Abstract
Purpose: To compare the applicability of fusion imaging between contrast-enhanced ultrasound (CEUS) and computed tomography (CT) or magnetic resonance imaging (MRI) (CT/MRI-CEUS fusion imaging) and fusion imaging between CEUS and ultrasound (US-CEUS fusion imaging) in the assessment of treatment response during liver cancer ablation.
Methods: From August to December 2015, patients who underwent US-guided thermal ablation of liver tumors at our hospital with available CT/MRI images were enrolled consecutively. Both CT/MRI-CEUS and US-CEUS fusion imaging were performed in all patients to evaluate treatment responses. The applicable rate, success rate of registration and duration time were recorded. Complications were monitored in the follow-up period, and CECT/MRI within three months were taken as the standard reference of technical efficacy.
Results: A total of 157 liver tumors (19 ± 8 mm, range 8–55 mm) in 115 patients (54 ± 11 years old, range 2 7∼ 84 years old) were enrolled. The applicable rate of US-CEUS fusion imaging was 61.1% (96/157) because of inconspicuous lesions in US, lower than that of CT/MRI-CEUS fusion imaging (99.7% (155/157)) (p < .05). However, the success rate of registration in US-CEUS fusion imaging (93.8% (90/96)) was superior to that of CT/MRI-US fusion imaging (81.3% (126/155)) (p < .05), especially for cases combined with alternative preablation surgeries or procedures (p < .05). The technical efficacy rate was 99.3% (150/151) according to the CECT/CEMRI.
Conclusions: Both CT/MRI-CEUS and US-CEUS fusion imaging are feasible means for intraprocedural immediate evaluation of treatment response for liver thermal ablation. US-CEUS fusion imaging is preferred because of its convenience and higher success rate of registration.
1. Introduction
Currently, ultrasound (US) is the most popular imaging modality for the guidance, monitoring and immediate assessment of the treatment response for liver thermal ablation because of its convenience, portability, low cost and real-time performance [Citation1]. However, compared with other imaging modalities, such as computed tomography (CT) or magnetic resonance imaging (MRI), US cannot provide an accurate evaluation of the treatment response for thermal ablation because it is unable to reflect the blood perfusion of the ablation zone [Citation2]. Therefore, a secondary procedure may be needed if the postprocedural CT or MRI indicates that the target tumor has not been completely ablated, which may increase the patient’s trauma and lengthen the hospital stay. The introduction of contrast-enhanced ultrasound (CEUS) has partially solved these problems. CEUS can reflect the real-time micro-vascular blood perfusion of the ablation zone and has been listed as a useful means of immediate treatment response assessment [Citation3–6]. However, the target tumor typically becomes inconspicuous after ablation [Citation7], and it is difficult to estimate whether the target tumor is completely covered by the ablation zone or whether a sufficient ablative margin has been achieved [Citation8]. Additionally, for tumors with poor blood supply, it is hard for CEUS to detect residual tumor. All of these factors can result in incomplete ablation as well as an increase in the incidence of local tumor progression (LTP) [Citation9].
The development of fusion imaging techniques has shown promise for overcoming these limitations. As a novel technique, fusion imaging with US refers to the overlapping and alignment of real-time US images and preprocedural multi-planar reconstructed images (CT/MRI or US) using an electromagnetic positioning system and three-dimensional (3D) reconstruction data [Citation10–13]. Several studies have reported on the application of fusion imaging between CT/MRI and US (CT/MRI-US fusion imaging) for the detection, guidance and evaluation during liver cancer thermal ablation [Citation13–20]. Our previous studies [Citation16,Citation21,Citation22] have also demonstrated that CT/MRI-CEUS fusion imaging is a feasible and accurate method for immediately assessing the treatment response of thermal ablation. The target tumor and 5-mm ablative margin can be depicted in preprocedural CT/MRI images; by overlapping these images with real-time CEUS, operators can intuitively determine whether the target tumor and its ablative margin can be encompassed by the avascular zone. However, this technique requires the acquisition of CT/MRI images in the Digital Imaging and Communications in Medicine (DICOM) format, and the registration process requires an operator with experience in both CT/MRI and US images, limiting the widespread clinical application of this technique.
