 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose: This prospective study was to evaluate clinical outcomes of microwave ablation (MWA) of benign breast tumors with minimum 12 months follow up.
Methods: With approval of the institutional ethics committee and written informed consent, 56 patients with 107 biopsy-proved breast benign tumors were recruited from November, 2013 to April, 2017. MWA with ultrasound (US) guidance was performed under local anesthesia. During the procedure, pull-back technique was used for tumors larger than 1.0 cm in diameter and hydro-dissection technique was used for tumors adjacent to skin, pectoralis and areola. Clinical outcomes were followed up by physical examination and medical images including US, contrast enhanced US and MR.
Results: The maximum diameter of these tumors was 1.6 ± 0.8 cm. MWA was successfully performed with the median 120 s of duration (ranging 20–1100 s). Technical success was achieved in all patients. At the median follow-up of 20.5 months (ranging 12–53 months), the mean volume reduction ratios (mVRRs) of tumors were 77.1 ± 8.2%, 84.3 ± 10.6%, 93.3 ± 8.2% at follow-up of 12, 18, 24 months (p < .0001), respectively. Compared with 92% of masses were palpable before ablation, mass palpabilities were 40%, 11%, 5% at follow-up of 12, 18, 24 months (p < .001), respectively. Cosmetic satisfaction was reported excellent or good in 100% of patients.
Conclusions: As a safe and effective minimally invasive modality for inactivating benign breast tumors in situ, MWA achieved optimistic clinical outcomes on volume reduction and cosmetic satisfaction after minimum 12 months follow-up.
Introduction
Benign tumors with palpable or impalpable lumps are the most common complaints in breast for women [Citation1–3]. Owning to the high incidence, significant influence on life quality, and potential malignant progress of breast benign tumors, more attention should be deserved in clinic [Citation1,Citation3]. Nevertheless, the most frequent therapeutic approach by now is still surgical resection. In that case, patients either have to accept operation following several complications with distortion, asymmetry and non-ignorable scars, leaving a significant cosmetic defect especially for young women [Citation4]. Either choose to dynamic observation accompanied in part with anxiety and (or) nervous on intermittent development of palpable prominence, localized discomfort and potential canceration [Citation5]. To overcome the surgical dilemma described above, vacuum-assisted biopsy is widely developed to remove breast benign tumors during past decades [Citation2,Citation6]. However, the complete efficiency is still limited by regional hemorrhage during the procedure and following complications such as hematoma and skin laceration [Citation7,Citation8]. Therefore, alternative techniques with more therapeutic efficiency, less postoperative complications and better cosmetic outcomes are urgently demanded.
Owning to many superiorities of minimally invasive properties, the image-guided thermal ablations have been more and more important in treatment of benign thyroid nodules during the past several years [Citation9–13]. Specifically, some of them including cryoablation, radiofrequency ablation (RFA) and microwave ablation (MWA) are coming to spotlight for treatment of breast tumors [Citation14–17]. However, these studies almost use cryoablation and frequently focus on the breast cancers. Patients with benign breast tumors are seldom enrolled based on the major concern of the unsolved mass effect after local ablation. Whether the therapeutic outcomes of benign breast tumors can achieve the physio-psychological satisfaction of patients is not yet known. As the relevant follow-ups are no more than 12 months in the previously published literatures, it is still to be defined whether more significant volume reduction would happen in an extended period. Therefore, we performed in this study to assess the patients, technique and outcomes for MWA in the minimum 12-month follow-up for treatment of breast benign tumors. To the best of our knowledge, MWA of breast benign tumors have not been reported elsewhere on the relative long-term prognosis observation (more than 12 months) of therapeutic efficiency, tumor volume reduction and cosmetic satisfaction.
Materials and methods
Patient enrolment
With approval by the institutional ethics committee of Chinese PLA General Hospital, a total of 56 patients between November 2013 and April 2017 diagnosed with breast benign tumor by US-guided core needle biopsy were enrolled in this study. The study has been registered in Clinical-Trails. gov (Identifier No. NCT 02860104) and written informed consent was received from each patient.
