Abstract
Objective: To evaluate the long-term efficacy and prognostic factors of ultrasound-guided percutaneous radiofrequency ablation (RFA) for breast cancer liver metastasis (BCLM).
Methods: Between 2000 and 2015, 69 patients who underwent ultrasound-guided percutaneous RFA for BCLM and had regular follow-up examinations were included. All patients had undergone resection of the primary breast cancer and had received chemotherapy, endocrine therapy or both after surgery. The sample included two males and 67 females with an average age of 50.3 ± 10.0 years (31–76 y). The mean maximum diameter of metastatic lesions in the liver was 2.9 ± 1.4 cm (1.0–6 cm). Thirty-five patients had a single metastasis, while 34 patients had multiple liver metastases (2–5 lesions). Survival results were generated using Kaplan-Meier estimates and a multivariate analysis was performed using the Cox regression model.
Results: In total, 92 RFA sessions were performed and 135 BCLM lesions were treated. Major complications occurred in one of the 92 sessions (1.1%). Technical efficacy was achieved in 92.6% of lesions (125/135 lesions). Local tumor progression occurred in 11.6% (8/69) of patients and new intrahepatic metastasis occurred in 55.1% (38/69) of patients. From the time of initial RFA, the median overall survival was 26 months, and the one-, two-, three- and five -year survival rates were 81.8, 50.1, 25.3 and 11.0%, respectively. Based on the multivariate analysis, the following three factors were identified as independent prognostic factors for overall survival: tumor size (p = .017), positive estrogen receptor status (p = .009) and extrahepatic metastatic disease (p = .001). The median progression-free survival was 24 months, and the one-, two-, three- and five -year survival rates after RFA were 77.4, 47.0, 23.7 and 8.5%, respectively. Additionally, the independent prognostic factors for progression-free survival included tumor size (p = .011), ER positivity (p = .001), margin size (p = .017) and extrahepatic metastatic disease (p < .001).
Conclusion: The results of this study showed that RFA is a safe and locally effective method for the treatment of BCLM, especially in patients with lesions measuring less than 3 cm in diameter, a single liver metastasis, positive estrogen receptor status and no extrahepatic metastases. Also, patients with margin size >10 mm had no local tumor progression.
Introduction
Breast cancer is one of the most common malignant tumors in women. The incidence and mortality rates of breast cancer have been increasing in most of Africa and Asia [Citation1]. Additionally, approximately 20% of breast cancer patients experience disease recurrence [Citation2] and the most common sites of advanced breast cancer metastases include lung, bone and liver, among others [Citation3]. According to NCCN guidelines, advanced breast cancer is primarily treated systemically [Citation4]. Systemic treatment of advanced breast cancer is complex and anti-cancer agents or endocrine therapy are based on hormone status [Citation4]. Although liver resection of breast cancer liver metastasis (BCLM) was previously regarded with considerable skepticism, surgery may be useful for a select subset (<5%) of patients with isolated BCLM [Citation5,Citation6]. BCLM is a complex process with multiple factors, steps and involved genes. In the absence of treatment, the prognosis for BCLM remains poor, although extensive research has sought to identify the mechanism responsible for this disease [Citation7].
Previously, local ablation techniques have been proven to be safe and effective for isolated colorectal liver metastasis [Citation8]; however, the biological behavior of breast cancer differs from that of colorectal cancer [Citation9]. Therefore, clinical studies of radiofrequency ablation (RFA) for the treatment of BCLM are limited because of controversy regarding its effectiveness. Several studies have shown promising results for RFA in BCLM [Citation10–14], but more data are needed to confirm long-term outcomes. Moreover, little evidence is available regarding the effectiveness of various treatment techniques in patients with BCLM, a high-risk population with an unfavorable prognosis [Citation15,Citation16]. As outlined above, the purpose of this study was to explore the local control and long-term outcomes of RFA for BCLM.
Materials and methods
Patients
The Institutional Review Board provided a waiver of authorization of the protocol because of the retrospective nature of this study. All patients signed an informed consent form before RFA treatment. Between January 2000 and December 2015, 76 BCLM patients underwent ultrasound-guided percutaneous RFA in our center. Seven patients were excluded for the following reasons: four patients were lost to follow-up, one had widespread extrahepatic metastases, and two patients had metastatic lesions in the liver with diameters >6 cm. The remaining 69 patients were included in this study. The inclusion criteria for this study were as follows: (1) tumor size ≤6 cm in diameter and number of tumors ≤5; (2) conventional ultrasound or contrast-enhanced ultrasonography (CEUS) showing hepatic metastasis; (3) a liver function classification of Child-Pugh A or B; (4) no concomitant extrahepatic metastatic disease or extrahepatic metastasis with stable control before RFA treatment; (5) absence of significant direct tumor invasion of adjacent organs or tumor thrombi in the main or lobar portal system; (6) no tumor invasion of the main bile duct or an obviously exophytic tumor; (7) an international normalized ratio <1.6 and platelet count >50 000/μl and (8) primary breast cancer lesions that had been surgically removed;
In this group, all primary tumors were diagnosed as breast cancer and all primary breast cancer tumors had been surgically removed. The pathological types of 54 patients were recorded, including 48 invasive ductal carcinomas, two invasive lobular carcinomas, and four other types of breast cancer. The hormone receptor status of the primary breast cancers was shown in . Among 69 patients, 43 patients were estrogen receptor (ER) positive, 37 patients were progesterone receptor (PR) positive, and 22 patients were epidermal growth factor receptor-2 (HER-2) positive. Liver metastasis was confirmed in 34 patients by biopsy before RFA treatment. Among these patients, receptor status was assessed in 23 patients and no discrepancy with the original primary status was found. The liver lesions were evaluated by oncologists based on typical imaging findings, laboratory tests (e.g., tumor markers) or clinical history. Among 69 patients, one patient was diagnosed with colorectal cancer after resection of breast cancer, and one patient was diagnosed with lung and thyroid cancer after resection of breast cancer. Liver metastasis in these two patients was confirmed by pathology as BCLM.