Recently, another type of fusion imaging using only US images has been introduced [Citation23–27]. Three-dimensional US images (3DUS) are used to substitute the CT or MRI images as reference images, and these images are fused with real-time US through an electromagnetic positioning system. A few reports [Citation23–25,Citation27] have validated the value of US–US fusion imaging in the assessment of treatment responses in liver tumor ablation. In a previous study [Citation27], we certified that US–US fusion imaging combined with CEUS is feasible in the intraoperative immediate evaluation of treatment responses for liver thermal ablation and the guidance of supplementary ablation.
However, to our knowledge, comparative studies of these two types of fusion imaging techniques in the assessment of treatment responses are lacking. How to select the appropriate fusion imaging technique in clinical practice remains inconclusive. The purpose of the present study is to compare the applicability of CT/MRI-CEUS and US-CEUS fusion imaging in the treatment response assessment during ablation procedure and to provide a reference for selecting proper fusion imaging techniques.
2. Materials and methods
2.1. Study population and lesions
This prospective research was approved by the institutional ethics review board, and the present study was in compliance with the Declaration of Helsinki. Informed consent was obtained from every participant.
From August to December 2015, patients who underwent US-guided thermal ablation in our hospital and met the inclusion criteria were included consecutively. The following inclusion criteria were considered in the present study: (1) diagnosed as a liver malignant tumor; (2) indication of liver tumor thermal ablation; and (3) available CT/MRI images in DICOM format.
The diagnosis of hepatocellular carcinoma was based on typical imaging or pathological results [Citation1], while the diagnosis of other liver malignancies was based on pathological results.
The following indications for thermal ablation [Citation28] were considered: (1) Child–Pugh A or B liver function status; (2) the absence of intractable ascites and uncorrectable coagulopathy; (3) prothrombin activity above 40% and a platelet count of more than 500,000/L; and (4) patients unsuitable for or refusing hepatectomy.
The following exclusion criteria were considered: (1) patients with a history of pacemaker implantation; and (2) equipment failure during the procedure.
2.2. Equipment
2.2.1. Equipment for fusion imaging
MyLab 90 and MyLab Twice ultrasound machine (Esoate, Genoa, Italy) with Virtual Navigator (VN) and three-dimensional software were employed. A magnetic field generator was positioned next to the right shoulder of the patient to emit a magnetic field. A magnetic sensor was attached to the convex probe CA541 (frequency range from 1 to 8 MHz) or CA431 (frequency range from 1 to 8 MHz) for US guidance and assessment of the treatment response. The magnetic signal detected by the sensor was analyzed to obtain the precise spatial location and orientation of the probe.
2.2.2. Equipment for thermal ablation
Radiofrequency ablation (RFA) and microwave ablation (MWA) were used in the present study. RFA was performed with a cooled-tip RFA system (Covidien, Mansfield, MA) using a 17-gauge, internally cooled-tip electrode with a 3-cm tip. Generally, the RF generator was set in the impedance mode with maximum output. Twelve minutes was used in each RF electrode insertion. For MWA, an internally cooled microwave antenna (Kangyou Cor., Nanjing, China) with an MW generator (Kangyou Cor., Nanjing, China) of 2450 MHz was employed. The MW generator was set at 60 watts, and 4–6 min was used in each MW antenna insertion.
2.2. Research design
In the present study, both CT/MRI-CEUS and US-CEUS fusion imaging were applied on the same enrolled patient by two senior sonographers (X.E.J and L.K.) with more than 5 years of experience in fusion imaging when participating in this study. The sequence of two fusion imaging was randomly defined. when one of these two kinds of fusion imaging techniques was failed, we would use the other one of them which was successful to assess the treatment response. When both of these two fusion imaging techniques failed, treatment response was evaluated by CEUS only. The applicable rate, registration success rate and duration of CT/MRI-CEUS and US-CEUS fusion imaging were recorded by another independent observer.
2.3. Sample size
We used the success rate of registration to be the outcome measurement to estimate the sample size. According to the past data, we estimated that the success rate of registration would be 80% with CT/MRI-CEUS fusion imaging and 90% with US-CEUS fusion imaging. A sample size of at least 136 lesions was calculated to be needed to detect a difference at 5% type-I error and 95% power for a 1-tailed log-rank test.
2.4. Thermal ablation
The thermal ablation procedure was performed under general anesthesia by two US interventional doctors with more than 8 years of experience in ablation procedures when participating in this study. RFA or MWA were selected according to the size and location of the target tumor. Usually, the lesion close to the vital organs and structures or in the difficult puncture location, RFA would be preferred. Conversely, for the lesion with larger size or the patient with poorer coagulation function, MWA would be prior. For the inconspicuous lesions on US images, CT/MRI-US fusion imaging was employed for guidance of puncture. In all cases, single or overlapped multiple punctures were applied to encompass the target tumor and its circumferential 5-mm ablative margin, if possible.
