Abstract
Background: Hepatic hemangioma is a common benign liver tumor. The majority of cases are asymptomatic and require no specific treatment. The aim of this study was to evaluate the feasibility, safety and efficacy of microwave ablation (MWA) for symptomatic or enlarging giant hepatic hemangioma (≥10 cm).
Methods: From December 2013 to June 2016, 12 patients with giant hepatic hemangioma (≥10 cm) underwent ultrasound-guided percutaneous MWA, and ablation-related complications were observed. All patients were followed up with magnetic resonance or enhanced CT imaging at one month postoperatively to evaluate efficacy.
Results: This study included a total of 13 giant hepatic hemangiomas (mean: 11.7 ± 1.6 cm) in 12 patients who initially underwent 16 sessions of MWA; three lesions were treated with two sessions of planned ablation. The average ablation time for a single hepatic hemangioma was 39.0 ± 14.4 minutes. Two patients had acute postoperative non-oliguric renal insufficiency without intra-abdominal hemorrhage, liver failure or other complications. Initially, complete ablation was achieved in ten lesions in nine patients (76.9%, 10/13). One patient underwent a second session of MWA at 5 months postoperatively due to fast growing residual tissue; complete necrosis was achieved after treatment. The remaining two cases did not receive any invasive treatment due to small residual volumes. The total complete ablation rate was 84.6% (11/13).
Conclusion: Image-guided MWA is a safe, feasible, effective treatment for giant hepatic hemangioma; these findings may open a new avenue for treatment.
Introduction
Hepatic hemangioma is the most common benign mesenchymal tumor of the liver, with an incidence rate of 5%–7%, accounting for 73% of all benign liver tumors [Citation1,Citation2]. Most hepatic hemangiomas have small volumes, are asymptomatic, and generally require no intervention [Citation3–5]. However, due to rapid growth, hepatic hemangiomas with large volumes and obvious symptoms may have complications, such as abdominal pain, anemia, bleeding, hemorrhage, jaundice, thrombocytopenia, and hyperfibrinogenemia; moreover, a palpable mass may result in pressure on adjacent organs or partial infarction within the tumor, and the serious mental burden caused by hemangiomas must be treated [Citation5–9].
To date, the most common therapy for hepatic hemangioma is surgical resection. Surgical resection offers precision and efficacy but is often accompanied by substantial trauma and high risk. Generally, the difficulty and risk of surgery will increase with increased hemangioma volume, especially for giant hepatic hemangiomas with diameters ≥10 cm [Citation5,Citation9–13]. Recently, many studies have explored transcatheter arterial chemoembolization (TAE) [Citation14,Citation15], radiotherapy [Citation16,Citation17] and other non-surgical therapies [Citation18,Citation19] for hepatic hemangioma, but the majority of these methods have uncertain efficacy and are prone to major complications (Clavien–Dindo: III and above) [Citation14–23]. In recent years, many studies have explored radiofrequency ablation (RFA) for the treatment of hepatic hemangioma and have achieved satisfactory efficacy and safety [Citation24]; however, reports on giant hepatic hemangiomas with diameters exceeding 10 cm are rare [Citation25].
With the clinical application of microwave ablation (MWA) technology in large malignant liver tumors, some studies have started to explore the efficacy, safety, and feasibility of the application of MWA to large hepatic hemangiomas. At present, unfortunately, relevant reports are rare.
In this study, we attempted to apply MWA to 12 patients with 13 giant hepatic hemangiomas (≥10 cm) in our center and analyzed the feasibility, efficacy, and safety of this treatment.
Patients and methods
Ethics statement
The study protocol conformed to ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Renji Hospital, Shanghai Jiaotong University. All patients provided written informed consent.
Patients
From December 2013 to June 2016, a total of 12 patients (four men and eight women) with 13 giant hepatic hemangiomas larger than 10 cm in diameter received ultrasound (US)-guided percutaneous MWA in our center. The average patient age was 41 ± 6 years old (range: 31–67 years old). Please refer to for detailed information.
Table 1. Information on the 12 cases of patients with giant hemangiomas.
