Abstract
Purpose: Comparison between different thermal ablation systems for thyroid nodules regarding their different procedural characteristics such as treatment-time, number of shots and energy transmission in the context of their clinical performance such as complication rate and volume reduction after three months.
Methods: A total of 60 patients with 65 nodules underwent thermal ablation of thyroid nodules with either microwave ablation (MWA) (9 male, 15 female and mean age 57 ± 13 years) or radiofrequency ablation (RFA) (12 male, 24 female and mean age 54 ± 12 years).
Results: Mean initial volume (MWA: 23.90 ± 17.35 ml; RFA: 29.44 ± 30.09 ml), energy transmission (MWA: 13.56 ± 10.17 kJ; RFA: 15.12 ± 13.45 kJ), energy transmission per ml (MWA: 0.85 ± 1.01 kJ/ml; RFA: 0.65 ± 0.32 kJ/ml), power (MWA: 22.69 ± 12.32 J/s; RFA: 20.97 ± 7.86 J/s) and duration of ablation (MWA: 618 ± 304 s; RFA: 695 ± 463 s) were not statistically different (p > .05). MWA required significantly less shots (MWA: 3 ± 1; RFA: 6 ± 3) than RFA (p < .05). At three-months follow-up a significant mean nodular volume reduction of 53.54 ± 15.40% after MWA and 51.21 ± 16.58% after RFA (p < .05) was measured. However, mean nodular volume reduction was not significantly different between both systems (p > .05). One patient treated by MWA reported a transient Horner’s syndrome, which recovered without any further treatment. Major complications such as nodule rupture, infection or persisting nerve injuries did not occur.
Conclusion: Both systems are suitable to treat thyroid nodules and show no significant difference in the duration of application, energy transmission and volume reduction. However, MWA requires less shots to treat the whole nodule.
Introduction
Minimal-invasive treatments as an alternative to thyroid surgery for benign thyroid lesions are increasingly used in clinical everyday practice [Citation1,Citation2]. Besides radiofrequency ablation (RFA), common minimal-invasive treatments such as laser (LA) and ethanol ablation (EA) or novel systems like microwave ablation (MWA) are used to treat benign thyroid nodules. In comparison to RFA, ethanol ablation is reported to be superior in treating dominantly cystic nodules but showed less effectiveness in solid nodules [Citation3,Citation4]. A recent meta-analysis evaluating RFA and LA reported that both modalities are effective in treating solid nodules, with RFA being superior to LA in decreasing thyroid nodule volume after six months [Citation5]. Concerning MWA and RFA, several studies have shown that they are effective in treating both cystic and solid nodules with volume reduction rates between 56–74.4% [Citation6,Citation7] and 54.23–74% after three months [Citation8,Citation9], respectively. A recent propensity score matching study compared bipolar RFA and MWA and reported that the volume reduction ratio (79.4 vs. 77.2%, p = .108 after six months and 83.6 vs. 81.6%, p = .144 after 12 months, for RFA and MWA, respectively), therapeutic success rate, symptom and cosmetic score and complications related to treatment for the two techniques were equivalent [Citation10]. Further results were recently published in a prospective multicenter study, that compared monopolar RFA and MWA. They reported no significant differences in volume reduction rate (VRR) between RFA and MWA at the three-month-follow-up (67.6 ± 20.3 vs. 64.4 ± 43.5% (p = .143), respectively), whereas at the six-month-, 12-month- and last follow-up the VRR of the RFA group and MWA group were significantly different (84.1 ± 13.5 vs. 78.4 ± 48.2% (p = .016), 89.6 ± 20.0 vs. 82.5 ± 49.7% (p = .035) and 91.3 ± 12.6 vs. 81.1 ± 70.4% (p = .045), respectively) [Citation11].
RFA can either be performed using monopolar or bipolar systems [Citation12], whereby most interventions are still performed using monopolar systems that have been thoroughly investigated and proved to be effective [Citation13]. In contrast, only little attention has been paid to bipolar thyroid nodule ablations. In fact, bipolar systems are promising, as they do not require additional grounding pads, thereby providing several advantages like less systemic heating effects and a higher electrical current density between electrodes, which leads to larger and more reproducible ablation zones [Citation14].
Besides RFA, MWA represents one of the latest thermal ablation techniques and is mainly used to treat hepatocellular carcinoma [Citation15]. Several authors assume that MWA also provides advantages over RFA. Since MWA uses electromagnetic radiation to heat the nodular tissue, several authors assume, that MWA (in comparison to RFA) is less susceptible to local differences in impedance, provides a faster temperature rise and higher temperatures and thereby results in larger ablation zones [Citation16,Citation17].