Alternative preablation surgeries and procedures: For the target tumor in the dome whose acoustic window was affected by lung gas, artificial hydrothorax was applied to help improve the acoustic window immediately before ablation. For the target tumor in a high-risk location close to critical structures, such as the gastrointestinal tract, gallbladder or diaphragm, artificial ascites or hydrothorax was injected as a protection immediately before ablation. Open or laparoscopic surgery was combined when cholecystectomy or splenectomy was performed in the same section, and hepatectomy or gastrointestinal primary malignant tumor resection was needed to cure multiple liver cancers or a gastrointestinal tumor.
2.5. CEUS
SonoVue (Bracco, Milan, Italy) was injected for CEUS examination as a rapid bolus of 1.5–2.0 ml via the antecubital vein, followed by 5 ml saline solution. When necessary, SonoVue could be injected repeatedly. CEUS was usually performed 5∼15 min after the thermal ablation when the gas produced by thermal ablation mostly disappeared.
2.6. Fusion imaging
The steps of CT/MRI-CEUS fusion imaging included the acquisition of CT/MRI volume images, registration, fine-tuning and assessment of ablative margin (AM). The steps of US-CEUS fusion imaging included acquisition of 3DUS volume images, fine tuning and assessment of AM. The registration algorithm of internal anatomic landmarks was used. All the fusion imaging procedures were performed under general anesthesia. For a better registration performance, all the procedures of registration and evaluation were performed at the end expiratory status during a normal respiratory cycle by pausing ventilator. The detailed steps of these two fusion imaging techniques have been previously described [Citation22,Citation27].
2.7. Technical success of liver thermal ablation
After liver thermal ablation, the technical success of liver thermal ablation was immediately assessed. Supplemental ablation was performed if at least one type of fusion imaging technique indicated that the target tumor was not completely covered by the ablation zone. When at least one type of fusion imaging technique was successfully applied, technical success was considered achieved if the target tumor was completely covered by the ablation zone. When both types of fusion imaging failed, technical success was defined as achieved if no focal hyper-enhancement and wash-out were observed in the ablation zone margin by CEUS.
2.8. Imaging follow-up
The occurrence of complications was monitored and recorded during the follow-up period. US examination was performed within 72 h after ablation to exclude early complications. Contrast-enhanced CT/MRI was performed within three months after ablation and taken as the standard reference of the technical efficacy of the ablation procedure.
2.9. Data analysis
2.9.1. Data analysis
The applicable rate: A specific fusion imaging technique was considered as applicable if both the target tumor and the registration markers (such as portal vein or hepatic vein) were clear on the reconstructed volume image for alignment. Otherwise, the specific fusion imaging was considered inapplicable. The applicable rate refers to the percentages of lesions applicable to a specific fusion imaging technique.
The success rate of registration: A registration was considered successful when the target tumor, adjacent registration landmarks and organ contour were fully matched between the real-time US image and volume image with a registration error of less than 3 mm. The success rate of registration refers to the percentage of lesions considered with a successful registration.
The duration time required for CT/MRI-CEUS fusion imaging includes the time for importation of the CT/MRI images in DICOM format, outlining of the target tumor and its AM, process of co-registration and assessment of treatment response. The duration time required for US-CEUS fusion imaging includes the time for acquisition of 3DUS volume images, outlining of the target tumor and its AM, fine tuning and assessment of treatment response.
The technical success rate of liver thermal ablation refers to the percentage of lesions that technical success was achieved according to fusion imaging or CEUS.
The technical efficacy rate of liver thermal ablation refers to the percentage of lesions that the ablation zone completely covers the target tumor according to the CECT or CEMRI within three months after ablation.
2.9.2. Statistical analysis
SPSS 22.0 was employed, and the measurement data are presented as the average ± standard deviation if accorded with normal distribution or the median (range) if the data were not normally distributed. The duration required for fusion imaging was compared between the two types of fusion imaging techniques by a t test between two independent samples. The applicable and success rates of registration were compared between the two types of fusion imaging by x2 test and Fisher’s exact test. p values below .05 are considered significant differences.
