid and face. All patients had received appropriate fluid replacement, diuresis, urine alkalinization and other normal treatments, and 9 of them entered the diuretic phase within 15 days after treatment and had decreased levels of urea nitrogen and creatinine in the blood. Three patients received hemodialysis treatment two to four times due to significant clinical symptoms and a high creatinine level, after which their clinical symptoms gradually disappeared and renal function slowly improved. Three months later, all patients had completely normal renal function without long-term sequelae.
Incidence and risk factors
After risk factor analysis for AKI using preventive measure A (double electrodes for ablation), higher levels of RBC, HGB and Alb were found to increase in the case of AKI (p = .043, p < .001, p = .002, p = .003 and p = .023) (). Furthermore, the results showed that the preventive measure and the levels of RBC, HGB and Alb mainly affected the incidence of oliguric AKI. Patients who received preventive measures B had a significantly lower oliguric AKI rate than did those who received preventive measures A (0% vs. 3.7%, p = .018) (). The levels of RBC, HGB and Alb in patients with oliguric AKI were significantly higher than those in patients with nonoliguric AKI (RBC: Oliguric vs. Nonoliguric, 4.82 ± 0.52 vs. 4.17 ± 0.59, p = .001, ; HGB: Oliguric vs. Nonoliguric, 147.09 ± 13.88 vs. 129.26 ± 16.81, p = .001, ; Alb: Oliguric vs. Nonoliguric, 44.78 ± 3.33 vs. 40.68 ± 5.94, p = .003, ), but the levels were not significantly different between patients with nonoliguric AKI and patients without AKI. For electrode number, the use of double electrodes significantly increased the incidence rate of nonoliguric AKI compared to the use of a single electrode (36.4% vs. 17.3%, p < .001, ). Additionally, ROC curves indicated that these factors had predictive values for the cases of nonoliguric and oliguric AKI (p < .05), especially the levels of RBC, HGB and Alb in the case of oliguric AKI with values of AUC larger than 0.7 (Figure S1).
Figure 1. The impact of risk factors among patients without AKI, with nonoliguric and oliguric AKI. (*p value was calculated using the Chi-square test for categorical variables and the independent t-test for continuous variables.) A. The impact of preventive measure; B. The impact of the number of electrodes; C. The impact of red blood cell (RBC) level; D. The impact of hemoglobin (HGB) level; E. The impact of albumin (Alb) level.

Table 2. The incidence and risk factors of AKI.
Clinical serology examination after ablation
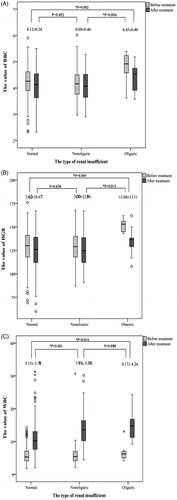
Patients with AKI (both oliguric and nonoliguric) had significantly a higher white blood cell (WBC) count (p < .001), alanine aminotransferase (ALT) (p < .001), aspartate aminotransferase (AST) (p = .001), total bilirubin (TB) (p = .014), serum creatinine (SCr) (p < .001) and blood urea nitrogen (BUN) (p < .001) on the first day after ablation ().
Table 3. The distribution of biochemical parameters after treatment, and their effects on the incidence of AKI.
For patients with oliguric AKI, the values of RBC and HGB declined significantly more than those in patients with either nonoliguric AKI or normal renal function (RBC: Nonoliguric vs. Oliguric, 0.09 ± 0.40 vs. 0.45 ± 0.40, p = .006; Normal renal function vs. Oliguric, 0.12 ± 0.34 vs. 0.45 ± 0.40, p = .002) (HGB: Nonoliguric vs. Oliguric, 3.00 ± 12.86 vs. 13.60 ± 13.11, p = .015; Normal renal function vs. Oliguric, 3.62 ± 10.67 vs. 13.60 ± 13.11, p = .004). In the analysis of white blood cells, the increased level was significantly higher in patients with AKI compared to that in patients with normal renal function (Normal renal function vs. Nonoliguric, 5.15 ± 3.78 vs. 7.91 ± 5.30, p < .001; Normal renal function vs. Oliguric, 5.15 ± 3.78 vs. 8.17 ± 4.24, p = .014). However, there was no significant difference between the levels in patients with nonoliguric and patients with oliguric AKI (p > .05) ().