Inclusion criteria were as followed: (a) breast tumors identified score 3 or less by Breast Imaging Recording and Data System (BI-RADS) and proved benign by core needle biopsy; (b) sizes of benign tumors persistent growth during a half year follow-up; (c) patients with tumor-related symptoms including pain, discomfort and oppression; (d) patients with evidently psychological pressure due to the existence of breast tumors; (e) patients refuse to accept other treatments; (f) Karnofsky Performance Status (KPS) greater than 70%.
Exclusion criteria included: (a) patients with contraindications on examination of CEUS or CEMR; (b) patients in pregnancy or lactation; (c) patients with evidence of coagulopathy, severe cardiopulmonary dysfunction, chronic liver diseases and (or) renal failure; (d) patients during menstrual period; (e) patients referring to other therapies such as surgical resection and mammotome.
MWA procedure
The ultrasound (US) guided MWA procedure was performed following our previous published literatures [Citation18]. Briefly, a well suitable ablation scheme was designed firstly including the orientation and routine of needle penetration (the distributions of MW antenna in tumors), ablation range, and duration time, etc., according to tumor characteristics and its surroundings based on the preoperative US and MR examinations. Patients were positioned supine or latericumbent, sterilized and locally anesthetised using a mixture of 1% ropivacaine and 2% lidocaine at a volume ratio of 1:1. MW antenna was then accurately placed at the desired site via percutaneous insertion under conventional US guidance (). After that, MWA was started immediately with 20 W of output and 3 mm of active tip for tumors less than 2.0 cm in diameter, and 30 W of output and 5 mm of active tip for the rest. When tumor size of less than 1.0 cm in diameter, fixed technique was adopted. When tumors measuring 1.0 cm or greater, the pull-back technique was used. In each tip site, the emitting duration of MW energy was 10–30 s. For all tumors, only one antenna was used for insertion and ablation under US guidance. US guidance was used via the LOFIQ E9 US instrument (GE Medical) with a convex array transducer (5.0–9.0 MHz). Microwave equipment (KY-2000, Canyon Medical, China) consisted of a microwave generator (2450 MHz) and a water-cooled needle antenna. The needle antenna has a size of 16 gauge (G) in diameter and 10 cm in length with an active tip of 3 or 5 mm in length.
Figure 1. Microwave ablation (MWA) procedure under ultrasound (US) guidance (A 18-year-old woman with a right breast fibroadenoma of 2.5 × 1.3 × 1.5 cm in size). (A) US guidance showed the accurate placement of the antenna tip in the tumour at the beginning of MWA session; (B, C) Hydro-dissection technique: PTC needle (yellow arrows) was firstly inserted into the interval between tumour margin and adjacent tissues to infusion of saline. Then MW antenna (red arrow) was inserted into the tumour for ablation; (D–F) Pull-back technique: antenna tip was firstly inserted into the deepest site of tumour for ablation (red arrow in D). Then pull back till the bottom section was completely ablated. After that, antenna tip was again inserted to ablate the centre section (red arrow in E). And again, antenna tip was inserted to ablate the top section (red arrow in F). Repeatedly, the ablated zone was through dots, lines, planes, and solids till to completely cover the entire tumors.
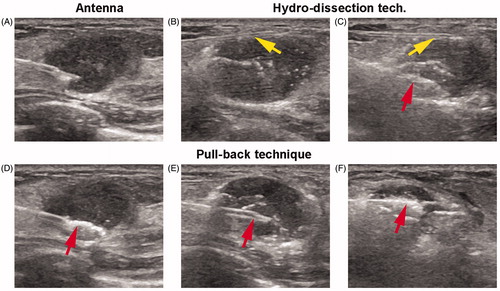
During ablation, the ablation would occasionally be suspended and the mixture of ropivacaine and lidocaine was local-injected again for the patients who could not tolerate the pain in the process. Visual analogue scale (VAS, a numerical rating scale: 0–10) was used for pain assessment following: score 1–3 was assorted in slight pain, score 4–6 was assorted in moderate pain and score 7–10 was assorted in severe pain.
