Abstract
Purpose: To evaluate the safety and efficacy of transarterial chemoembolization (TACE) combined with cryoablation (TACE-cryoablation) in large (main tumor ≥5 cm in diameter) hepatocellular carcinomas (HCCs).
Methods: From January 2010 to December 2015, 56 patients were treated with combination therapy via a single TACE session followed by one to three percutaneous cryoablation sessions twice a week (TACE-cryoablation group). A total of 54 patients were treated with TACE alone for two to six sessions once a month (TACE group). The decision between TACE and TACE cryoablation was based on patient choice. Outcomes of patients in two groups were compared according to the largest tumor diameter (subgroup): Group A (5 cm ≤ tumor <10 cm), Group B (10 cm ≤ tumor <15 cm), and Group C (tumor ≥15 cm).
Results: The mean number of cryoablation sessions per patient was 2.3 (range: 1–6). Within Group B, TACE-cryoablation significantly improved survival compared with TACE alone (11.0 vs 6.0 months; p = .008). This was also seen in Group C (8.0 vs 5.0 months; p = .001). However, no significant difference was noted in Group A (17.0 vs 13.0 months; p = .674). The complications related to TACE were comparable between the two groups. Two adverse events of grade 3 − 4 related to cryoablation occurred in two patients (3.6%). The independent prognostic factors for survival included: TACE cryoablation, AFP level, main tumor size and extrahepatic metastasis.
Conclusions: TACE-cryoablation may improve overall survival in patients with HCC who presented with a tumor diameter ≥10 cm, with minimal complications, when compared with TACE alone.
Introduction
Hepatocellular carcinoma (HCC) is one of the most serious life-threatening malignancies worldwide. In general, 80–90% of HCCs are related to cirrhosis, regardless of aetiology [Citation1]. In the United States and in many countries of Europe, the main aetiological factor is the hepatitis C virus (HCV), while hepatitis B virus (HBV) is the most common cause in parts of Africa and Asia [Citation2]. The majority of HCC tumors, particularly in China, are large- or giant- sized (over 5 or 10 cm in the largest diameter of the main lesion), owing to a lack of screening and awareness programs for early detection [Citation3]. Tumor size is a predictor of tumor stage progression, which needs to be considered in patient management and understanding a patient’s prognosis [Citation4]. Most HCCs over 10 cm are at an intermediate and advanced stage (Barcelona Clinic Liver Cancer [BCLC] stages B and C), with intra- or extra-hepatic metastases and portal vein invasion [Citation3]. In addition, the membrane surrounding the tumor is always incomplete, which leads to a high rate of recurrence [Citation3,Citation4]. The recurrence, in turn, can accelerate local spread and lead to deterioration of liver function [Citation5,Citation6]. The presence of large HCC limits treatment options, such as liver transplantation and resection, and the management remains a major medical challenge.
Transcatheter arterial chemoembolization (TACE) by using various combinations of chemotherapeutic drugs and embolic agents has been widely used in the management of large HCCs [Citation7]. However, residual tumor cells often remain viable after TACE treatment, and complete tumor necrosis occurred only in 16.9% of the patients [Citation8]. Repetition of the procedures is necessary to control tumor growth. However, repeated TACE may cause liver function to worsen because noncancerous liver parenchyma is also damaged [Citation7]. The response rates of TACE are generally poor and the 5-year survival rate is less than 20% [Citation9]. As a result, tumor ablation is still a necessary component in the treatment of large tumors as a possible locally curative therapy after effective TACE treatment [Citation10].
Cryoablation has become one of the most promising loco-regional therapies for the treatment of HCC, which is believed to ablate cancer cells with many advantages, including the following: formation of a visual ice-ball [Citation11], activation of cryoimmunology in cancer [Citation12], lack of severe damage to large blood vessels [Citation13], and no association with severe pain [Citation14,Citation15]. A recent study has reported that, for the treatment of one or two HCC lesions ≤4 cm, cryoablation was equally safe and effective, and resulted in a significantly lower local tumor progression when compared with radiofrequency ablation (RFA) [Citation16]. Although cryoshock is a serious complication in patients with cirrhosis who undergo cryoablation when the diameter of the area frozen exceeds 6 cm, the incidence of the phenomenon is rare and has garnered much attention from clinical researchers [Citation17,Citation18]. Previous studies have also reported that cryoablation is a promising approach with acceptable complications for the treatment of large HCCs. This contrasts with other local ablations, such as RFA, which may not reliably destroy tumors greater than 5 cm in diameter [Citation19–22]. However, few reports have compared combined TACE and cryoablation (TACE cryoablation) with TACE alone for the treatment of large HCCs. Here, we present our retrospective study of 110 patients with large HCCs. The purpose of our research was to evaluate the safety of combination therapy and compare the associated survival benefits with those of patients receiving TACE alone.