Currently, the most commonly used ablation technique for MWA and RFA of benign thyroid nodules is the moving shot technique (MST) [Citation10,Citation18]. A large multicenter study compared MWA with RFA using MST; both methods efficiently treated thyroid nodules [Citation11]. In previous studies, ablation techniques such as the multiple overlapping shot technique (MOST) have been reported to show promising results in thyroid as well [Citation6,Citation19,Citation20]. In comparison with MST, MOST does not require continuous movement of the antenna/electrode. Thus, MOST is less challenging for the operating physician and may reduce the rate of complications. However, to date there has not been any study comparing MST and MOST for ablation of thyroid nodules. While both systems (RFA and MWA) are feasible to treat thyroid nodules [Citation11], their operating principles are entirely different. The aim of this study was to compare two thermal ablation systems for thyroid nodules regarding their procedural characteristics such as treatment-time, number of shots and energy transmission in context of clinical characteristics such as complications and volume reduction after three months.
Material and methods
This retrospective study was approved by the local ethics committee and written informed consent was obtained from each patient.
Patients
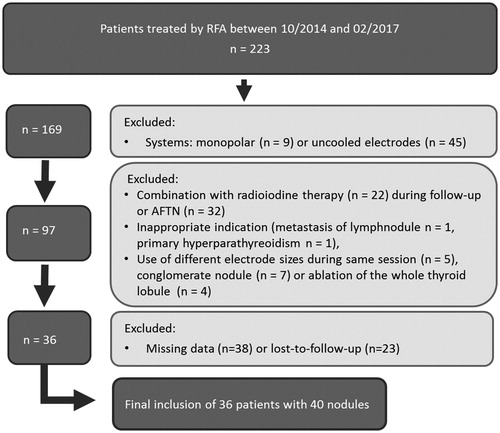
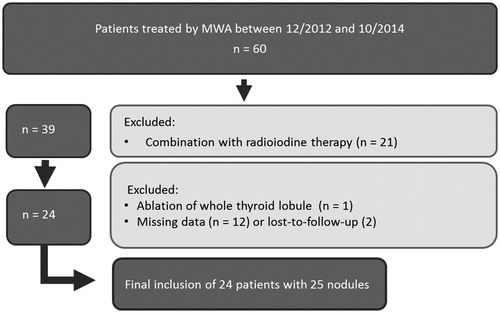
During the enrolment period (MWA: 12/12 – 10/14; RFA: 10/14 – 02/17), 60 patients were treated by MWA and 223 by RFA. Due to exclusion criteria, a total of 60 patients with 65 nodules were included in this study (). Among them, 24 patients (nine male, 15 female, mean age 57 ± 13 years) with 25 nodules were treated by MWA and 36 patients (12 male, 24 female, mean age 54 ± 12 years) with 40 nodules were treated by bipolar RFA. Ablations were performed by a single, experienced physician. Patients included in this study did not receive any further thyroid treatment during the three month follow-up period to avoid confounding variables.
Treatment condition for percutaneous thermal ablation
Inclusion criteria (≥ 1)
(I.) Mainly non-symptomatic thyroid nodules only causing cosmetic problems; (II.) Symptomatic patients complaining about swallowing problems, hoarseness, breathlessness or pain and (III.) Refusal of surgery or contraindications against surgery
Exclusion criteria
(I.) Excessive thyroid volume with retrosternal growth including contiguous position to vessels, esophagus, trachea or nerves; (II.) Fine needle aspiration biopsy (FNAB) with cytological signs of follicular dysplastic proliferations; (III.) Pathologic mismatch in 99mTc MIBI-scintigraphy as a sign of malignancy and (IV.) Absence of symptoms
Microwave system
MWA was performed using a system (MedWaves Avecure™ Microwave Generator, MedWaves, San Diego, CA), that operates at a frequency between 902 to 928 MHz and achieves a maximum temperature of up to 140 °C. Different needle antennas (MedWaves Avecure™ Microwave Probes, MedWaves) were used: small (width: 16 G; antenna length: 1 cm), medium (width: 14 G; antenna length: 1.6 cm) or large antennas (width: 14 G; antenna length: 2 cm; ). The system operates without an additional internal cooling system. To induce maximum cell damage and minimize the risk of unintended harms, a temperature of up to 80 °C with an output of 24–36 W was aimed for.