Figure 2. The comparison of the values of RBC, HGB and WBC before and after treatment (*p value was calculated using the independent t-test. The value marked in the picture represents the change in levels before and after the treatment. A. The value of red blood cells (RBC); B. The value of hemoglobin (HGB); C. The value of white blood cells (WBC).
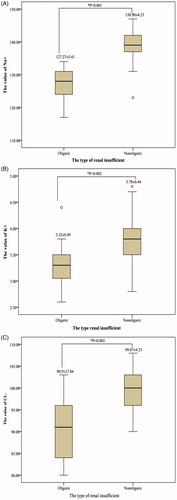
The electrolyte index (Na+, K+ and Cl−) after ablation significantly decreased among patients with oliguric AKI compared to patients with nonoliguric AKI (Na+: Oliguric vs. Nonoliguric, 127.27 ± 5.41 vs. 138.90 ± 4.23, p < .001; K+: Oliguric vs. Nonoliguric, 3.32 ± 0.49 vs. 3.78 ± 0.44, p = .002; Cl−: Oliguric vs. Nonoliguric, 90.91 ± 7.84 vs. 99.87 ± 4.33, p = .003) ().
Clinical effect of preventive measures
Postoperative hospital stays after ablation
For patients with oliguric AKI, the postoperative hospital stays were significantly longer than for patients with either normal renal function or nonoliguric AKI (None vs. Nonoliguric vs. Oliguric: 4.72 ± 4.46 vs. 5.29 ± 3.38 vs. 15.00 ± 5.57 days, Nonoliguric vs. None: p = .260, Oliguric vs. None: p < .001, Nonoliguric vs. Oliguric: p < .001). Among the patients with acute kidney injury, the patients who received preventive measure B had shorter postoperative hospital stays than patients who received preventive measure A (Total patients: B vs. A, 4.40 ± 3.48 vs. 5.45 ± 4.99 days, p = .023; Patients with AKI: B vs. A, 3.97 ± 1.29 vs. 7.51 ± 5.34 days, p < .001) ().
Table 4. Information on the duration of postoperative hospital stay.
Effects of preventive measure on serology
In comparing preoperative and postoperative serological indicators, those patients who received preventive measure B had higher RBC levels, which were statistically significant (B vs. A: 4.14 ± 0.59 vs. 3.85 ± 0.65, p = .030). In comparing renal function indicators, SCr (96.63 ± 27.70 vs. 301.07 ± 377.61) and BUN (8.39 ± 2.65 vs. 17.79 ± 10.89), on the day when the creatinine level was maximum, patients who received preventive measure B had significantly lower SCr and BUN levels than did those who received preventive measure A (p < .05) ().
Table 5. The comparison of biochemical parameters in AKI patients treated with and without preventive measures.
Discussion
Oliguric AKI caused by thermal ablation is usually accompanied by severe clinical symptoms, and thus its prevention and treatments should be the focus of clinicians. Different from what has been previously reported about the onset of hyperkalemia after liver tumor ablation [Citation17,Citation18], in our study, there was no significant elevation of serum potassium (mean; range) in all eleven cases of oliguric AKI. Even the three patients who received hemodialysis due to the severity of the clinical and laboratory framework did not have hyperkalemia. The levels of Na+, K+ and Cl− were significantly lower in patients with oliguric AKI compared to patients with nonoliguric AKI. The mechanism of this feature is still unclear. Due to the aforementioned treatment strategies of AKI after thermal ablation, this complication could be easily handled and were unlikely to cause more serious damages to the body.