Figure 1. A 51-year-old male. (a, b) Contrast-enhanced CT indicates a liver tumor located in segment 7. Arterial enhancement and delay wash-out were observed. (c) A hypoechoic lesion in US. (d) Real-time US was successfully matched with immediately acquired preablation 3DUS. (e) Real-time US was matched with preablation CT volume images successfully. (f) Ablation was applied under the guidance and monitoring of US. (g) CEUS was performed and fused with preablation 3DUS. The ablation zone completely covered the target tumor and the 5-mm ablative margin. (h) CEUS fused with preablation CT indicated the ablation zone completely covered the target tumor and the 5-mm ablative margin. (i) Subsequent contrast-enhanced CT within three months confirmed that the tumor had been completely ablated.
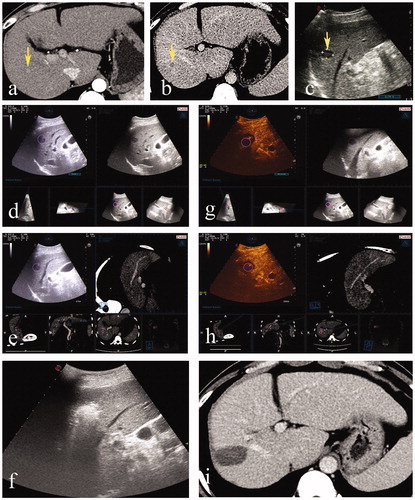
Figure 2. A 43-year-old male. (a, b) Contrast-enhanced CT indicated a massive hepatocellular carcinoma in the left liver with a small lesion located in segment 8. (c) Hepatectomy was performed before ablation, and the small lesion was hypoechoic on US. (d) Deformation of liver after hepatectomy led to the failure of registration of US and preablation CT volume images. (e) Immediately acquired 3DUS volume images were successfully matched to the real-time US. (f) After the ablation procedure, CEUS was performed and fused with preablation 3DUS. The ablation zone completely covered the target tumor and the 5-mm ablative margin. (g) Subsequent contrast-enhanced CT within three months confirmed that the tumor had been completely ablated.
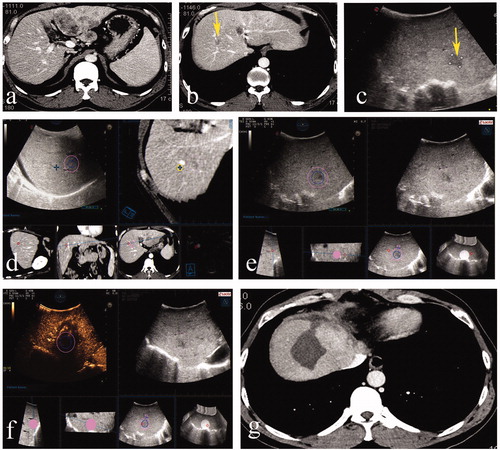
Figure 3. A 46-year-old male. (a,b) Contrast-enhanced MRI indicated a small recurrent tumor located in segment 4. (c) The lesion was inconspicuous in the approximated location on US. (d,e) Arterial enhancement and delay wash-out in Contrast-enhanced US was observed. (f) Real-time US was matched with preablation MRI volume images successfully. (g) Ablation was performed. (h) CEUS fused with preablation MRI indicated the ablation zone completely covered the target tumor and the 5-mm ablative margin. (i) Subsequent contrast-enhanced CT within three months confirmed that the tumor had been completely ablated.
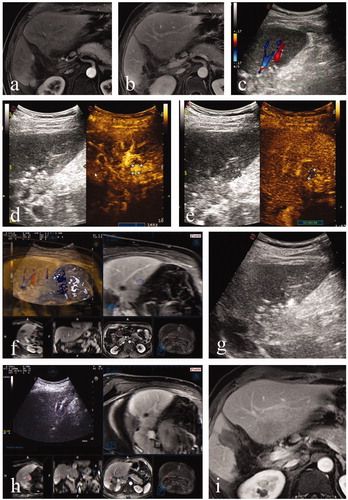
3. Results
3.1. Enrollment
From August to December 2015, a total of 182 liver tumors in 130 consecutive patients underwent ultrasound-guided thermal ablation in our department. Among the 128 patients (175 liver tumors) who satisfied the inclusion criteria, 13 patients (18 liver tumors) were excluded due to fusion imaging equipment failure during the ablation procedure. Finally, 157 liver cancers in 115 patients were enrolled in the present study. The 115 patients included 94 males and 21 females, aging 27–84 years (mean age 54 years). The tumor diameter ranged from 8–55 mm, with a mean diameter of 19 ± 8 mm. The baseline characteristic features of the study population are shown in .