Table 1. Clinical characteristics of primary breast cancer and liver metastasis.
The sample included two males and 67 females with an average age of 50.3 ± 10 y (range: 31–76 y). The mean maximum diameter of the metastases was 2.9 ± 1.4 cm (range: 1.0–6 cm). Thirty-five patients had a single liver metastasis, while 34 patients had multiple liver metastases (range: 2–5). BCLM was defined as metachronous if the liver metastasis was diagnosed after the treatment of primary breast cancer and synchronous if the liver metastasis was diagnosed at the same time as the primary breast cancer. Six patients had synchronous liver metastasis, and 63 patients had metachronous liver metastasis. The interval between the primary breast cancer diagnosis and the liver metastasis diagnosis ranged from 5–215 months (mean: 47.6 ± 50 months). After resection, all patients received systemic chemotherapy, endocrine therapy, or both before RFA. The adjuvant chemotherapy included cyclophosphamide, 5-fluorouracil, Adriamycin, Xeloda, docetaxel or methotrexate. Thirty-three of the 69 patients received endocrine therapy, including tamoxifen, letrozole, or aminoglutethimide. All post-RFA treatments were performed by the oncologist based on the patient’s hormone receptor status and follow-up examinations. A total of seven patients underwent local treatment for liver lesions, including two patients with resection, two patients with RFA in other hospitals, and three patients with trans-catheter arterial chemoembolization (TACE). After RFA, systemic therapy was continued by the oncologist. Among the 69 patients, 32 patients experienced extrahepatic metastasis with stable disease control before RFA treatment, including 20 patients with a single extrahepatic metastatic site and 12 patients with multiple extrahepatic metastatic sites ().
Pre-procedural examination and preparation
All patients underwent a baseline evaluation including ultrasound and enhanced computerized tomography (CT) or a magnetic resonance imaging (MRI) scan of the abdomen within one month prior to RFA. Imaging parameters included tumor size, shape, number, border and location. Additionally, chest CT scanning, head MRI or radionuclide bone imaging was performed on patients with extrahepatic metastatic disease. Serum laboratory tests included a complete blood count, coagulation test, screening for infection, and hepatic and renal functional tests, all of which were performed in the two weeks prior to RFA. If the intrahepatic nodules were questionable regarding whether they originated from breast cancer metastasis, a liver biopsy specimen was required. All patients underwent regular CEUS after 2004 to assess the size, number, and location of the tumor before treatment. All related pre-procedural examinations were performed to determine whether patients were suitable candidates for RFA treatment.
RFA methods and instruments
All RFA procedures were performed by four radiologists (CMH, YK, WW and YW) who each had more than 10 years of experience in ultrasound-guided interventional procedures. CT/MRI imaging data were routinely obtained within the two weeks prior to RFA and were used as a reference for ultrasound-guided ablation. Treatments were planned based on a combination of CT/MRI and ultrasound before the procedure; treatment response was assessed with ultrasound or enhanced ultrasound after the procedures were completed. Targeting, monitoring, and intraprocedural modification were all performed during the procedure with real-time ultrasound and enhanced ultrasound if necessary. Multiple overlapping ablations were performed for larger tumors (> 3 cm) [Citation17]. A circumferential ablative margin covering at least 0.5 cm (ideally 1 cm) beyond the target tumor was planned in the preoperative protocol except for tumors adjacent to major structures such as the diaphragm, bowel, gallbladder, kidney or large blood vessels. For these cases, the ablative margin was limited; therefore, an individualized protocol was developed [Citation18–20]. In the RFA process, the hyperechoic bubbles caused by ablation could be visualized and monitored by real-time ultrasound. Roughly assessing the hyperechoic zone covering the target tumor was important for the completion of ablation. Overlapping and modification of ablation could be performed during the procedure to ensure a sufficient ablation zone. CEUS was immediately performed after RFA to evaluate the ablative margin if the tumor was larger than 3 cm or close to important structures [Citation21].