Table 1. Measured data of uncooled MW antennas.
Radiofrequency system
The system used in this study (CelonLab POWER, Olympus, Hamburg, Germany) operates at a frequency of 470 ± 10 kHz and uses an impedance feedback mechanism to adjust the transmitted energy that can be increased to a maximum output of 250 W. The energy was applied using cooled electrodes with a probe length of 100 mm and a width of 18 G. The active tip length ranged between 20 and 40 mm (CelonProSurge, Olympus; ()). The active tip contains two electrodes, which are separated by an isolator. In consequence, no additional grounding pads need to be applied. In order to cool the electrode, water runs through two additional lumina within the electrode at a rate of up to 30 ml/min.
Table 2. Measured data of cooled RF electrodes.
Ultrasound equipment
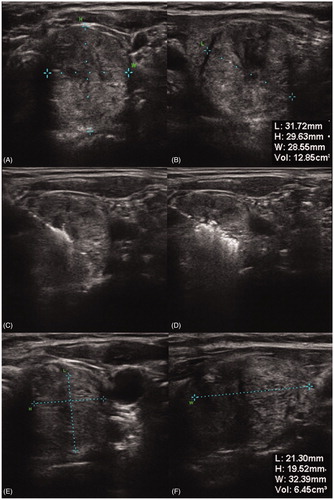
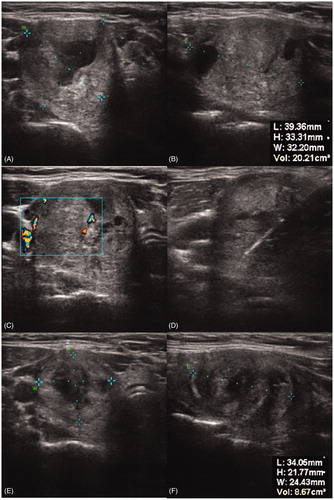
The ultrasound system SonixTOUCH (Ultrasonix Medical Corporation, Richmond, BC, Canada) was used to determine the shape, size and location of the thyroid nodule. Furthermore, ultrasound elastography was used as an additional tool to predict the dignity of the nodule, where low-strain values highly correlate with an increased risk of malignancy [Citation21]. During the treatment it was used to assure a correct electrode/probe placement and monitor the induced ablation zone.
Ablation protocol – multiple overlapping shot technique
Both, MWA and RFA were performed using the multiple overlapping shot technique.
Prior to the procedure, the largest possible active tip length was chosen according to the diameter of the nodule. After choosing the appropriate electrode/antenna, the whole length of the active tip was placed within the nodule. As the ablation was started, the antenna/electrode was not moved, until the ablation was stopped due to local impedance increase or if the margin of the nodule was reached. Afterwards the antenna/electrode was repositioned next to the previous ablation zone and the procedure was repeated until the whole nodule was treated.
Assessment
During each treatment, the number of shots, power output, energy transmission per shot and the total application of energy was measured. Additionally, the duration of each shot, as well as the duration of energy application was recorded.
Statistical analysis
Statistical results were obtained using BiAS, version 10.04 (Epsilon Verlag, Germany). Wilcoxon-Mann-Whitney-U-test was performed to evaluate differences between the measured data of RFA and MWA. The level of significance was set at <.05. All values are reported as mean ± standard deviation.
Results
Procedure
The groups mean initial volume (MWA: 23.90 ± 17.35 ml; RFA: 29.44 ± 30.09 ml) did not significantly differ (p > .81). The total energy transmission (MWA: 13.56 ± 10.17 kJ; RFA: 15.12 ± 13.45 kJ), the power (MWA: 22.69 ± 12.32 J/s; RFA: 20.97 ± 7.86 J/s), as well as the total duration of ablation (MWA: 618 ± 304 s; RFA: 695 ± 463 s) was not statistically different (p > .96, > .64 and >0.64, respectively). Based on the initial volume, the mean transmitted energy per ml, the ‘specific energy’, was calculated (MWA: 0.85 ± 1.01 kJ/ml; RFA: 0.65 ± 0.32 kJ/ml), which also did not significantly differ between the groups (p > .97). MWA required significantly less shots (MWA: 3 ± 1; RFA: 6 ± 3) than RFA to treat the whole nodule (p < .01; ).
Volume reduction
With a mean volume of 11.51 ± 9.33 and 14.45 ± 14.56 ml after three months, corresponding to a relative volume reduction of 53.54 ± 15.40 and 51.21 ± 16.58% in MWA and RFA, respectively, both systems reached a significant (p < .001) volume reduction at follow-up, although the differences between both systems were not significant (p > .65) ().