Materials and methods
Study design
Between January 2010 and December 2015, the medical records of consecutive patients who underwent TACE cryoablation or TACE alone for large HCC were retrospectively analysed at the First Affiliated Hospital of Sun Yat-sen University. Accessing the database and methods of data retrieval and analysis was approved by the ethics committee of our hospital. The study was carried out in accordance with the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective design.
The diagnosis of HCC was based on non-invasive criteria according to the disease guidelines developed by the European Association for the Study of the Liver and the American Association for the Study of Liver [Citation23]. Based on the tumor diameter, these patients were divided into three subgroups: Group A (5 cm ≤ tumor size <10 cm), Group B (10 cm ≤ tumor size <15 cm), and Group C (≥15 cm). Patients who met all of the following criteria were included in the analysis: (1) age between 18 and 75 years, (2) main lesion with a diameter of over 5 cm measured by computed tomography (CT) or magnetic resonance imaging (MRI), with or without daughter lesions, (3) Child-Pugh classification A or B, (4) Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–2, and (5) no history of previous treatment. Exclusion criteria were patients who had a secondary malignancy and those who underwent other treatment methods (sorafenib, RFA, systemic chemotherapy) during this study.
Before these patients received the initial TACE, the combination TACE-cryoablation treatment strategy was recommended by the attending physicians (J.L. and Y.W.) with 20 and 16 years of experience, respectively, in interventional radiology. If the patient agreed to the physician’s recommendation, computed tomography (CT)-guided percutaneous cryoablation treatments were administered after the first TACE. Patients who refused cryoablation underwent TACE only. According to the treatment strategy, patients included in this study were divided into two groups: the TACE-cryoablation group and the TACE group.
TACE procedure
TACE was carried out by using techniques we described previously [Citation24] by our three radiologists (J.L., Y.W., and W.F.), who had 8–20 years of experience in TACE. The chemotherapeutic emulsion which consisted of 10–20 ml lipiodol (Lipiodol; Guerbet, Roissy, France) and 20–40 mg of epirubicin (Farmorubicin; Pfizer, Wuxi, China) was slowly injected for chemoembolization by using a 5-Fr Yashrio catheter or 2.7-Fr micro-catheter (Progreat; Terumo) until the blood flow slowed. Embolization using 150–350-560 μm of gel foam (Ailikang Medicine, Hangzhou, China) mixed with contrast medium was injected to reduce the residual blood flow if necessary until there was no longer any tumor staining after repeat angiography. The tumor-feeding artery was selected or super-selected whenever possible.
Percutaneous cryoablation procedure
In the TACE-cryoablation group, a single TACE procedure was performed in each patient as the first therapeutic step. Two weeks after the initial TACE, percutaneous cryoablation was performed under CT guidance. Cryoablation sessions were administered at a rate of one cryoablation twice a week. The number of cryoablation sessions in each patient was mainly determined by the size of the targeted area. The tumor section in main tumor that indicated a defect in lipiodol uptake was the targeted area that was frozen. Before the ablation procedures, a therapy plan was made for each patient: generally, patients in Group A were treated with a cycle of one session, Group B underwent two sessions and Group C underwent three sessions. The aim of the treatments was cytoreduction (to ablate the greatest amount of residual viable tumor to control growth). The residual viable tumor was assessed using contrast-enhanced CT or MRI done previously.
All the cryoablation procedures were performed with local anaesthetic, which was the practice at our institution. The cryoablation was performed with the Cryo-Hit™ (Galilmedical, Israel) using argon gas as a cryogen. First, a CT (INFX-8000C combined with Aquilion 64 channel CT scanner; Toshiba Medical Systems Corporation, Otawara, Japan) scan was performed to localize the site of the main tumor mass and to confirm its diameter. Then, local anesthetic (1% lidocaine) was injected from the insertion site in the skin down to the peritoneum along the planned puncture track. The skin was incised with small lancets; cryoprobes were then advanced into the targeted area. Next, two or three freeze-thaw cycles were performed, each reaching a temperature of –180 °C at the tip of the probe. The placement and numbers of cryoprobes (probe diameter, 2.1–2.4 mm), appropriate approach, and ablation time were determined on the basis of residual active tumor size, shape, proximity to vulnerable structures and the achievement of a visible ‘ice-ball’. The tumor was frozen at a maximum flow rate for about 15 min, thawed for 5 min and then was refrozen for another 15 min [Citation22]. Finally, the cryoprobes were removed when the tip temperature reached above 0 °C, and a CT scan was performed to detect liver hemostasis after removal of the cryoprobes. Considering the risk of myoglobinuria-induced acute tubular necrosis, three ampules of sodium bicarbonate (50 mEq per ampule) were added to 150 ml of 5% dextrose in water which was routinely administered at the end of each procedure (this may be an unnecessary step). Twelve hours of electrocardiographic monitoring was performed after all procedures.