The inclusion criteria are as follows:
hemangioma diameter between 10 and 15 cm.
obvious symptoms, such as abdominal pain, anemia, bleeding, hemorrhage, jaundice, thrombocytopenia, and hyperfibrinogenemia, or serious mental burden caused by hemangiomas;
hemangioma with a tendency to enlarge (enlarged more than 0.5 cm within one year);
a distance greater than 0.5 cm between the hemangioma and hollow viscera (especially the gastrointestinal tract and gallbladder);
Child-Pugh A or B level; no significant irreversible coagulopathy;
no active infection, important organ (heart, lung, kidney, brain, etc.) dysfunction nor serious organic disease;
diagnosis of hepatic hemangioma confirmed by imaging and serological examinations (alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA199)), which were used to exclude malignant tumors.
Percutaneous MWA procedures
MWA was performed percutaneously under general anesthesia by using a real-time US-guided and water-cooled MWA system according to the standard protocol. Vital signs were continuously monitored during the procedure and within one hour after the procedure. Microwave energy was delivered using a 15-gauge electrode (25 cm in length; Vision-China Medical Devices R&D Centre, Nanjing, Jiangsu Province, China). The power and time of ablation were set based on the tumor size and location according to the manufacturer’s instructions.
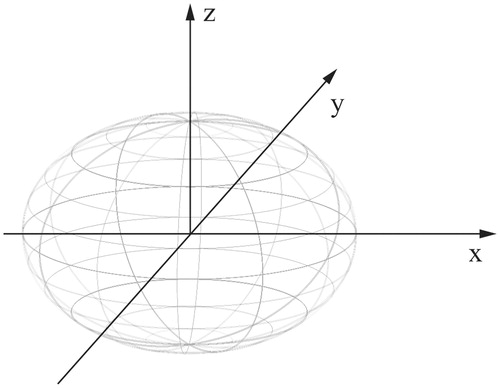
According to our MWA protocol, two electrodes were inserted into the tumor parallel to each other with an inter-electrode distance of 2.5–3.0 cm in the same plane using a 3.5-MHz probe (MyLab 60; Esaote, Genoa, Italy). The microwave generator was turned on, and the temperature of the electrode tip was maintained at 100 °C for 3–10 min. The distal margin of the lesion was ablated first, and the electrodes were then withdrawn by 1–1.5 cm for multipoint ablations until the tumors were completely covered by the gasification zone. The ablation was completed with 0.5 cm of adjacent liver to ensure a tumor-free margin. After the ablation procedure, another one or two needle tracts were ablated at the same or adjacent inter-costal space, if necessary. Generally, for a giant hepatic hemangioma, the number of needle tracts in a plane, ablation sites in a single needle tract, and the number of ablation planes were determined according to the tumor transverse diameter (X-axis), longitudinal axis (Y-axis) and vertical axis (Z-axis), respectively (). Patients with multiple hepatic hemangiomas or with two lesions more than 10 cm in diameter underwent two sessions of MWA.
After MWA, all patients were closely monitored for procedure-related acute or chronic complications. Peripheral blood tests and liver and renal function examinations were performed one and three days after MWA. Approximately 1–2 h after the procedure when patients recovered from the general anesthesia, short-term administration of low-dose dexamethasone or methylprednisolone as well as hemostasis was applied for liver and kidney protection. One or two kinds of hepatoprotective agents, such as glutathione and/or polyene phosphatidyl choline, combined with dexamethasone (10 mg) were applied for 2–3 days to decrease the level of transaminase. According to the level of creatinine on the first day after ablation, 125 ml 5% sodium bicarbonate solution was administered for 2–3 days.
Clinical data collection and follow-up
All patient clinical data were collected and recorded in a computerized database. The complication rate, mortality, complete ablation rate, volume of the ablated lesion, length of hospitalization, patient symptoms and quality of life after MWA were analyzed.
Complications were defined as any adverse event that occurred after MWA, excluding pain or transient febrile response to MWA, and were evaluated based on the Clavien–Dindo grading system. MWA-related mortality was defined as any death that occurred within 30 days after MWA. The follow-up evaluation was performed based on a contrast-enhanced magnetic resonance imaging (MRI, preferred) or computed tomography (CT) scan. The response to ablation was assessed one month post-ablation. Complete ablation was defined as the absence of any contrast-enhancing lesion that was suspicious for a residual tumor at the ablation site on the post-ablation MRI or CT scan. Patients who had a residual tumor on the post-ablation scan underwent a repeated ablation, if feasible; alternatively, they were followed up every six months for any changes in the tumor to determine whether additional treatments were required. If complete ablation was achieved, contrast-enhanced MRI (preferred) or CT re-examination was conducted every year for three years.