Complications
Both treatments were well tolerated, with the most common symptom being a slight heat sensation in the neck, which was reported by most of the patients. Sympathetic nerve injury occurred in one patient treated by MWA. The patient showed a transient Horner’s syndrome after the procedure, which recovered within three months. Further major complications such as nodule rupture, infection or persisting nerve injuries did not occur.
Discussion
As shown in both groups consisted of nodules with comparable nodule size, which allows an adequate evaluation of characteristics such as energy transmission, number of applications and treatment time. The mean transmitted energy, as well as the specific energy were statistically similar between RFA and MWA, indicating that nodules in both groups received a comparable amount of energy. Besides that, the duration of application as well as the mean power showed no significant differences.
These results are in conflict with previous studies describing MWA as the more powerful and more effective system [Citation16,Citation22]. As microwaves are penetrating through biological tissue with less dependency on electrical conductivity, higher temperatures and faster ablations are possible [Citation16,Citation22]. This has been confirmed in a previously published comparison between MWA and bipolar RFA, showing that MWA created larger lesions in less time and applied more energy than bipolar RFA [Citation23]. According to this study, a significant difference in energy transmission and duration of application would have been suggested. However, the problem is that these advantages are rather theoretical and not completely applicable in thyroid ablation. In practical use, the average power is more similar in MWA and RFA (MWA: 22.69 ± 12.32 J/s; RFA: 20.97 ± 7.86 J/s), if it is adapted on the patients tolerance. Despite higher power settings would have been possible, patients would not have tolerated an increase. With a maximum of 80 °C, the operating temperature during ablation was limited below its maximum of 140 °C [Citation24,Citation25]. An average power output of 24–36 W during the ablation was targeted. This yielded a maximum temperature of 80 °C, which is substantially below the maximum of 140 °C. Whereas the higher temperature range is acceptable for hepatic tumours, those temperatures would not have been tolerated by patients in this scenario. In our study, we reported one nerve injury resulting in a Horner’s Syndrome, which was seen to recover within three months without any further treatment. Horner’s Syndrome is typically caused by an injury of the middle cervical sympathetic ganglion (CSG), which is usually located at the lower part of the thyroid. It results in an ipsilateral combination of ptosis, miosis and enophthalmos as well as an anhydrosis of the ipsilateral face [Citation26]. In order to prevent an injury of the middle CSG, ultrasound examination prior to ablation is mandatory. Although it can be visualized in only 41%, it is usually located either lateral or in some cases medial to the common carotid artery [Citation27]. Therefore, cautious tracing of the electrode and antenna is important, which should never exceed the margin of the targeted nodule. Additionally, hydrodissection can be performed to increase the distance between vulnerable structures and the targeted nodule [Citation28,Citation29]. In RFA thermal convective heat-loss through large cervical vessels, the so called ‘heat-sink-effect’, may protect vulnerable structures around vessels, especially nerves such as the medial CSG or vagus nerve. In contrast, MWA is less affected by heat-sink-effects, thus the surrounding tissue is more at danger () [Citation22,Citation30]. While the additional convective heat-loss through contiguous vessels limits thermal ablation of malignant neoplasia due to incomplete treatment, vital thyroid tissue and surrounding structures may benefit from its thermal protection. Additionally, heating phenomena along the shaft pose a problem during ablation and is reported in MW systems () [Citation22,Citation31]. Some of the emitted microwaves are reflected and expand along the shaft in reverse direction (‘comet effect’). This effect can be reduced by using additional chokes behind the active tip or internal cooling systems to reduce the heat spread and complications [Citation32–34]. As the MW system used in this study neither used chokes nor internal cooling systems, the ablation was observed more critically. Also RFA benefits from internal cooling systems as used in this study, because they may help to reduce the risk of unintended damage through shaft heating as well as to limit overshooting temperatures and carbonization [Citation14].
Figure 3. Illustration of different shapes and sizes of thermal ablative zones in thyroid. Left part of the illustration: Cooled RF electrode producing ellipsoid shaped ablation zones. Less heating along the shaft, which results in less damage of passed structures like the skin or M. Sternocleidomastoideus. Heat-sink-effects by large vessels (A and V) protecting contiguous nerve structures such as Vagus nerve (III) or middle cervical sympathetic ganglion (II). Right part of the illustration: uncooled MW antenna inducing ‘tear-drop’ shaped ablation zones as a result of additional heating effects along the shaft. Less heat-sink-effects next to vulnerable structures like middle cervical sympathetic ganglion (II) or Vagus nerve (III) with possible higher risk of unintended harms. I: recurrent laryngeal nerve; II: middle cervical sympathetic ganglion; III: vagus nerve; A: common carotid artery; V: internal jugular vein.