Follow-up and assessments
All patients were followed up with a physical examination, routine blood tests, α-fetoprotein (AFP) measurements, liver and kidney function tests, and contrast-enhanced CT or MRI scanning at a two-month interval after the previous TACE. In the TACE group, two TACE sessions were performed in each patient in a 4–6-week interval. The therapeutic response of tumors was evaluated one month after the second session (i.e. approximately two months after the initial TACE session) by using CT or MRI imaging. In the TACE-cryoablation group, the therapeutic response of tumors was evaluated at four to six weeks after the end of cryoablation (i.e. approximately 2 months after TACE). All follow-up CT or MRI scans were interpreted by three radiologists (K.Z, Z.L. and S.F.) with 30, 28 and 16 years of experience, respectively, in imaging diagnosis. The radiologists were from our institution and were aware of the clinical history of the patients.
In both groups, tumor response was assessed based on radiological evaluation according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [Citation25]. Criterion for re-treatment of the main tumor was any response less than partial response (PR) on follow-up imaging. Patients with a residual viable tumor or recurrent tumor underwent repeated TACE-cryoablation in the TACE-cryoablation group and re-TACE in TACE group if the liver function and performance status was tolerable.
The adverse events of TACE and cryoablation that occurred within one month were recorded according to Common terminology criteria for adverse events (CTCAE, version 3.0) [Citation26]. All complications were observed clinically when patients were admitted and assessed by telephone interview after patients were discharged. The information was recorded separately by two authors (W.F and M.L.). The cumulative overall survival time (OS) was calculated from the beginning of the first TACE treatment until death or the last follow-up.
Statistical analyses
All statistical analyses were performed using SPSS software (version 19.0; IBM, Chicago, IL). To determine significant differences between the two groups, the continuity correction and independent-sample t, Pearson χ2, and Fisher exact tests were used. Survival curves were calculated for both groups using Kaplan–Meier methods. Univariate analyses were performed with the log-rank test. Multivariate analyses using the Cox model were performed to identify risk factors that affected OS. A value of p < .05 was considered a significant difference.
Results
Study population
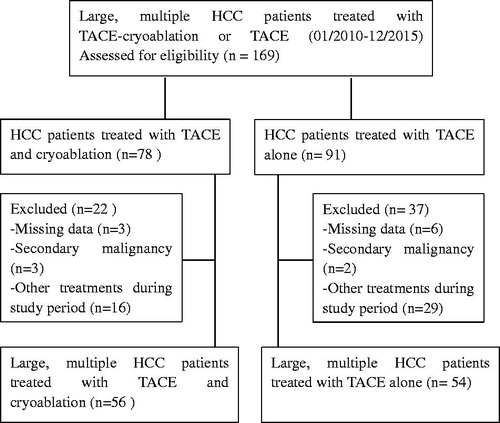
Between January 2010 and December 2015, a total of 169 consecutive patients who had large HCC and received combined TACE-cryoablation or TACE alone were retrospectively observed during the study period, with 59 patients excluded from the analysis based on our exclusion criteria. Of the remaining patients, 56 had undergone TACE-cryoablation, and the other 54 patients underwent TACE alone. A flowchart of this study is shown in . The baseline characteristics of patients are summarized in . Baseline characteristics were comparable between the groups in terms of the cause of liver disease, tumor characteristics and liver function. The majority of the patients were male, and hepatitis B virus and cirrhosis were the common underlying diseases.
Table 1. Baseline patient characteristics.