During RFA, moderate intravenous sedation was administered to all patients using 2.5–5 mg of midazolam (Roche; Basel, Switzerland) and 50–100 µg of fentanyl (Fentaini; Renfu, Yichang, China). The puncture points were determined by ultrasound guidance before the procedure, and local infiltration of anesthesia for the puncture points was achieved with 5–15 ml of 1% lidocaine (Liduokayin; Yimin, Beijing, China). The patients were conscious when the RFA electrode was placed and the anesthesiologist continuously monitored the heart rate, blood pressure and oxygen saturation during the procedure. If a tumor was located adjacent to the diaphragm, bowel, gallbladder, kidney or large vessels, injections of D5W were sometimes required to protect or separate the tissues and organs from the heat. Track ablation was performed in all patients when withdrawing the RFA electrodes. After RFA, the patient remained in the hospital overnight for observation. Ultrasonography examination was performed 24 h after RFA to monitor complications. Patients were then discharged if no evidence of active bleeding was found.
The RFA systems used in this study were as follows: multi-tined RITA electrodes (RITA Medical Systems, Martinez, CA) and multi-polar electrodes (Celon Lab Power, Germany). The RITA RFA system is a 460 kHz generator (model 1500, RITA Medical Systems) consisting of a 14 gauge, 15 cm-long outer insulated needle and nine hook-shaped prongs that can be deployed from the central needle cannula and retracted by a movable hub with a deployment diameter of 3–5 cm. High-frequency power was passed through the deployed prongs to the tumor tissue. Approximately 20 min were needed to produce a 5 cm ablation sphere during RFA. The Celon Lab Power system used in this study provides a maximum power output of 250 W and can connect one to three 15- to 20 cm-long electrodes with an exposed tip of 3–4 cm. In bipolar and multi-polar modes, the size and shape of coagulation depend on the length, number and distance between the power and deploying time of the electrodes.
The Prosound-10 (ALOKA, Japan) and Vivid E9 4 D cardiovascular ultrasound systems (GE Healthcare, Chicago, IL) were used for ultrasound guidance and blood flow observation. Sonovue (Bracco, SPA, Italy) was used as a contrast medium in CEUS.
Assessment of therapeutic efficacy and follow-up
Technical success was defined when the tumor was treated and completely replaced by RFA zones and tumor coverage was assessed either during or immediately following the procedure by ultrasound or CEUS [Citation22]. Technical efficacy refers to a prospectively defined time point at which complete ablation was achieved [Citation22]. In this study, abdominal contrast-enhanced CT/MRI was performed one month after RFA treatment to assess the technical efficacy. As described in a previous report [Citation23], we used the same method to evaluate the margin size. The pre- and one-month post-ablation CT images (portal venous phase) were reviewed side by side to compare the index tumor and the ablation zone/defect. The minimal margin size was the minimum of multiple margin values on CT. The margin size was assessed and categorized as 0–5 mm, 6–10 mm or >10 mm [Citation23]. The patients were then regularly followed with abdominal and breast ultrasound, enhanced CT/MRI and lab tests every 2–3 months for the first year and every 4–6 months thereafter. Local tumor progression was defined as the appearance of a tumor along the edge of the ablation zone [Citation22]. When local tumor progression or a new tumor was confirmed, another RFA session was performed according to the patient’s condition. Progression-free survival (PFS) was defined as the time from the first RFA treatment to local tumor progression or death, whichever occurred first [Citation22]. The time between the diagnosis of liver metastasis and the last follow-up ranged from 4–131 months (median: 36 months). The time between RFA and the last follow-up ranged from 4– 100 months (median: 26 months). The time between the diagnosis of liver metastasis and RFA ranged from 0–121 months (mean: 11.4 ± 18.2 months).
Statistical analysis
Ten potential prognostic factors were analyzed in this study, including age, tumor size, the number of tumors, tumor locations, the distribution of tumors, extrahepatic metastasis, hormone receptor status, proximity to large vessels, the interval between breast cancer and liver metastasis, the number of RFA sessions and margin size. These factors were also analyzed for local PFS. Tumor interphase was defined as the interval between the diagnosis of breast cancer and the diagnosis of BCLM. The overall survival time was counted in months from the date of the first RFA to death. The Kaplan-Meier model and log-rank tests were used for the univariate analysis. The Cox proportional hazards model was used for the multivariate analysis. SPSS 21.0 software (SPSS, Chicago, IL,) was used, and a p value < .05 was considered statistically significant.
Results
Technical efficacy
A total of 92 RFA sessions (range: 1–5 sessions) were performed and 135 liver metastases (range: 1–11 metastases) were treated in 69 patients. Technical efficacy was achieved in 92.6% (125/135) of liver metastases after RFA. Sixty-one liver metastases had a maximum diameter larger than 3 cm. The technical efficacy rate for cases with a metastatic lesion larger than 3 cm was 95.1% (58/61). Sixty-six lesions were close to major structures and technical efficacy was achieved in 89.4% (59/66) of cases. Residual tumors were observed in 10 lesions in 10 patients, including one lesion close to the kidney, two tumors close to large blood vessels, two tumors close to the liver capsule, two lesions close to the diaphragm, and three lesions larger than 4 cm. Nine of these 10 patients achieved secondary technical success after repeated RFA sessions. One patient received TACE because of the occurrence of multiple lesions.