Results
Of the 12 patients, 6 had single lesions. One patient had two tumors ≥10 cm. The average diameter of giant hepatic hemangiomas was 11.7 ± 1.6 cm (range: 10–14.5 cm). Ten giant lesions were located in the parenchymal region; two giant lesions were close to the diaphragm, while another was located near the intestinal tract. Three patients experienced sustained abdominal pain or other discomfort, seven patients had persistent hemangioma growth, and the remaining two patients had both persistent abdominal pain and tumor growth. Preoperatively, the coagulation, liver function and renal function indices of all patients were examined. The liver function (albumin (Alb), alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TB) and direct bilirubin (DB)) and renal function (serum creatine (Scr)) indices were all within the normal range. The average ablation time was 39.0 ± 14.4 min (range: 20–62 min). Two needles were used for all 13 giant lesions.
Postoperative discomfort and complications
All postoperative discomfort and complications are shown in . A total of 11 patients experienced low fever, constipation, slight wound pain, stomach discomfort, and other MWA postoperative reactions. All these issues self-healed and did not need special treatment.
Table 2. Discomfort and complications after MWA for 13 giant hemangiomas.
Two cases had acute renal insufficiency that required medical treatment. There was a significant increase in postoperative Scr, and the absolute increased values were ≥26.4 μmol/L within 48 h or increased by ≥50% above the baseline values; however, there was no significant decrease in urine output, which is indicative of non-oliguric acute renal insufficiency. Treatments such as hydration, urine alkalization, and diuretics were administered. Intravenous injection of 20 mg furosemide and intravenous infusion of 125 ml 5.0% sodium bicarbonate solution were performed once a day to improve the renal microcirculation until the level of Scr recovered. Both patients recovered within 7 days.
All patients exhibited different degrees of liver function damage after ablation, as indicated by significantly high levels of bilirubin (TB and DB), increased transaminase (ALT and AST) and decreased Alb (p < .01). Detailed changes in liver function are shown in . After ablation, the combination of 2 or 3 kinds of liver preservation drugs (such as reduced glutathione injection (240 mg), magnesium isoglycyrrhizinate injection (200 mg) and polyene phosphatidyl choline injection (929 mg)) and low-dose hormones were used each day for hepatoprotection. Additionally, liver function was examined once every 3–4 days. Liver function indicators for all patients gradually recovered to normal levels within 7 days, and no irreversible liver failure occurred.
Table 3. Liver function changes after ablation.
No serious complications, such as intra-abdominal hemorrhage or infection of the ablated lesion, were observed, and no deaths occurred (see ).
Complete ablation rate
Postoperative follow-up periods ranged from 7 to 38 months. Initially, thirteen giant hemangiomas received 16 sessions of MWA, and 4 patients underwent two planned sessions of ablation. The initial postoperative review indicated the complete ablation of 10 giant hemangiomas, accounting for 76.9% (10/13).
Classic case 1
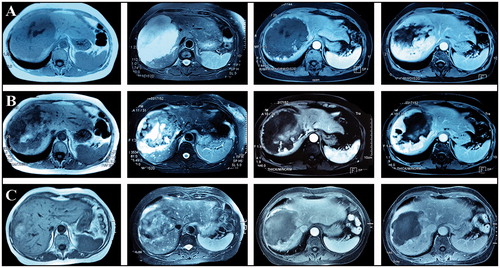
Female, 67 years old. A giant hepatic hemangioma (13 cm in diameter) was located on the right side of the liver. Two scheduled sessions of US-guided MWA were performed half a month apart. One month after ablation, an MRI scan indicated complete necrosis of the tumor ().