Treatment
Collectively, in the TACE-cryoablation group, patients with large HCC underwent repeated TACE-cryoablation with a median of 2 (range 1–3) cycles. The mean number of cryoablation procedures per patient in the TACE-cryoablation group was 2.3 (range 1–6): patients in Group A had a mean of 1.1 sessions (range 1–2), Group B had a mean of 2.1 sessions (range 2–4) and Group C had a mean of 3.2 sessions (range 3–6). Patients in the TACE group underwent repeated TACE with a median of 4 (range, 2–6) TACE procedures.
Tumor response
The details of tumor responses in patients within different groups are shown in . The objective response rate for the TACE-cryoablation group was 48.2%, which was significantly higher than the 22.2% observed in the TACE group (p=.004). Subgroup analysis revealed that objective response rates in Groups B and C patients in the TACE-cryoablation group were 47.6% and 23.8%, respectively, which were significantly higher than the rates observed in the TACE group (9.1%, p = .005, 0%, p = .027, respectively). However, in Group A patients, the objective response rate was comparable between the groups (21.4% vs 18.5%, p = .357).
Table 2. Tumor responses in different groups of tumor sizes for the two groups.
Safety and adverse events
Adverse events related to cryoablation for the TACE-cryoablation are detailed in . The most common Grades 1–2 adverse events were abdominal pain and thrombocytopenia. Grades 1–2 adverse events also included fever, local superficial partial-thickness frostbite near the skin puncture site, liver haemorrhage without blood transfusion and pleural effusion. Grades 3 and 4 adverse events related to cryoablation occurred in two (3.6%) of 56 patients. One patient who had grade 4 thrombocytopenia was assisted recovery by recombinant human interleukin-11. The other patient who had grade 3 liver haemorrhage recovered after receiving 2 U of red blood cells through a transfusion. No severe complications related to cryoablation, such as cryoshock, bile leak were identified post-procedure.
Table 3. Complications of cryoablation in the TACE-cryoablation group.
Adverse events related to TACE within four weeks after treatment are shown in . Post-embolisation syndrome and liver dysfunction were the most common adverse events. The occurrence rates of adverse events related to TACE between the two groups were not significantly different.
Table 4. Complications related to TACE for the two groups.
Survival analysis
At the end-point of the study, 11 (19.6%) patients in the TACE-cryoablation group and six (11.1%) patients in the TACE group were still alive. The mean follow-up duration was 23 months (range, 2–49 months) for all patients, with no patients lost to follow-up. Kaplan–Meier survival analysis () identified a significant between-group difference in survival (p < .001), with a median survival of 11.0 months (95% CI: 7.3, 14.7) for the TACE-cryoablation group compared to 6.0 months (95% CI: 5.1, 7.0) for the TACE group. With the exception of patients in Group A, the median OS of patients in Group B and Group C was significantly improved in the TACE-cryoablation group when compared with the OS in the TACE group (p = .008, p = .001, respectively) (). The univariate and multivariate analysis showed that the treatment method, AFP level, and extrahepatic metastasis were independent prognostic factors for OS ().
Figure 2. Kaplan–Meier curves of OS in patients with HCC who underwent TACE-Cryoablation or TACE. (a)Whole study population (TACE cryoablation group: n = 56, median OS =11.0 months; TACE group: n = 54, median OS =6.0 months; p < .001). (b) Patients with 5–10 cm in the largest diameter of tumor (TACE cryoablation group: n = 14, median OS =17.0 months; TACE group: n = 14, median OS =13.0 months; p = .674). (c) Patients with 10–15 cm in the largest diameter of tumor (TACE-Cryoablation group: n = 21, median OS =11.0 months; TACE group: n = 22, median OS =6.0 months; p = .008). (d) Patients with over 15 cm in the largest diameter of tumor (TACE-Cryoablation group: n = 21 median OS =8.0 months; TACE group: n = 18, median OS =5.0 months; p = .001).
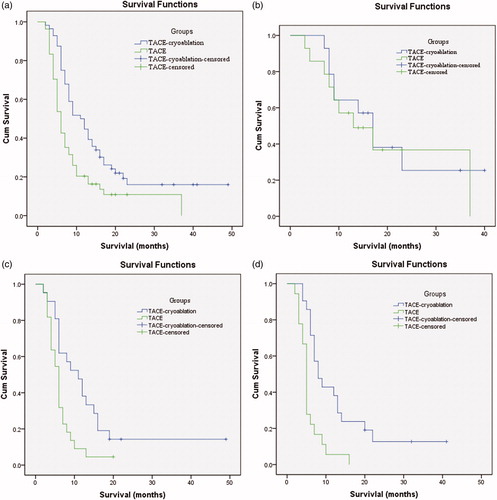
Table 5. Univariate and multivariate analysis of prognostic factor for OS.