Local tumor progression and new tumor development
During the follow-up period, local tumor progression (LTP) was observed in eight of the 69 patients (11.6%), 4–8 months after RFA. The LTP rates for tumors with a margin of 0–5 mm, > 5–10 mm and >10 mm were 38.9 (7/18), 3.6 (1/28) and 0% (0/23), respectively. Among these eight patients, seven underwent repeated RFA sessions and one patient had extrahepatic metastases and received repeated chemotherapy. Thirty-eight patients (55.1%) presented new intrahepatic metastasis during the follow-up period. Among these, 28 patients had new multiple intrahepatic metastases and 10 patients had a new solitary lesion. The 38 patients with intrahepatic metastases received the following treatments: chemotherapy (19 patients), repeated RFA sessions (2–5 sessions;16 patients), and TACE (three patients).
Survival rate and prognostic analysis
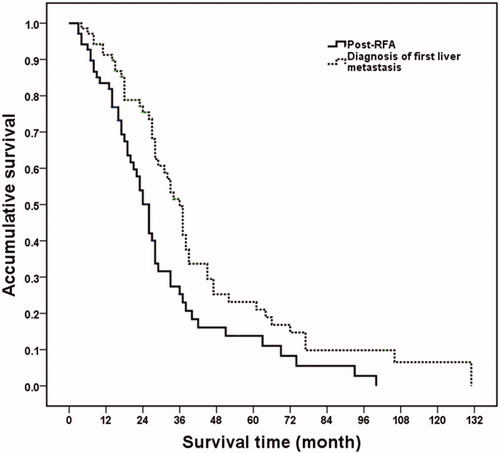
At the last follow-up, five patients were alive, while the remaining 64 patients had succumbed to their condition because of disease progression during the follow-up period. The median overall survival and cumulative the one-, two-, three- and five-year survival rates after RFA were 26 months and 81.8, 50.1, 25.3, and 11.0%, respectively. From the time that the first liver metastasis was diagnosed, the median overall survival was 36 months, and the one-, two-, three-, and five-year survival rates were 92.6, 75.2, 48.7, and 20.7%, respectively. A significant difference in overall survival was identified after diagnosis and after RFA treatment of BCLM (p = .004) (). The median PFS and the one-, two-, three- and five -year survival rates after RFA were 24 months and 77.4, 47.0, 23.7 and 8.5%, respectively.
Figure 1. Overall survival curves in breast cancer liver metastasis patients after RFA and diagnosis of liver metastasis. From the time of the first RFA, median overall survival time and one-, two-, three- and five-year survival rates were 26 months and 81.8, 50.1, 25.3 and 11.0%, respectively. From the time the liver metastasis was diagnosed, the median overall survival time was 36 months and the one-, two-, three- and five-year survival rates were 92.6, 75.2, 48.7 and 20.7%, respectively (p = .004).
Based on the univariate analysis, the following four factors were identified as related to post-RFA overall survival: tumor size (p = .003), number of tumors (p = .030), ER positivity (p < .001) and extrahepatic metastatic disease (p = .001). The factors that were statistically significant in the univariate analysis were further analyzed by multivariate analysis. Among them, tumor size (p = .017), ER positivity (p = .009) and extrahepatic metastatic disease (p = .001) were independent prognostic factors for overall survival (; and ).
Figure 2. Post-RFA overall survival curves in breast cancer liver metastasis patients with different maximum diameter of metastasis lesions. Patients with maximum diameter of metastasis ≤3 cm had higher overall survival rate than those patients with 3.1–6 cm lesions (p = .003).
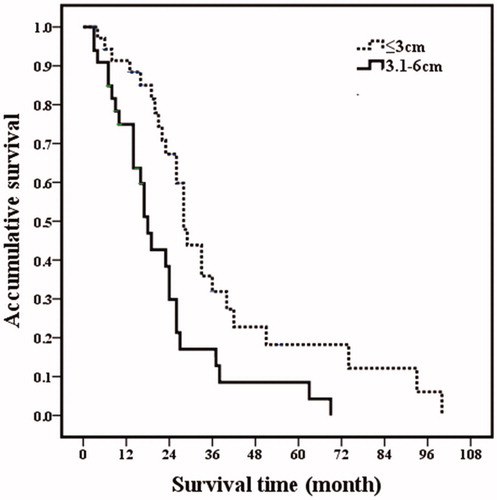
Figure 3. Post-RFA overall survival curves in breast cancer liver metastasis patients with different number of metastasis. Patients with 1–2 metastases had higher overall survival rates than those patients with 3–5 metastases (p = .030).
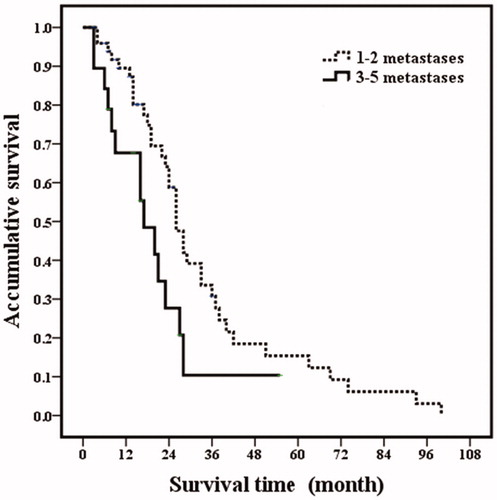
Figure 4. Post-RFA overall survival curves in breast cancer liver metastasis patients with extrahepatic metastatic disease. Patients with extrahepatic metastatic disease had poor overall survival rates than those patients without extrahepatic metastatic disease (p = .001).