Classic case 2
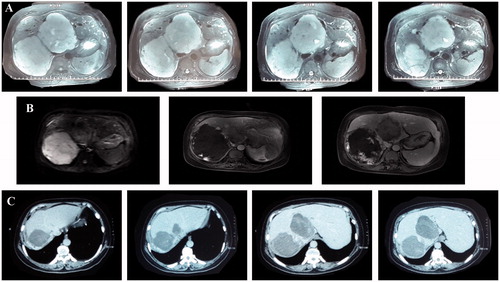
Male, 37 years old. Two giant hepatic hemangiomas (12.5 and 11.7 cm in diameter) were located in the right posterior lobe and middle lobe of the liver, respectively, and constricted the inferior vena cava. Two scheduled sessions of US-guided MWA were performed half a month apart. The lesion in the middle lobe was ablated first. After one month, MWA was performed on the lesion in the right posterior lobe. One month after the two MWA sessions, an MRI scan indicated complete necrosis of both giant tumors ().
Residue and follow-up treatments
For two of the three patients in whom initial MWA failed to achieve complete ablation, one had a hemangioma located near the intestinal tract (<2 mm), while the other had a hemangioma located on the diaphragmatic dome. Therefore, a portion of the hemangioma was left untreated in order to protect the surrounding organs. These two patients did not receive any further treatment because the residual tumors showed no change during the follow-up periods. Due to fast growing residual tissue, the other patient underwent a second session of MWA at 5 months postoperatively, and the follow-up review indicated complete necrosis. Thus, the total complete ablation rate was 84.6% (11/13).
Length of hospital stay
Patients were not permitted to leave hospital until all liver and renal function indices returned to normal to ensure safety. The average postoperative hospital stay was 5.0 ± 1.5 days (range: 3–7 days). The hospitalization time of the two patients in whom acute renal insufficiency occurred and two patients with a TB level over 50 was 7 days. All remaining patients recovered and left the hospital within 3–5 days.
Long-term follow-up
During the follow-up, the clinical condition of the patient was evaluated during a clinic visit, which included assessments of abdominal pain, hemorrhage, jaundice, intraperitoneal adhesion, and intestinal obstruction. Additionally, if a residual tumor was present, the size and change were evaluated. At the final follow-up, all patients were alive, and there were no symptoms or long-term complications.
Discussion
Compared with RFA, MWA has the advantages of a broader thermal range and a more even spread; moreover, the internal tumor temperature can reach approximately 130 °C. In addition, MWA has a fast heating speed and is less affected by carbonization and blood-flow perfusion, while multiple needles can conduct ablation simultaneously without interference and exert a synergistic effect, thus resulting in a broader ablation range and shorter ablation time. The potential typical cause of hemangioma formation is abnormal vascular development during embryonic development due to a lack of smooth muscle tissue in the abnormal blood vessels, leading to slow blood flow, blood stagnation, and slow heat dissipation. Thus, MWA is more suitable for treating hepatic hemangioma.
From August 2010 to September 2013, we treated 46 cases of large hepatic hemangioma (5–10 cm in diameter) with MWA. MWA achieved a 95.7% complete ablation rate, 10.9% severe complication rate, and a shorter ablation time than did RFA. Therefore, we inferred that MWA was suitable for giant hepatic hemangiomas [Citation26] and tried to apply MWA to 12 patients with 13 giant hepatic hemangiomas (≥10 cm) in this study.
Based on our clinical record, the average ablation time for hemangiomas ≥10 cm was only 39.0 ± 14.4 min (range: 20–62 min). In comparison, Gao et al. [Citation27] reported 36 cases of patients with hepatic hemangiomas who underwent RFA, with an average tumor diameter of 10 ± 4 cm (5.0–21.5 cm) and a RFA time of 103 ± 27 min (range: 40–160 min). Zou et al. [Citation28] reported 30 cases of patients with hepatic hemangioma who underwent RFA treatment, and the average RFA time was 60.5 ± 36.2 min (range: 27–208 min). Fan et al. [Citation29] reported 68 cases of patients with hepatic hemangioma who underwent RFA; of these, 19 cases underwent percutaneous punctures, and 49 cases had laparoscopic or open RFA combined with the Pringle maneuver. The average tumor diameter was 5.6 cm (2.5–11 cm). The average RFA of the Pringle maneuver group was 29 ± 7.5 min and that of the non-Pringle group was 55.4 ± 12.4 min. Additionally, the result of a MWA study reported by Timothy [Citation30] was consistent with our results, with a mean treatment time of 11.6 min for hemangiomas, which ranged from 3.4 to 12.2 cm in diameter. The largest hemangioma (12.2 cm mean diameter) in their study was treated in only 24.5 min. Thus, MWA has the advantage of a short treatment duration.