Table 1. Baseline features of the study population.
3.2. The applicable rates and the success rates of registration
The applicable rates and the success rates of registration in CT/MRI-CEUS and US-CEUS fusion imaging are listed in , and the differences are statistically significant. The causes for inapplicable cases of the two fusion imaging techniques are listed in , and the causes for the failure of registration are listed in .
Table 2. The applicable rate and success rate of registration in CT/MR-CEUS and 3DUS-CEUS fusion imaging techniques.
Table 3. Causes for inapplicable CT/MRI-CEUS and US-CEUS fusion imaging.
Table 4. Causes for the failure of registration in CT/MRI-CEUS and US-CEUS fusion imaging techniques.
Finally, among the 157 liver tumors in 115 patients included in the present study, 87.9% (138/157) of the liver tumors were successfully assessed by at least one type of fusion imaging, and 12.1% (19/157) of the liver tumors were assessed by CEUS only.
3.3. Influence of alternative preablation surgeries or procedures on the success rate of registration
The success rate of registration was significantly higher in US-CEUS fusion imaging than in CT/MRI-CEUS fusion imaging for cases with alternative preablation surgeries or procedures, as shown in and . There was no significant difference between CT/MRI-CEUS and US-CEUS fusion imaging in cases that were not combined with other surgeries or procedures.
Table 5. Influence of combined operations or procedures during the ablation procedure on the success rate of registration in two fusion imaging techniques.
Table 6. Influence of different combined operations or procedures during ablation procedure on success rate of registration in two fusion imaging techniques.
3.4. Duration of registration and assessment
The duration time for co-registration and assessment was 5.7 ± 2.7 min in CT/MRI-CEUS fusion imaging and 3.7 ± 1.7 min in US-CEUS fusion imaging (p < .001).
3.5. Technical success rate
There were 39 lesions in 31 patients received supplemental ablation to achieve sufficient ablation zone after the immediate assessment using fusion imaging during the ablation procedures. Finally, 157 liver tumors were completely covered by the ablation zone, yielding a technical success rate of 100% (157/157).
3.6. Follow up
3.6.1. Major complications
No major complication was observed during the follow-up period for any of the enrolled patients.
3.6.2. Technical efficacy rate
The results of CECT/CEMRI within three months after the ablation procedure were obtained in 110 patients with a total of 151 tumors. A total of 150 tumors achieved complete ablation, yielding a technical efficacy rate of 99.3% (150/151) ().
4. Discussion
In the present study, the applicable rate of US-CEUS fusion imaging was obviously inferior to that of CT/MRI-CEUS fusion imaging. The major reason was the inconspicuous lesions on conventional US images. There were 26.7% (41/157) of inconspicuous lesions observed in the enrolled patients, similar to a previous report [Citation29–31]. Without conspicuous margins, the 3DUS images were insufficient as references for fusion imaging. Moreover, if the lesion was inconspicuous after 3DUS reconstruction, then it could not be applied for US-CEUS fusion imaging either. There were 13.4% (21/157) lesions inapplicable for this reason. In contrast, the applicable rate of CT/MRI-CEUS fusion imaging was as high as 98.7%, and only two lesions were inconspicuous in the CT/MRI sequences and could not be used as reference images for fusion imaging.
However, if the lesion was applicable for US-CEUS fusion imaging, the success rate of registration was superior to that for CT/MRI-CEUS fusion imaging. Since the reference images of 3DUS were acquired intraoperatively before ablation [Citation27] and due to the mono-modality imaging of US images, the alignment using US-CEUS fusion imaging was easier to realize. The anatomical consistency of US images between preablation and postablation was better. In CT/MRI-CEUS fusion imaging, the inherent imaging deformations between CT/MRI and US images were inevitable. Moreover, the CT/MRI images were usually obtained several days before the ablation procedure. The anatomical structures between CT/MRI and US images would be more or less changed, especially for patients who underwent ablation with alternative preablation surgeries or procedures [Citation16], such as artificial hydrothorax or ascites, laparoscopic surgery or open surgery. According to the present results, if the ablation was combined with alternative preablation surgeries or procedures, then the success rate of registration using US-CEUS fusion imaging was higher than that of CT/MRI-CEUS fusion imaging (p = .018). However, for patients who underwent ablation without alternative preablation surgeries or procedures, the success rates of registration for these two fusion imaging techniques were comparable (p = .312). Moreover, according to the results, the failed cases of the registration of CT/MRI-CEUS fusion imaging mainly occurred in patients with open surgery. While, the majority of patients with artificial hydrothorax or ascites or laparoscopy were successful in the registration of CT/MRI-CEUS fusion imaging. The possible reason were that the changes of liver anatomical structures were not too much in these situations and an experienced sonographer could improve the registration success rate with patient and careful alignment.