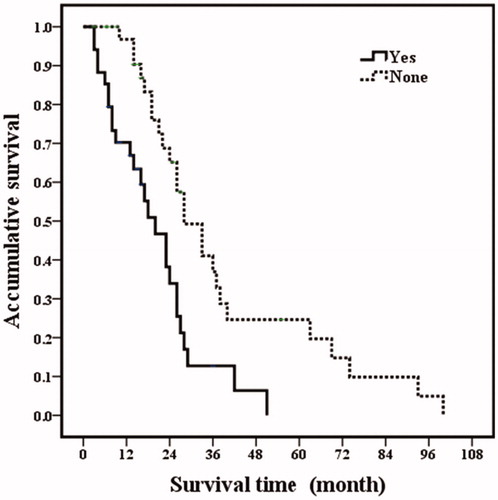
Figure 5. Overall survival curves in breast cancer liver metastasis patients with estrogen receptor status. Patients with estrogen receptor negative had poorer overall prognosis (p <.001).
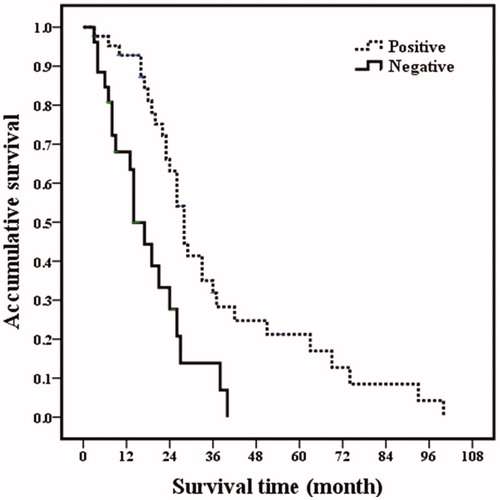
Figure 6. A 54-year-old woman with breast cancer liver metastasis (BCLM) underwent radiofrequency ablation (RFA) treatment. (A) Ultrasound before RFA showed a 3.1 cm tumor in segment II of liver (↑). The tumor was diagnosed as BCLM-moderately differentiated adenocarcinoma by ultrasound-guided biopsy. (B) Contrast-enhanced CT before RFA showed a 3.1 cm tumor in segment II of liver. (C) Ultrasound in 53 months after first RFA showed the second RFA was performed for the new liver metastasis in segment III. (D) Contrast-enhanced CT in one month after RFA showed that ablation zone had non-enhancing. (E) Ultrasound in 80 months after first RFA showed multiple new intrahepatic metastases (↑). The patients underwent chemotherapy. (F) Magnetic resonance imaging (MRI) in 80 months after first RFA confirmed that ablation area had non-enhancing (↑). (G) Ultrasound in 92 months after first RFA showed massive pleural effusion. (H) MRI in 95 months showed the ablation zone had non-enhancing but other liver metastasis had no response to chemotherapy treatment. This patient survived more than eight years post treatment and died due to the disease progression.
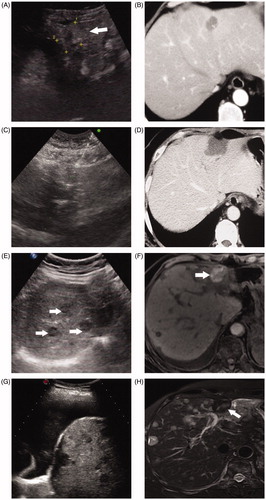
Table 2. Univariate analysis for BCLM overall survival prognosis after RFA.
Table 3. Multivariate analysis of overall survival prognostic factors with Cox proportional hazards in BCLM patients after RFA.
Based on the univariate analysis for PFS, tumor size (p = .005), number of tumors (p = .025), ER positivity (p < .001), PR positivity (p = .020), margin size (p = .023) and extrahepatic metastatic disease (p = .001) were identified as factors affecting PFS. Further multivariate analysis revealed that the following four variables were independent prognostic factors for PFS: tumor size (p = .011), ER positivity (p = .001), margin size (p = .017) and extrahepatic metastatic disease (p < .001; ). Especially, the one-, two-, three- and five-year PFS for margin of 0–5, 6–10 and >10 mm were 54.2, 23.7, 5.9 and 0%; 72.9, 44.8,12.8 and 4.3% and 95.5, 65.8, 35.4 and17.7%, respectively ().
Figure 7. Kaplan-Meier curves for PFS of margin size after radiofrequency ablation. the one-, two-, three- and five-year PFS for margin of 0–5 , 6–10 and >10 mm were 54.2, 23.7 and 5.9%; 72.9, 44.8, 12.8 and 4.3% and 95.5, 65.8, 35.4 and 17.7%, respectively.