Discussion
The management of large HCCs is challenging, and the prognosis of patients is extremely poor. The treatment results of TACE alone for these patients are rarely satisfying. To enhance the therapeutic effect of TACE, several treatments have been applied in clinical practice, such as combined use of cryoablation or RFA, and ethanol injection. In the present study, we demonstrated that the efficacy and safety of TACE cryoablation were better than those of TACE alone in patients with HCC tumors measuring ≥10 cm in diameter, but comparable in those with tumors 5–10 cm in diameter. Data on the tolerability and efficacy of TACE and cryoablation for unresectable large HCC tumors is limited. Our findings add to the growing body of literature that suggests that TACE cryoablation is a safe and effective method and may provide a reasonable alternative to select patients.
In the management of large HCCs, cryoablation offers two important potential advantages compared with RFA. First, multiple cryoprobes can be used simultaneously to generate a large ice-ball. Second, the size and shape of the cryotherapy-generated ice-ball can be readily visualised using intraprocedural CT [Citation27]. The potential therapeutic effect of combined TACE and cryoablation has only been evaluated in a few studies. Xu et al. [Citation28] have reported that in their study of 420 patients, 18 of those with larger HCC tumors (>5 cm in diameter) survived more than five years after TACE cryoablation while no patients survived after receiving cryoablation alone. Huang et al. [Citation29] have reported a significantly longer median survival for patients with large HCC tumors treated with TACE cryoablation compared to TACE alone. In our study, treatment with combined TACE and cryoablation improved OS and tumor objective response, compared to treatment by TACE only. Therapeutically, TACE and cryoablation offers several benefits. Foremost, TACE was performed first to interrupt the tumor blood supply, which not only induced tumor necrosis but also reduced the loss of energy caused by the ‘heat sink’ effect [Citation28]. Most of our patients had an objective response, as shown by extensive tumor necrosis, which might translate into improving survival. Moreover, TACE cryoablation improves a patient’s immune system and other beneficial treatment effects when compared to treatment with TACE alone. After treatment, the CD4+ cells, CD4+/CD8+ ratio, and NK cells were dramatically increased in HCC patients, while CD8+ cells were significantly decreased [Citation30]. In addition, large-volume hepatic freezing is associated with a significant release of the cytokines interleukin-6 and tumor necrosis factor [Citation31].
These results indicate that therapy with TACE cryoablation is safe in patients with large HCC. However, the results show that patients with tumors 5–10 cm in diameter had a comparable OS in the two groups. We suggest the reasons for this were as follows: first, the number of patients in this group is relatively small. Second, according to our univariate analysis, a tumor ≤10 cm in diameter was a significant prognostic factor for survival. Regardless of the type of therapy, patients with a tumor 5–10 cm in diameter had a relatively good outcome.
Cryoablation was generally well-tolerated and had manageable adverse events, such as pain, slight hepatic bleeding, myoglobin, transient thrombocytopenia, and pleural effusions. Most of these changes were usually self-limited and correlated with ablative margin volume and site. The incidence of other complications in our study was similar to those reported in previous studies that compared the combined and individual ablation therapy [Citation28]. One previous study reported that cryoablation can alleviate the pain of unresectable hepatic tumors [Citation32], and TACE prior to cryoablation can also decrease the incidence of bleeding.
There are several limitations to this study. First, this is a retrospective study without randomization, therefore selection bias may not be negligible. Our results may have been skewed because of the selection bias. For example, patients receiving TACE cryoablation more likely had financial support compared to those who were treated with TACE alone as initial treatment; therefore patients within each group may not be representative of the population as a whole. Second, we did not evaluate the cost-effectiveness of the two treatments for select patients. In addition, the size of this study is relatively small. Especially, the size of patients in group A is relative smaller than that in groups B and C, which may have reduced the statistical power. Other limitations include the following: a limited period of follow-up, inconclusive verification of the optimal time interval from TACE to cryoablation, and use of a gel foam embolizing agent rather than bead particles (which are preferred in western countries) for performing TACE procedures.
Conclusion
Overall, the findings suggest that there may be increased survival for patients with HCC ≥10 cm treated with combination compared to those treated with TACE alone. This requires confirmation in a randomized study.
Acknowledgements
We thank all the patients, clinicians and support staff who participated in this study. English language editorial support was provided by Elizabeth of Editage, a brand of Cactus Communications (https://www.editage.cn/).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223–238.