Not only the success rate of registration but also the duration time of US-CEUS fusion imaging was superior to that of CT/MRI-CEUS fusion imaging. In the present study, the duration time of CT/MRI-CEUS fusion imaging (5.7 ± 2.7 min) was similar to that of previous reports [Citation16,Citation21,Citation22]. The duration time of US-CEUS fusion imaging was reduced to 3.7 ± 1.7 min. The time saving of US-CEUS fusion imaging might contribute to its intraoperative application. The intraoperative acquired 3DUS reference images before ablation could be automatically matched with the real-time US images using a magnetic positioning system and no further co-registration was needed. After the ablation, a minor registration error between 3DUS reference images and real-time US images might occur. However, the mono-modality fusion imaging of only US made the alignment easier and faster. Compared with CT/MRI-CEUS fusion imaging, US-CEUS fusion imaging required less experience with image co-registration and CT/MRI image reading skills. Thus, this technique would be more suitable for intraoperative application, particularly for doctors without sufficient experience in fusion imaging.
Due to the immediate evaluation using fusion imaging, all the lesions were treated with 100% technical success rates, without major complications. According to the results of CECT/CEMRI within three months after ablation, complete ablation was achieved in 150 of 151 lesions. The technique efficacy rate was as high as 99.3%. This result indicated that both CT/MRI-CEUS and US-CEUS fusion imaging techniques might be feasible and valuable for improving short-term outcomes.
In our opinion, for the intraoperative immediate evaluation of liver thermal ablation, US-CEUS fusion imaging is preferred if the lesions are conspicuous on US images, especially for cases combined with alternative preablation surgeries or procedures, and this procedure is more convenient, faster and more accurate. If the lesions are inconspicuous on US images, then CT/MRI-CEUS fusion imaging could be employed instead. CT/MRI-CEUS fusion imaging would be helpful not only in the evaluation but also in the detection and guidance of these inconspicuous lesions during thermal ablation. In the present study, 87.9% of lesions could be successfully assessed for immediate therapeutic responses using at least one type of these two fusion imaging techniques. When both fusion imaging techniques combined with CEUS failed to be registered, CEUS only was recommended to assess the immediate therapeutic response to liver thermal ablation, which is more common in many other institutions.
There are some limitations of the present study. First, CT/MRI-CEUS and US-CEUS fusion imaging were both performed to evaluate the therapeutic response, which was comparable to a paired study that showed reduced grouping bias. However, the evaluation results of these two fusion imaging methods could influence each other and have an influence on the evaluation result and the supplemental ablation, which would be unable to investigate the differences in the efficacy of these two fusion imaging techniques. Further case-control studies are needed to clarify this problem. Second, the follow-up period was short, and long-term outcomes, such as local tumor progression, recurrence and survival, should be considered in the future. Lastly, only one type of fusion imaging equipment was investigated and the results may not be generalizable to other fusion imaging systems are used. Other equipment would be employed in the further research.
In conclusion, both CT/MRI-CEUS and US-CEUS fusion imaging are feasible methods for the intraoperative immediate evaluation of the therapeutic response to liver thermal ablation. US-CEUS fusion imaging is preferred because of its convenience and higher success rate of registration.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;10:761–780.
- Minami Y, Nishida N, Kudo M. Therapeutic response assessment of RFA for HCC: contrast-enhanced US, CT and MRI. World J Gastroenterol. 2014;20:4160–4166.
- Minami Y, Kudo M. Imaging modalities for assessment of treatment response to nonsurgical hepatocellular carcinoma therapy: contrast-enhanced US, CT, and MRI. Liver Cancer. 2015;4:106–114.
- Du J, Li HL, Zhai B, et al. Radiofrequency ablation for hepatocellular carcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in guiding and assessing early therapeutic response and short-term follow-up results. Ultrasound Med Biol. 2015;41:2400–2411.