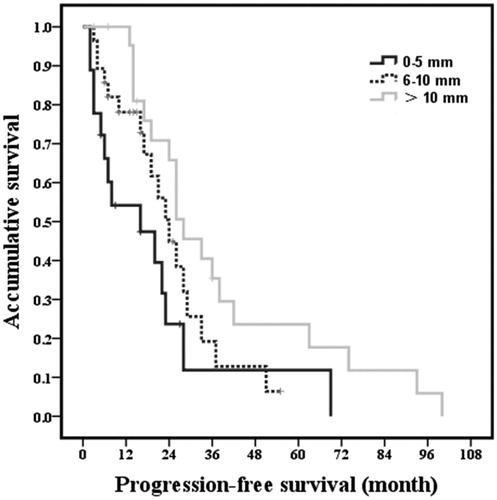
Table 4. Multivariate analysis of PFS with Cox proportional hazards in BCLM patients.
Complications
No patients experienced death related to RFA treatment. The occurrence of major complications was 1.1% (1/92 RFA sessions). One patients experienced cholecystitis; she had a history of gallstones and swelling of the gallbladder wall was observed one day after RFA. Antibiotic treatment was administered and the patient recovered after three days. Minor complications included mild skin rash (n = 1), mild needle-tract bleeding (n = 2) and moderate pleural effusion (n = 5).
Discussion
Breast cancer-associated morbidity and mortality are still increasing in many countries worldwide [Citation1], posing a challenge to the prevention and treatment of breast cancer. With the development and improvement of surgical techniques and neoadjuvant chemotherapy, the prognosis of breast cancer has significantly improved. According to previous reports, the five-year survival rate of breast cancer is 87–90.5% [Citation24, Citation25]. Studies have reported metastasis confined to the liver in approximately 12–15% of patients with breast cancer [Citation26]. BCLM patients without treatment have a poor prognosis with a median survival of only 3–15 months [Citation27,Citation28]. Therefore, clinical treatment for BCLM should be timely and effective.
The detection rates and level of treatment for BCLM have recently improved [Citation29]. Treatment options for BCLM include systemic chemotherapy, surgical resection, TACE, and RFA, among others. Several studies [Citation30,Citation31] on surgical resection of BCLM have shown that the median overall survival time and the five-year survival rate were 17–57 months and 23–37%, respectively. Major complications occurred in 3.4–34% of patients. Surgical resection was performed for selected patients and these patients achieved clinical benefit [Citation32,Citation33]. In general, the inclusion criteria for surgery in BCLM patients are as follows [Citation30–33]: no extrahepatic metastasis or complete control of extrahepatic metastasis, normal liver function and normal performance conditions. However, many BCLM patients are ineligible for surgery due to extrahepatic metastatic disease, multiple liver metastases, and poor physical condition, among other factors [Citation30–33]. Therefore, the number of patients with BCLM who are eligible for surgery is limited [Citation34].
RFA was recently introduced as a minimally invasive therapy for liver metastasis, with improved survival outcomes in colorectal carcinoma liver metastasis [Citation35,Citation36]. RFA is less expensive, less invasive and has fewer contraindications than surgery. RFA treatment is also associated with lower major complication rates (surgery: 3.4–34 vs. RFA: 4–10%) in the treatment of BCLM [Citation12–14,Citation30–33]. Therefore, RFA can help control tumor progression and potentially prolong survival in patients with unresectable BCLM or in patients who reject surgery. In our study, we retrospectively analyzed the long-term overall survival and prognosis of 69 patients with BCLM who underwent percutaneous RFA treatment. These data may provide important information for clinical practice.
Technical efficacy was achieved in 90–95% of liver metastases in previous studies [Citation12,Citation14,Citation37,Citation38], which is similar to the 92.6% technical efficacy rate in our study. Furthermore, the local tumor progression rate was 11.6% in our study (the time between RFA and the last follow-up: median 26 months), which is slightly better than that observed in previous studies (18–25%; (follow-up: median 16.0–33.5 months). Regarding survival of BCLM after RFA, as previously reported [Citation12–14,Citation38], the median overall survival time and survival rates at one and three years were 29.9–33.5 months and 68–90 and 43–75%, respectively. In our study, the median overall survival time and survival rates at one and three years were 26 months and 81.3 and 25.3%, respectively. Survival outcomes in our study were less favorable than those in previous studies, possibly because 49.3% of the patients (34/69) in our group had multiple liver metastases (no more than four lesions) at the time of the initial RFA session, including 15 patients with two metastases and 19 patients with 3–5 liver metastases. In previous studies [Citation12,Citation14,Citation38], the mean number of metastases was 1.0–1.6, which is fewer than that in our study. Furthermore, the mean diameter of the BCLM tumors in our study was 2.9 ± 1.4 cm, which is larger than the 2.0–2.5 cm diameter reported in other studies [Citation12,Citation14,Citation38]. In addition, nearly half of the patients (46.4%) in our study had extrahepatic metastases, including 12 patients with multiple extrahepatic metastatic sites prior to RFA. Extrahepatic metastases predicted poor survival, even in the patients exhibiting local control of liver metastases.