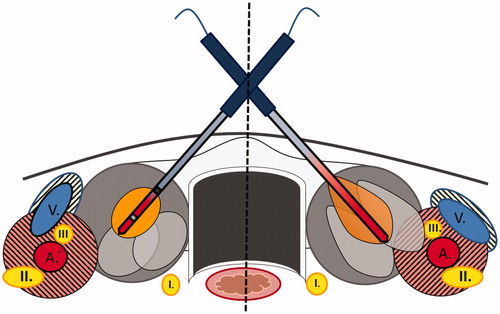
The main procedural difference between both systems are the number of shots. MWA required significantly less shots than bipolar RFA to treat an entire nodule. Microwaves, especially with a wavelength around 915 MHz are reported to show a high penetration depth, thereby creating large ablation zones [Citation22]. In contrast, the RF power deposition is reported to attenuate rapidly away from the RF electrode [Citation22]. Furthermore, microwaves are less depending on local water content or increasing resistance as much as RF systems [Citation16], thus the ablation was still performed after some parts of the tissue charred or desiccated. In consequence, MWA is able to treat thyroid nodules with less shots than RFA. After every shot, the antenna/electrode is repositioned, which may cause pain or sensible sensations, thus a low number of shots is associated with a decrease of discomfort.
Concerning the volume reduction after three months, both systems can provide a significant volume reduction of thyroid nodules, although a significant difference between both systems was not found (e.g., ). The similar volume reduction may be a result of the similar energy transmission. A previously published study reported a positive correlation between energy transmission and volume reduction after three months and assumed, that the energy transmission plays an important role in predicting the therapeutic response [Citation19]. These results are in accordance to a recently published study by Yue et al. [Citation10], who found no significant differences between bipolar RFA and MWA in volume reduction ratio. They explained this by the fact that both modalities operated at a similar ablation power (RFA 20–25 W, MWA 20–35 W), which lessens the difference in thermal effects between both systems. In comparison to this study, we used MOST technique instead ofMST. Despite MST being the more common ablation technique, MOST resulted in a significant volume reduction in both systems; similar results were observed compared to prior reports [Citation10,Citation35]. The use of MOST in combination with internally cooled, bipolar electrodes as well as MW antennas enabled a sufficient treatment of both small- and large nodules with a volume greater than 50 ml (). While no major complications in patients treated by RFA were reported, MWA resulted in one transient Horner’s syndrome. MOST is relatively simple because the antenna/electrode is not moved during the procedure. Further, eliminating electrode movement may reduce complications. However, because, the electrode is left longer at the same position, this may increase the risk of thermal damage as a result of an enlarging ablation zone. MWA is less effected by heat-sink effects, but such effects may protect vulnerable structures during ablation [Citation16,Citation22]. A clinical comparison between MST and MOST, which includes RFA and MWA has yet to be reported. Thus a final decision about the best ablation technique in each system is not yet determined. This study has several limitations. First, we included various sized devices. In order to adequately compare the treatment characteristics, further studies evaluating the respective sizes are necessary to finally determine the treatment characteristics of RFA and MWA. Second, this is a retrospective study with a follow-up of just three months. A recent multicenter study that compared MWA and RFA showed that the volume-reduction after three months was similar, but RFA achieved significantly greater maximal diameter reduction ratio and volume reduction ratio at the six-month, 12-month and last follow-up [Citation11]. Therefore, further studies with a prospective design including long-term follow-ups and larger populations are necessary as significant differences between both systems may only occur at long-term follow-ups. Furthermore, a longer follow-up is necessary as recurrence of thyroid nodules after thermal ablations is a current issue. Previous long-term studies (3–5 y) of LA [Citation36,Citation37] and RFA [Citation38] revealed, that the regrowth of treated nodules is commonly detected 1–2 y after ablation and already suggested the factors related to the recurrence of treated nodules after LA, RFA or MWA [Citation39–41]. Lastly, also patients with cystic nodules were enrolled. Despite previous studies reporting the efficacy of both thermal ablation and percutaneous ethanol ablation [Citation3,Citation4,Citation7], current medical guidelines only recommend ethanol ablation for relapsing benign cystic nodules as a first-line therapy [Citation2].