- Kim CK, Choi D, Lim HK, et al. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: Utility of contrast-enhanced agent detection imaging. Eur J Radiol. 2005;56:66–73.
- Claudon M, Dietrich CF, Choi BI, et al. World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound: Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Der Medizin. 2013;39:187–210.
- Leyendecker JR, Dodd GD, Halff GA, et al. Sonographically observed echogenic response during intraoperative radiofrequency ablation of cirrhotic livers: pathologic correlation. Ajr Am J Roentgenol. 2002;178:1147–1151.
- Zhou P, Kudo M, Minami Y, et al. What is the best time to evaluate treatment response after radiofrequency ablation of hepatocellular carcinoma using contrast-enhanced sonography? Oncology. 2007;72:92–97.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33:227–239.
- Makino Y, Imai Y, Igura T, et al. Usefulness of the multimodality fusion imaging for the diagnosis and treatment of hepatocellular carcinoma. Dig Dis. 2012;30:580–587.
- Toshikuni N, Tsutsumi M, Takuma Y, et al. Real-time image fusion for successful percutaneous radiofrequency ablation of hepatocellular carcinoma. J Ultrasound Med. 2014;33:2005–2010.
- Wood BJ, Kruecker J, Abi-Jaoudeh N, et al. Navigation systems for ablation. J Vasc Interv Radiol. 2010;21:S257–S263.
- Lee MW, Rhim H, Cha DI, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012;198:1438–1444.
- Krücker J, Xu S, Venkatesan A, et al. Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol. 2011;22:515–524.
- Zhong-Zhen S, Kai L, Rong-Qin Z, et al. A feasibility study for determining ablative margin with 3D-CEUS-CT/MR image fusion after radiofrequency ablation of hepatocellular carcinoma. Ultraschall in Med. 2012;33:E250–E225.
- Park HJ, Lee MW, Lee MH, et al. Fusion imaging-guided percutaneous biopsy of focal hepatic lesions with poor conspicuity on conventional sonography. J Ultrasound Med. 2013;32:1557–1564.
- Makino Y, Imai Y, Igura T, et al. Feasibility of extracted-overlay fusion imaging for intraoperative treatment evaluation of radiofrequency ablation for hepatocellular carcinoma. Liver Cancer. 2016;5:269–279.
- Ahn SJ, Lee JM, Lee DH, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66:347–354.
- Mauri G, Cova L, De Beni S, et al. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143–151.
- Li K, Su Z, Xu E, et al. Evaluation of the ablation margin of hepatocellular carcinoma using CEUS-CT/MR image fusion in a phantom model and in patients. BMC Cancer. 2017;17:61.
- Li K, Su ZZ, Xu EJ, et al. Improvement of ablative margins by the intraoperative use of CEUS-CT/MR image fusion in hepatocellular carcinoma. BMC Cancer. 2016;16:9.
- Hiraoka A, Hirooka M, Koizumi Y, et al. A modified novel technique for determining therapeutic response to radiofrequency ablation therapy for hepatocellular carcinoma using US-volume system. Gastroenterology. 2010;23:493–497.
- Minami Y, Minami T, Chishina H, et al. US-US fusion imaging in radiofrequency ablation for liver metastases. Dig Dis. 2016;34:687–691.
- Toshikuni N, Shiroeda H, Ozaki K, et al. Advanced ultrasonography technologies to assess the effects of radiofrequency ablation on hepatocellular carcinoma. Radiol Oncol. 2013;47:224–229.
- Park HJ, Lee MW, Rhim H, et al. Percutaneous ultrasonography-guided radiofrequency ablation of hepatocellular carcinomas: usefulness of image fusion with three-dimensional ultrasonography. Clin Radiol. 2015;70:387–394.
- Xu EJ, Lv SM, Li K, et al. Immediate evaluation and guidance of liver cancer thermal ablation by three-dimensional ultrasound/contrast-enhanced ultrasound fusion imaging. Int J Hyperthermia. 2018;34:870–876.
- The Ministry of Health of The People’s Republic of China. Diagnosis and therapy criterion for hepatocellular carcinoma (version 2011). Chin Clin Oncol. 2011;2011:929–946.
- Lee MW, Rhim H, Cha DI, et al. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1-3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013;24:958–965.
- Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol. 2007;33:1515–1526.
- Lee MW, Kim YJ, Park HS, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010;194:W396–W400.