Regarding the safety of MWA, the 12 patients in this study did not have intra-abdominal hemorrhage, irreversible liver failure, ablation lesion infection or other severe complications, with the exception of 2 patients who had reversible non-oliguric renal insufficiency. Moreover, no deaths occurred; thus, the safety of this procedure is satisfactory.
Importantly, acute oliguric renal insufficiency should be the focus for complications with the application of MWA and RFA for treating large hepatic tumors. This complication occurred more often in thermal ablation treatment for large hepatic tumors, which may be related to complication factors, such as long continuous thermal damage of the cell contents in blood circulation, tumor cell disintegration and unknown cell-factor stimulations. Acute renal function insufficiency was caused by rare hyperkaliemia and other electrolyte disorders caused by thermal ablation; however, patients achieved self-recovery with general medical treatment, and very few patients required hemodialysis assistance. From September 2013, we changed our protocol for preventing acute renal insufficiency after the patient was returned to the ward after completion of ablation and recovery from anesthesia. We provided rapid preoperative rehydration and supplied sodium carbonate for urine alkalinization halfway during the ablation process to maintain urine formation and excretion and prevent renal tubular formation and thermal damage to endothelial cells. With the above treatments, there were no cases of acute oliguric renal insufficiency, which is common following ablation of large tumors under 10 cm.
Unlike the ‘completely and entirely’ principle for treating malignant hepatic tumors, appropriate adjustments can be made for ablation of hepatic hemangioma. Sacrificing the safety of treatment for pursuing a ‘safe ablation edge’, which is required for malignant tumor ablation, is unnecessary, especially for hemangiomas located near the diaphragm, hilar, and hallow viscera. In fact, as ablated lesions shrink, the edge of a hemangioma moves farther away from high-risk organs during the ablation process. Thus, complete ablation for hemangiomas near a ‘high-risk location’ is possible. This conclusion was consistent with the results of RFA for hepatic hemangioma in previous studies [Citation26–29].
Although the complete ablation rate for this group was not 100%, two lesions with small volumes of residual tissue without significant growth were not subjected to a repeated intervention, unlike malignant tumors. After ablation with mild damage, the majority of hepatic hemangiomas exhibited necrosis. Additionally, tissue contraction occurred following high-temperature ablation, consistent with current studies [Citation31,Citation32], and did not affect liver function due to rapid growth, consistent with the treatment purpose for benign liver tumors.
Overall, MWA is a safe, easy, and effective minimally invasive treatment. MWA is a potential first choice or alternative therapy for giant hepatic hemangiomas.
Acknowledgements
Zhi Wang collected patient data, designed the analysis pipeline and drafted the manuscript. Tang Xiaoyin, Qi Xingxing, Shi Yaoping, Chi Jiachang, and Li Ping revised the manuscript. ZhaiBo conceived and coordinated the overall study and revised the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Belli L, De Carlis L, Beati C, et al. Surgical treatment of symptomatic giant hemangiomas of the liver. Surg Gynecol Obstet. 1992;174:474–478.
- Ishak KG, Rabin L. Benign tumors of the liver. Med Clin North Am. 1975;59:995–1013.
- Farges O, Daradkeh S, Bismuth H. Cavernous hemangiomas of the liver: are there any indications for resection? World J Surg. 1995;19:19–24.
- Yeh WC, Yang PM, Huang GT, et al. Long-term follow-up of hepatic hemangiomas by ultrasonography: with emphasis on the growth rate of the tumor. Hepatogastroenterology. 2007;54:475–479.
- Schnelldorfer T, Ware AL, Smoot R, et al. Management of giant hemangioma of the liver: resection versus observation. J Am Coll Surg. 2010;211:724–730.
- Kumashiro Y, Kasahara M, Nomoto K, et al. Living donor liver transplantation for giant hepatic hemangioma with Kasabach-Merritt syndrome with a posterior segment graft. Liver transplantation: official publication of the American Association for the Study of. Liver Dis Int Liver Transplant Soc. 2002;8:721–724.