Previously, some scholars inferred that thermal ablation could cause oliguric AKI, myoglobinuria and tumor lysis syndrome [Citation14,Citation19]. They believed that rapid thermal inactivation of malignant tumors might generate a large number of related factors and even cause extensive damage to myoglobin, thereby causing injuries to renal tubules or renal tubular cells or leading to the formation of urinary casts, resulting in oliguric AKI. However, from the data of this study, four of the 11 oliguric AKI cases were caused by thermal ablation of benign tumors (large hepatic hemangiomas and focal nodular hyperplasia). Such cases should not be associated with tumor lysis syndrome, and it had no connections with myoglobinuria because thermal ablation of abdominal viscera rarely causes muscle tissue necrosis. Therefore, there was no pathophysiological basis that links post-ablation AKI to tumor lysis syndrome and myoglobinuria. However, the levels of related indicators were not measured in this study, and more evidence is needed to confirm this conclusion.
Until now, there has been no report of oliguric AKI in patients with small liver tumors who received thermal ablations, and thus it may be a specific complication of ablation for large tumors. Long preoperative fasting, water deprivation and loss of body fluids caused by long and intensive intraoperative thermal ablation can lead to a variety of pathophysiological changes affecting glomerular filtration rate, such as hypovolemia, increased blood viscosity, increased destruction of blood cell components in unit volume, acid–base imbalance, damage of renal microvascular endothelial cells and formation of urinary casts, which eventually lead to oliguric AKI. Curley et al. [Citation20] believed that the temperature of tissues surrounding the electrode could reach 50–100 °C during thermal ablation of liver tumors, and hemolysis could occur when temperatures were over ≥50 °C [Citation21]. When hemolysis occurred, a large number of red blood cells (RBC) were damaged, and the RBC fragmentation and free hemoglobin could block microscopic tubules and even cause AKI. Similar to the results reported by Keltner et al. [Citation13], our study found that after ablation, the mean levels of red blood cell and hemoglobin decreased significantly in patients with oliguric AKI compared to patients with normal renal function or nonoliguric AKI. Therefore, hemolysis caused by prolonged intensive thermal ablation might be one of the main causes of oliguric AKI; however, this still needs further verification.
In addition, double electrode ablation had been proved safe and effective in previous studies [Citation22], but this study suggested that patients who received double electrode ablation had higher risks of nonoliguric AKI than patients who received a single electrode. It is inferred that the intensity of ablation per unit time might be an important risk factor for nonoliguric AKI. For ablation of an equal volume of tumor, ablation with a single electrode required more time than ablation with double electrodes, and cell components continued to be diluted in blood circulation with continuous replenishing of the fluid volume during ablation. Therefore, single electrode ablation had significantly lower damage to cellular components per unit time than short time ablation with double electrodes. Therefore, single-electrode, long-duration and multipoint ablation might be safer than double electrode ablation, at least for the case of nonoliguric AKI. Additionally, we found that high preoperative levels of RBC, HGB and Alb were risk factors for oliguric AKI. This might be because more cell components in blood circulation were damaged per unit time during the ablation process in patients with high preoperative levels of RBC, HGB and Alb. Obstructions are more likely to form in the renal tubules of patients with more damaged cells, and oliguric AKI is more likely to occur.