- Marcon P, Tovo CV, Kliemann DA, et al. Incidence of hepatocellular carcinoma in patients with chronic liver disease due to hepatitis B or C and coinfected with the human immunodeficiency virus: a retrospective cohort study. World J Gastroenterol. 2018;24:613–622.
- Ke S, Ding X, Gao J, et al. Solitary huge hepatocellular carcinomas 10 cm or larger may be completely ablated by repeated radiofrequency ablation combined with chemoembolization: Initial experience with 9 experience with 9 patients. Mol Med Rep. 2012;5:832–836.
- Xue T, Le F, Chen R, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015;32:64.
- Ariizumi S, Kotera Y, Takahashi Y, et al. Impact of hepatectomy for huge solitary hepatocellular carcinoma. J Surg Oncol. 2013;107:408–413.
- Yamashita Y, Taketomi A, Shirabe K, et al. Outcomes of hepatic resection for huge hepatocellular carcinoma (≥10 cm in diameter)). J Surg Oncol. 2011;104:292–298.
- Bartolozzi C, Lencioni R, Caramella D, et al. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812–818.
- Fan J, Tang ZY, Yu YQ, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674–678.
- Poon RT, Ngan H, Lo CM, et al. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114.
- Zangos S, Eichler K, Balzer JO, et al. Large-sized hepatocellular carcinoma (HCC): a neoadjuvant treatment protocol with repetitive transarterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT). Eur Radiol. 2007;17:553–563.
- Littrup PJ, Freeman-Gibb L, Andea A, et al. Cryotherapy for breast fibroadenomas. Radiology. 2005;234:63–72.
- Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11.
- Ladd AP, Rescorla FJ, Baust JG, et al. Cryosurgical effects on growing vessels. Am Surg. 1999;65:677–682.
- Arciero CA, Sigurdson ER. Liver-directed therapies for patients with primary liver cancer and hepatic metastases. Curr Treat Options in Oncol. 2006;7:399–409.
- Niu L, Li J, Xu K. Percutaneous cryoablation for liver cancer. JCTH. 2014;2:182–188.
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590.
- Wong WS, Patel SC, Cruz FS, et al. Cryosurgery as a treatment for advanced stage hepatocellular carcinoma: results, complications, and alcohol ablation. Cancer. 1998;82:1268–1278.
- Niu LZ, Li JL, Xu KC. Percutaneous cryoablation for liver cancer. J Clin Transl Hepatol. 2014;2:182–188.
- Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339. 1340.
- Alnaggar M, Niu L, Li J, et al. Cryoprotective therapy for huge hepatocellular carcinoma: a study of 14 patients with a single lesion. Cryobiology. 2014;69:457–461.
- Yang Y, Lu Y, Wang C, et al. Cryotherapy is associated with improved clinical outcomes of sorafenib therapy for advanced hepatocellular carcinoma. Cell Biochem Biophys. 2012;63:159–169.
- Xu KC, Niu LZ, He WB, et al. Percutaneous cryoablation in combination with ethanol injection for unresectable hepatocellular carcinoma. WJG. 2003;9:2686–2689.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
- Zhang Y, Fan W, Wang Y, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist. 2015;20:1417–1424.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:052–060.
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181.
- Hu KQ. Advances in clinical application of cryoablation therapy for hepatocellular carcinoma and metastatic liver tumor. J Clin Gastroenterol. 2014;48:830–836.
- Xu KC, Niu LZ, Zhou Q, et al. Sequential use of transarterial chemoembolization and percutaneous cryosurgery for hepatocellular carcinoma. WJG. 2009;15:3664–3669.
- Huang KB, WZ, Fan YY, Zhang Y, et al. Transarterial chemoembolization combined with cryoablation for unresectable large hepatocellular carcinoma: a controlled study. Zhonghua Yi Xue Za Zhi. 2016;96:2978–2982.
- Huang M, Wang X, Bin H. Effect of transcatheter arterial chemoembolization combined with argon-helium cryosurgery system on the changes of NK cells and T cell subsets in peripheral blood of hepatocellular carcinoma patients. Cell Biochem Biophys. 2015;73:787–792.
- Seifert JK, France MP, Zhao J, et al. Large volume hepatic freezing: association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J Surg. 2002;26:1333–1341.
- Long XA, Zeng J, Niu L, et al. Alleviating the pain of unresectable hepatic tumors by percutaneous cryoablation: experience in 73 patients. Cryobiology. 2013;67:369–373.