- Ferraz AA, Sette MJ, Maia M, et al. Liver transplant for the treatment of giant hepatic hemangioma. Liver Transpl. 2004;10:1436–1437.
- Terkivatan T, Vrijland WW, Den Hoed PT, et al. Size of lesion is not a criterion for resection during management of giant liver haemangioma. Br J Surg. 2002;89:1240–1244.
- Terkivatan T, de Wilt JH, de Man RA, et al. Indications and long-term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg. 2001;136:1033–1038.
- Hanazaki K, Kajikawa S, Matsushita A, et al. Giant cavernous hemangioma of the liver: is tumor size a risk factor for hepatectomy? J Hepato Biliary Pancr Surg. 1999;6:410–413.
- Yoon SS, Charny CK, Fong Y, et al. Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg. 2003;197:392–402.
- Lerner SM, Hiatt JR, Salamandra J, et al. Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg. 2004;139:818–821.
- Hamaloglu E, Altun H, Ozdemir A, et al. Giant liver hemangioma: therapy by enucleation or liver resection. World J Surg. 2005;29:890–893.
- Srivastava DN, Gandhi D, Seith A, et al. Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: a prospective study. Abdom Imaging. 2001;26:510–514.
- Sun JH, Nie CH, Zhang YL, et al. Transcatheter Arterial Embolization Alone for Giant Hepatic Hemangioma. PLoS One. 2015;10:e0135158.
- van Tilborg A, Dresselaars HF, Scheffer HJ, et al. RF ablation of giant hemangiomas inducing acute renal failure: a report of two cases. Cardiovasc Intervent Radiol. 2016;39:1644–1648.
- Zhang X, Yan L, Li B, et al. Comparison of laparoscopic radiofrequency ablation versus open resection in the treatment of symptomatic-enlarging hepatic hemangiomas: a prospective study. Surg Endosc. 2016;30:756–763.
- Cao X, He N, Sun J, et al. Interventional treatment of huge hepatic cavernous hemangioma. Chin Med J. 2000;113:927–929.
- Giavroglou C, Economou H, Ioannidis I. Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol. 2003;26:92–96.
- Huang XQ, Huang ZQ, Duan WD, et al. Severe biliary complications after hepatic artery embolization. Wjg. 2002;8:119–123.
- Issa P. Cavernous haemangioma of the liver: the role of radiotherapy. Br J Radiol. 1968;41:26–32.
- Park WC, Rhillips R. The role of radiation therapy in the management of hemangiomas of the liver. Jama. 1970;212:1496–1498.
- Gaspar L, Mascarenhas F, da Costa MS, et al. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol: J Eur Soc Therap Radiol Oncol. 1993;29:45–50.
- Wang YSZ. Advance of radiofrequancy ablation in liver surgery. Chin J Curr Adv Gen Surg. 2010;13:145–148.
- van Tilborg AA, Nielsen K, Scheffer HJ, et al. Bipolar radiofrequency ablation for symptomatic giant (>10 cm) hepatic cavernous haemangiomas: initial clinical experience. Clin Radiol. 2013;68:e9–e14.
- Tang XY, Wang Z, Wang T, et al. Efficacy, safety and feasibility of ultrasound-guided percutaneous microwave ablation for large hepatic hemangioma. J Digest Dis. 2015;16:525–530.
- Gao J, Ke S, Ding XM, et al. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2013;153:78–85.
- Fan RF, Chai FL, He GX, et al. Laparoscopic radiofrequency ablation of hepatic cavernous hemangioma. Surg Endosc. 2006;20:281–285.
- Zou HYJ, Wu YX, Ou X, et al. The new technology of enhanced radiofrequency ablation is safe and effective for treating giant hepatic hemangioma. Zhonghua Gan Zang Bing Za Zhi. 2012;20:261–265.
- Ziemlewicz TJ, Wells SA, Lubner MA, et al. Microwave ablation of giant hepatic cavernous hemangiomas. Cardiovasc Intervent Radiol. 2014;37:1299–1305.
- Liu D, Brace CL. CT imaging during microwave ablation: analysis of spatial and temporal tissue contraction. Med Phys. 2014;41:113303.
- Brace CL, Diaz TA, Hinshaw JL, et al. Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vas Int Radiol. 2010;21:1280–1286.