The pull-back technique was described as follows (): antenna tip was inserted into the deepest position of tumors firstly. As the hyperechoic region formed by MW irradiation, the tip was then moved back slowly and continuously along the long axis of the needle until reaching the margin of the mass. Repeatedly, the ablated zone was through dots, lines, planes and solids till to completely cover the entire tumors.
Hydro-dissection technique (), as an adjuvant method, was used for the treatment of breast mass adjacent to skin, pectoralis and areola less than 0.2 cm (measured by US). Before MWA, a PTC needle (HAKKO, Nagano, Japan) with 22 G in diameter and 7 cm in length was inserted in advance under US guidance into the interval between tumor margin and adjacent tissues. After that, 10–30 ml of saline was infused to dissection and protection of tissues (skin, pectoralis and areola) during the ablative procedure.
Peri-ablative imaging of MWA
Conventional US, contrast-enhanced US (CEUS) and contrast-enhanced MR (CEMR) were performed to evaluate the masses before and after ablation [Citation19,Citation20]. US equipment was the same as described above for the use of MWA guidance. Sonovue (Bracco, Itlay) as an intravenous agent for CEUS was used through bolus administration of 2.4 ml, followed by a 5-ml saline flush. CEMR was performed using a 3.0 T unit MRI system (Signa Echo-Speed, GE medical) with intravenous injection of Gd-DTPA as a contrast agent. The maximum diameters on three dimensionalities were determined by CEUS, which were almost the same by CEMR, and volume calculated following V = π/6 × a × b × c [Citation21].
Follow-up and outcomes
Physical examination and US were performed for patients at 1, 3, 6 months after MWA and then at 6 month intervals to evaluate the long-term efficacy. If necessary, CEUS or CEMR was used for evaluation of the suspicious. Patient information and ablation variables were documented, including demographics, tumor quantity, size and location by US, and pathological type for each patient and session, puncture, duration and power, technical success, complications, volume reduction ratio (VRR), palpability and cosmetic satisfaction during or after the ablation process.
Technical success was defined as that tumor was treated according to protocol and covered completely by the ablation zone [Citation22]. Complications were classified as major or minor according to classification of the Society of Interventional Radiology [Citation23]. Cosmetic satisfaction was recorded as excellent, good, acceptable, and poor by patient self-assessment. VRR was calculated by the equation as:
Statistical analysis
Statistical software SPSS (SPSS 22.0, Chicago, IL) was used for statistical analyses. Categorical variables were descripted as numbers (percentages) and compared using a χ2 or Fisher’s exact test. Continuous variables were descripted as mean ± standard deviation (SD) or medians (interquartile range (IR)), and compared using the unpaired t test (two-tailed) or Mann–Whitney U test, according to the normality result by using Kolmogorov–Smirnov test. p values less than .05 were considered statistically significant.
Results
Baseline characteristics
About 56 patients with a median age of 33 years (ranging 19–59 years) and a total of 107 tumors were enrolled in this study, including 26 of patients with tumor persistent growth, 12 of patients with tumor relevant symptoms, 30 of patients with evidently psychological pressure, and 5 of patients rejected to accept other treatments. All the patients had KPS scores more than 70% and BI-RADS scores 3 or less. Besides, a total of 16 tumors less than 1 cm in size were enrolled for the reasons of evidently psychological pressure and (or) rejection of other treatments.
The maximum diameter of these tumors was 1.6 ± 0.8 cm. Tumor numbers of less than 1 cm, between 1 and 2 cm, and larger than 2 cm in size were 16 (15%), 52 (49%) and 39 (36%), respectively. Before ablation, the masses could be palpable in 92% (98/107) of the cases. 62 (58%) tumors located in the parenchyma, 13 (12%) adjacent to skin, 12 (11%) adjacent to the pectoralis, 20 (19%) adjacent to the areola. All patients were identified by using core biopsy, 40 (71%) of them were diagnosed with fibroadenomas, 12 (21%) with adenosis, 3 (5%) with fibrous epithelioid tumor, and 1 (2%) with mastoplasia and collagen fiber hyperplasia ().
Table 1. Patients characteristics.