Conclusion
Both systems are suitable to treat thyroid nodules and show no significant difference in the duration of application and energy transmission. With a mean volume reduction of 53.54 ± 15.40 and 51.21 ± 16.58% in MWA and RFA (p < .001), respectively, both systems are effective in treating thyroid nodules, although there were no significant differences between both groups. In comparison to RFA, the number of shots needed to treat the whole nodule is significantly reduced in MWA (p < .01). The risk of unintended harms may be increased in uncooled MWA as the system is less affected to heat-sink-effects and shows an increased risk of shaft heating.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gharib H, Hegedüs L, Pacella CM, et al. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98:3949–3957.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract. 2016;22:1–60.
- Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013;269:293–300.
- Sung JY, Kim YS, Choi H, et al. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol. 2011;196:W210–W214.
- Ha EJ, Baek JH, Kim KW, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. 2015;100:1903–1911.
- Kohlhase KD, Korkusuz Y, Gröner D, et al. Bipolar radiofrequency ablation of benign thyroid nodules using a multiple overlapping shot technique in a 3-month follow-up. Int J Hyperth. 2016;32:511–516.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250.
- Korkusuz H, Happel C, Heck K, et al. Percutaneous thermal microwave ablation of thyroid nodules. Nuklearmedizin. 2014;53:123–130.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82:e11–e16.
- Yue W-W, Wang S-R, Lu F, et al. Radiofrequency ablation vs. microwave ablation for patients with benign thyroid nodules: a propensity score matching study. Endocrine. 2017;55:485–495.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7:9554.
- Nakada SY, Jerde TJ, Warner TF, et al. Bipolar radiofrequency ablation of the kidney: comparison with monopolar radiofrequency ablation. J Endourol. 2003;17:927–933.
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540.
- Bruners P, Lipka J, Günther RW, et al. Bipolar radiofrequency ablation: is the shape of the coagulation volume different in comparison to monopolar RF-ablation using variable active tip lengths?. Minim Invasive Ther Allied Technol. 2008;17:267–274.
- Groeschl RT, Pilgrim CHC, Hanna EM, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014;259:1195–1200.
- Ahmed M, Brace CL, Lee FT, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369.
- Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21:4278–4283.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125.
- Korkusuz Y, Kohlhase K, Gröner D, et al. Microwave ablation of symptomatic benign thyroid nodules: energy requirement per ml volume reduction. Rofo. 2016;188:1054–1060.
- Korkusuz Y, Mader A, Gröner D, et al. Comparison of mono- and bipolar radiofrequency ablation in benign thyroid disease. World J Surg. 2017;41:2530–2537.
- Vorländer C, Wolff J, Saalabian S, et al. Real-time ultrasound elastography—a noninvasive diagnostic procedure for evaluating dominant thyroid nodules. Langenbeck’s Arch. Surg. 2010;395:865–871.
- Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38:65–78.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79:124–130.
- Yang D, Converse MC, Mahvi DM, et al. Measurement and analysis of tissue temperature during microwave liver ablation. IEEE Trans Biomed Eng. 2007;54:150.
- Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874.
- Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015;16:749–766.
- Shin JE, Baek JH, Ha EJ, et al. Ultrasound features of middle cervical sympathetic ganglion. Clin J Pain. 2015;31:909–913.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25:163–170.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27:3128–3137.
- Bhardwaj N, Dormer J, Ahmad F, et al. Microwave ablation of the liver: a description of lesion evolution over time and an investigation of the heat sink effect. Pathology. 2011;43:725–731.
- Lubner MG, Brace CL, Ziemlewicz TJ, et al. Microwave ablation of hepatic malignancy. Semin Intervent Radiol. 2013;30:056–066.
- Longo I, Gentili GB, Cerretelli M, et al. A coaxial antenna with miniaturized choke for minimally invasive interstitial heating. IEEE Trans Biomed Eng. 2003;50:82–88.
- Cavagnaro M, Amabile C, Bernardi P, et al. A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans Biomed Eng. 2011;58:949–959.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology. 2009;251:933–940.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18:615–623.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20:1253–1261.
- Døssing H, Bennedbaek FN, Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011;165:123–128.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23:1044–1049.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int. J. Hyperth. 2017;33:911–919.
- Baek JH. Factors related to the efficacy of radiofrequency ablation for benign thyroid nodules. Ultrasonography. 2017;36:382–385.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int. J. Hyperth. 2017;33:905–910.