The prognostic analysis in our study indicated that the important factors affecting survival included the maximum diameter of liver metastases, the number of metastases and extrahepatic metastatic disease. Long-term survival was related to the maximum diameter of metastasis, which is consistent with previous studies [Citation12,Citation38]. Meloni et al. [Citation12] reported survival results associated with RFA in 52 patients with BCLM and found that patients with tumors larger than 2.5 cm in diameter had a worse prognosis than those with tumors less than 2.5 cm tumors in diameter (hazard ratio, 2.1; p = .007). Veltri et al. [Citation38] analyzed 45 patients with 87 BCLM lesions (mean size 2.3 cm) and concluded that a liver metastasis diameter larger than 3 cm was a potential prognostic factor for local effectiveness. These authors also reported that complete ablation was achieved in 81% of patients with a liver metastasis diameter of <3 cm versus 43% of patients with a liver metastasis diameter larger than 3 cm. In our study, the patients with metastatic lesions ≤3 cm showed a higher survival rate than those with a maximum metastatic lesion diameter of 3.1–6 cm (p = .003). Therefore, the maximum diameter of metastatic lesions is an important factor when considering RFA treatment. In addition to tumour burden, the ability to achieve complete ablation with sufficient margins is also important for patient outcomes. According to the study reported by Shady et al. [Citation39], ablation margins larger than 5 mm are important for local tumor control, with no LTP observed for margins over 10 mm. In this study, we observed a similar result that a smaller margin size was associated with lower PFS (p = .017) and patients with a margin size >10 mm had no LTP during the follow-up. Also, ablations with margins larger than 5 mm had a better PFS. Thus, these data indicated the significant role of sufficient ablation size for tumor control, and we should make effort to create margins at least larger than 5 mm and ideally larger than 10 mm beyond tumors.
Additionally, the patients with a single liver metastasis survived longer than those with multiple liver metastases. In our study, the patients with 3–5 liver metastases had a median overall survival of 17 months, while those with 1–2 liver metastases had a median survival of 29 months (p = .030). Zhou et al. [Citation40] published clinical results of microwave ablation in 32 patients with gastric adenocarcinoma liver metastasis and found that the number of liver metastases, therapeutic modalities and the presence of extrahepatic metastases were independent prognostic factors in a multivariate regression analysis. Multiple liver metastases were associated with poor biological behavior and treatment difficulty, ultimately influencing the patients overall survival [Citation41].
Our study confirmed that the presence of extrahepatic metastatic disease indicated a worse prognosis for patients with BCLM. Shady et al. [Citation42] showed that more than one site of extrahepatic metastatic disease (p < .001) was an independent predictor of shorter overall survival, which is similar to our results. Jakobs et al. [Citation10] found that solitary bone metastases did not negatively affect the survival probability after RFA. In our study, the patients with lung metastasis seemed to have worse outcomes than those with bone metastasis; however, no significant difference was observed. Therefore, these patients may need to be treated differently. In surgical reports, an interval between breast cancer diagnosis and BCLM of less than 24 months was associated with a poor prognosis [Citation43,Citation44]. In a study by Selzner et al. [Citation45], an interval between breast cancer diagnosis and BCLM of less than 12 months indicated a poor prognosis, with a median overall survival of only nine months. In our study, we observed no statistical effect on overall survival, which was most likely due to the high percentage of our patients who already had extrahepatic metastasis at the time of RFA treatment. However, the median overall survival after diagnosis and after RFA of BCLM was 36 and 26 months, respectively (p = .004). These data provide evidence that early effective treatment of BCLM is beneficial to overall survival.
Based on our experience using RFA in BCLM patients, we developed the following recommendations. (1) A close and regular follow-up is helpful for early detection of metastasis after breast cancer resection. (2) The focus should be on the high risk for relapse population after RFA treatment, including patients with liver metastatic lesions larger than 3 cm, multiple liver metastases or concomitant extrahepatic metastatic disease. (3) Standardized and effective adjuvant treatment strategies should be adopted before and after RFA.
There are several limitations to our study. First, this study was a retrospective study, and patient selection from the single specialized center may have inevitably led to bias. This study included a relatively small sample size and a certain level of heterogeneity within the study group (e.g., patient gender). Second, tumors larger than 3 cm were included in this study, which may have resulted in worse overall survival. A total of 47.8% of the BCLM patients (33/69) in our study had tumor diameters of 3.1–6 cm and this percentage of larger tumors is higher than that in other centers. Third, our study was conducted over a long period of time (from 2000–2015). Because clinical experience and skill in RFA may improve over time, patients who were enrolled later in the study may have received better treatment. Moreover, extrahepatic metastases were observed in nearly half of the patients in our study. However, these extrahepatic metastases were stable and controlled before RFA treatment. Furthermore, overall survival was more dependent on the disease course and systemic options than on local therapy alone. We should not ignore the effect of systemic treatment on long-term outcomes in BCLM patients.
In conclusion, our study demonstrated that adding percutaneous RFA to systemic chemotherapy achieved local control in patients with BCLM. BCLM patients with tumors less than 3 cm in diameter and a single liver metastasis without other metastatic sites showed better survival outcomes. In the study, patients with a margin size >10 mm had no local tumor progression. For patients diagnosed with large metastatic lesions in the liver (> 5 cm) or multiple liver metastases (> 5 lesions), intra-arterial therapies can be considered, including TACE, doxorubicin-eluting beat TACE and radioembolization.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67–78.
- Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624.
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN guidelines insights: breast cancer, Version 1.2017. J Natl Compr Cancer Netw. 2017;15:433.
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644.
- Belda T, Montalvá EM, Lópezandújar R, et al. Role of resection surgery in breast cancer liver metastases: experience over the last 10 years in a reference hospital. Cirugía Española. 2010;88:167–173.
- Ma R, Feng Y, Lin S, et al. Mechanisms involved in breast cancer liver metastasis. J Transl Med. 2015;13:64.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958.
- Jin K, Gao W, Lu Y, et al. Mechanisms regulating colorectal cancer cell metastasis into liver (Review). Oncol Lett. 2012;3:11–15.
- Jakobs TF, Hoffmann RT, Schrader A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32:38–46.
- Sofocleous CT, Nascimento RG, Gonen M, et al. Radiofrequency ablation in the management of liver Metastases from breast cancer. AJR Am J Roentgenol. 2007;189:883.
- Meloni MF, Andreano A, Laeseke PF, et al. Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation-intermediate and long-term survival rates. Radiology. 2009;253:861–869.
- Bergenfeldt M, Jensen BV, Skjoldbye B, et al. Liver resection and local ablation of breast cancer liver metastases – A systematic review. Eur J Surg Oncol. 2011;37:549–557.
- Lawes D, Chopada A, Gillams A, et al. Radiofrequency ablation (RFA) as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88:639.
- Kitagawa D, Horiguchi S, Yamashita T, et al. Comparison of outcomes between women with de novo stage IV and relapsed breast cancer. J Nippon Med Sch. 2014;81:139–147.
- Khanfir A, Lahiani F, Bouzguenda R, et al. Prognostic factors and survival in metastatic breast cancer: a single institution experience. Rep Pract Oncol Radiother. 2013;18:127–132.
- Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients-mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260.
- Chen MH, Yang W, Yan K, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006;17:671–683.
- Yan K, Yang W, Chen MH, et al. Clinical application of hepatic wedge ablation for treating liver malignancies of the inferior margin: a new ablation technique. Int J Hyperthermia. 2017;33:203–211.
- Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33:428–436.
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34: 11–29.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Ito Y, Miyashiro I, Ito H, et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 2014;105:1480–1486.
- Cowppli-Bony A, Uhry Z, Remontet L, et al. Survival of solid cancer patients in France, 1989–2013: a population-based study. Eur J Cancer Prev. 2017;26:461–468.
- Kamby C, Dirksen H, Vejborg I, et al. Incidence and methodologic aspects of the occurrence of liver metastases in recurrent breast cancer. Cancer. 1987;59:1524–1529.
- Bacalbaşa N, Balescu I, Dima S, et al. Long-term survivors after liver resection for breast cancer liver metastases. Anticancer Res. 2015;35:6919–6923.
- Rubino A, Doci R, Foteuh JC, et al. Hepatic metastases from breast cancer. Updates Surg. 2010;62:143–148.
- Ward EM, Desantis CE, Lin CC, et al. Cancer statistics: breast cancer in situ. Cancer J Clin. 2015;65:481–495.
- Bacalbasa N, Dima SO, Purtanpurnichescu R, et al. Role of surgical treatment in breast cancer liver metastases: a single center experience. Anticancer Res. 2014;34:5563.
- Abbott DE, Brouquet A, Mittendorf EA, et al. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery. 2012;151:710–716.
- Sadot E, Lee SY, Sofocleous CT, et al. Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg. 2016;264:147.
- Spolverato G, Vitale A, Bagante F, et al. Liver resection for breast cancer liver metastases: a cost-utility analysis. Ann Surg. 2017;265:792.
- Illing R, Gillams A. Radiofrequency ablation in the treatment of breast cancer liver metastases. Clin Oncol. 2010;22:781–784.
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818.
- Liu M, Huang G, Xu M, et al. Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia. 2017;33:446– 433.
- Gunabushanam G, Sharma S, Thulkar S, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol. 2007;18:67–72.
- Veltri A, Gazzera C, Barrera M, et al. Radiofrequency thermal ablation (RFA) of hepatic metastases (METS) from breast cancer (BC): an adjunctive tool in the multimodal treatment of advanced disease. Radiol Med. 2014;119:327–333.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29:268–275.
- Zhou F, Yu XL, Liang P, et al. Microwave ablation is effective against liver metastases from gastric adenocarcinoma. Int J Hyperthermia. 2017;33:1–23.
- Tabaries S, Dong Z, Annis MG, et al. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene. 2011;30:1318–1328.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278:601–611.
- Duan XF, Dong NN, Zhang T, et al. The prognostic analysis of clinical breast cancer subtypes among;patients with liver metastases from breast cancer. Int J Clin Oncol. 2013;18:26.
- Caralt M, Bilbao I, Cortés J, et al. Hepatic resection for liver metastases as part of the "oncosurgical" treatment of metastatic breast cancer. Ann Surg Oncol. 2008;15:2804.
- Selzner M, Morse MA, Vredenburgh JJ, et al. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127:383–389.