All the patients were achieved to the minimum follow-up of 12 months. The median follow-up was 20.5 months, ranging from 12 to 53 months. During follow-up, the period of observation was 12–24 months for 30 patients (54%), 24–36 months for 12 patients (21%), 36–48 months for 10 patients (18%), 48–60 months for 4 patients (7%) ().
Therapeutic outcomes
The median duration for MWA of all tumors was 120 s, ranging from 20 to 1100 s. The median power was 30 W, ranging from 20 to 50 W. 40 (71%) patients received hydro-dissection technique to protect proximal tissues and 51 (91%) patients were ablated by using pull-back technique. Technical success was achieved in all patients with one session under US guidance, one representative case was shown in . During the procedure, severe pain was occurred in 1 (2%) patient, slight to moderate pain was occurred in 11 (20%) patients ().
Figure 2. Technical success of MWA in one representative case (A 38-year-old woman with a left breast fibroadenoma of 1.2 × 1.1 × 1.0 cm in size). (A) US before MWA showed a hypoechoic tumour with clear boundary; (B) CEUS showed heterogeneous enhancement in the tumour; (C) CEMR showed the tumour (red arrow) was significant enhancement in arterial phase; (D) US showed the heterogeneous hypoecho in the ablated mass after MWA; (E) the ablated mass without enhancement on CEUS; (F) the ablated mass without enhancement on CEMR in arterial phase; (G–L) US variance of the breast benign tumour after MWA during follow up at 1, 3, 6, 12, 18, 24 months, respectively. Tumor size decreased gradually during the process and disappeared on the US image at the end; (M) no nodules were detected on CEMR at the follow-up of 24 month.
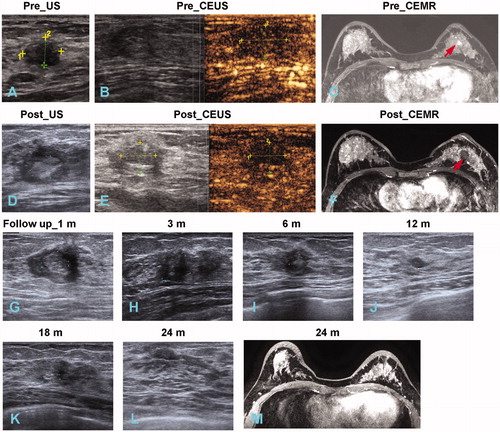
Table 2. Therapeutic response and clinical outcomes.
After ablation, no skin burn was observed in these 56 patients. A slight pain in the ablative site was reported by most patients at postoperative 1–2 days, and disappeared in the subsequent one week. No sedatives and antibiotics were given to any patients before and after ablation. No major complications including abscess, oedema, ecchymosis, hematoma and infection were induced by MWA in this study.
Tumor volume reduction
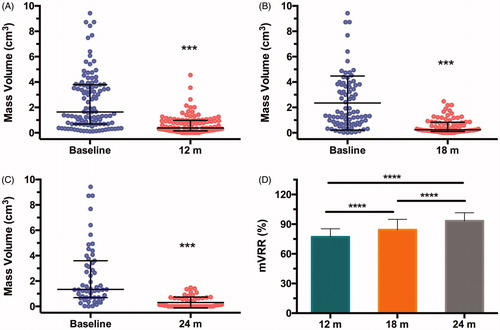
During the median follow-up of 20.5 months, mass volumes decreased from 1.64 cm3 (IR: 0.69, 3.79) before MWA to 0.38 cm3 (IR: 0.15, 0.98) at minimum 12-month follow-up for a total of 107 tumors (p < .001, ). For the 83 tumors in 44 patients with follow-up to 18 months, mass volumes decreased from 1.51 cm3 (IR: 0.7, 3.75) before MWA to 0.24 cm3 (IR: 0.11–0.84) at the 18-month after ablation (p < .001, ). For the 55 tumors in 26 patients with follow-up to 24 months, mass volumes decreased from 1.34 cm3 (IR: 0.69, 3.60) before MWA to 0.06 cm3 (IR: 0.00, 0.65) at the 24-month after ablation (p < .001, ). With the distinct decrease of masses in size, the mVRRs were 77.1 ± 8.2%, 84.3 ± 10.6%, 93.3 ± 8.2% at the follow-up of 12, 18 and 24 months, respectively, demonstrating a good performance on the tumor volume reduction (p < .0001, ).