In fact, we had conducted cognitive processes for preventing oliguric AKI after thermal ablation. Initially, (prior to September 2013), we applied preoperative catheterization, an intravenous injection of 20 mg of furosemide and an intravenous infusion of 125 ml of sodium bicarbonate solution at 1–1.5 h after anesthesia recovery from ablation (preventive measure A). We even applied a two-stage ablation strategy for patients with tumors larger than 7 cm, but oliguric AKI still occurred intermittently. For these reasons, we inferred that the timing for applying preventive measures might be too late. When patients recovered approximately 1 to 1.5 h after ablation, the tubular shape might have been formed, so the subsequent preventive measures could not be effective. After October 2013, the aforementioned preventive measures were applied during the ablation process (when half of the tumor was ablated), and rapid intravenous fluid was applied during ablation (preventive measure B). After that, there was not even one case of oliguric AKI. For those who had nonoliguric AKI, postoperative levels of SCr were clearly reduced with faster recovery times (). The efficacy of preventive measure B might be caused by the following reasons: (1) Preoperative or intraoperative rapid rehydration could dilute the circulating blood and reduce both the damage to cell components caused by thermal ablation and the tubular formation of the renal tubules; (2) The application of the diuretics during ablation could cause the fluid in circulating blood to pass continuously through the intrarenal vessels into the renal tubules to form excreted urine and to prevent the formation of the renal tubules; (3) The small dose of sodium bicarbonate was used to alkalinize the urine during ablation, which could also prevent the tubular formation of the renal tubules; and (4) Rapid rehydration and uninterrupted delivery of fluid to the renal tubule might reduce the degree of heat damage to the renal tubular epithelial cells.
In summary, a relatively small number of cases was used in this study without a deeper understanding of the mechanisms, but oliguric AKI was manageable if preoperative and intraoperative strategies were applied, including intravenous fluid administration, proper timing of diuresis and urine alkalinization and reasonable control of the volume of ablated tumor in each session. Even oliguric AKI could occur occasionally and could be easily handled without any long-term sequelae; thus, it should not be an obstacle for MWA of large liver tumors.
Conclusions
Patients who undergo MWA of large liver tumors are prone to develop AKI. The use of double needles for ablation was a risk factor for patients with nonoliguric AKI. In addition, high preoperative levels of RBC, HGB and Alb likely increased the incidence of oliguric AKI. AKI after ablation was related to damage of hemoglobin in red blood cells. Patients with AKI had abnormally high postoperative transaminase and renal function indicators. Implementation of intraoperative strategies during MWA can effectively prevent the occurrence of oliguric AKI, reduced renal damage caused by ablation and shortened hospital stay.
Supplemental Material
Download JPEG Image (763.1 KB)Acknowledgements
Ding Min designed the pipeline of the analysis, performed the statistical analysis and drafted the manuscript. Ma Sicong and Tang Xiaoyin collected the data of the patients, designed the pipeline of the analysis and drafted the manuscript. Wang Tao, Qi Xingxing, Zhang Yuan collected the data. Chi Jiachang, Wang Zhi, Cui Dan, Shi Yaoping and Li Ping revised the manuscript. Zhai Bo conceived and coordinated the overall study and revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692.
- Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97.
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802.
- Peng Z-W, Lin X-J, Zhang Y-J, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–1033.
- Vivarelli M, Guglielmi A, Ruzzenente A, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107.
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2007;47:82–89.
- Fan ST, Poon RT, Yeung C, et al. Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg. 2011;98:1292–1300.
- CM. Expert consensus on the norms of local ablation therapy for hepatocellular carcinoma. J Clin Hepatol. 2011;19(4):257–259.
- Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914–1923.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768.
- Liu Y, Zheng Y, Li S, et al. Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol. 2013;68:21–26.
- Keltner JR, Donegan E, Hynson JM, et al. Acute renal failure after radiofrequency liver ablation of metastatic carcinoid tumor. Anesth Analg. 2001;93:587–589.
- Rodriguez J, Tellioglu G, Siperstein A, et al. Myoglobinuria after laparoscopic radiofrequency ablation of liver tumors. J Gastrointest Surg. 2010;14:664–667.
- Ong SL, Gravante G, Metcalfe MS, et al. Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:599–605.
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- Metzner J, Evans JL, Domino KB. Life-threatening hyperkalemia during radiofrequency ablation of hepatocellular carcinoma. J Clin Anesth. 2010;22:473–476.
- Verhoeven BH, Haagsma EB, Appeltans BM, et al. Hyperkalaemia after radiofrequency ablation of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2002;14:1023–1024.
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:S1–S13.
- Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8.
- Mollison PL. Blood transfusions in clinical medicine. Anesth Analg. 1954;34:115–116.
- Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. Am J Roentgenol. 1998;170:1015–1022.