Figure 3. Mass volume evaluation during follow-up. (A) Mass volumes of 107 tumours in 56 patients with follow-up of 12 months. (B) Mass volumes of 83 tumours in 44 patients with follow-up of 18 months. (C) Mass volumes of 55 tumours in 26 patients with follow-up of 24 months. Baseline in (A–C) meant the time of MWA procedure. ***p < .001 by Mann–Whitney U test. (D) mean VRRs detection at the follow-up of 12, 18 and 24 months. ****p < .0001 by unpaired two-tailed t test. Data expression with mean ± SD or median (interquartile range), according to normality results.
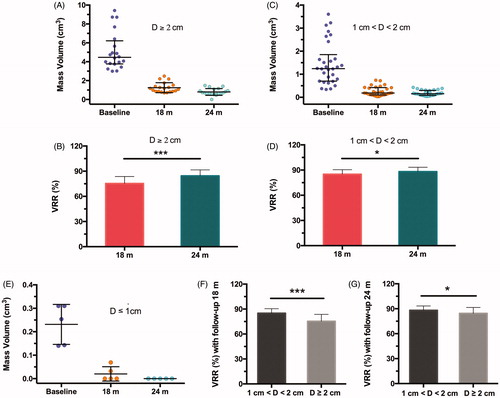
In a total of 55 tumors with follow-up more than 24 months, the volumes of masses with ≥2.0 cm in size decreased to 1.24 ± 0.52 cm3, 0.80 ± 0.36 cm3 from 4.46 cm3 (IR: 3.77, 6.22) (), and VRRs were 75.3 ± 8.3%, 84.4 ± 7.2% (p < .001, ) at the follow-up of 18, 24 months, respectively. For masses with the size of 1.0–2.0 cm, the volumes decreased from 1.24 cm3 (IR: 0.70, 1.85) to 0.19 cm3 (IR: 0.10, 0.42), 0.15 cm3 (IR: 0.08, 0.29) () and VRRs were 84.9 ± 5.4%, 88.2 ± 5.2%, respectively (p < .05, ). In addition, a total of five tumors less than 1 cm in size were all undetectable on US imaging, showing 100% of VRR at the follow-up of 24 months (). As shown in Figure F–G, VRR showed significant differences in tumors between 1.0 and 2.0 cm and ≥2.0 cm in sizes at follow-up of 18 (p < .001), 24 (p < .05) months, respectively, demonstrating a preferential volume reduction of smaller masses than that of larger masses.
Figure 4. Mass volume evaluation in different sizes of tumours during follow-up of 24 months. (A, C, E) Mass volumes of 20 tumors ≥2 cm (A), 30 tumors of 1–2 cm (C), 5 tumors of ≤1 cm (E) in diameter (D). Baselines meant the time of MWA procedure. (B, D) volume reduction ratios (VRR) of tumours in different sizes of D ≥ 2 cm (A), 1 cm < D < 2 cm (C) at 18- and 24-month follow-ups, respectively. (F, G) VRRs comparison of tumours at different sizes at 18-month (F) and 24-month (G) follow-ups. *p < .05, ***p < .001 by unpaired two-tailed t test. Data expression with mean ± SD or median (interquartile range), according to normality results.
Cosmetic outcomes
After ablation, no skin edema and thickening, fibrosis, retraction and distortion were observed in all patients. Mass palpability was determined through physical examinations. Because the ablated masses were mostly become smaller in size and softer in texture during follow-up, the percentage of palpability was decreased significantly (; ), resulting in 43 (40%), 11 (10%), 5 (5%) in the number of palpable masses at 12, 18, 24-month follow-up (p < .001 by χ2 test), respectively. In the subset of tumors on different sizes (), 14 (87%) of masses in the size of ≤1 cm were impalpable in the follow-up of 3 months and only two masses remained but disappeared at the follow-up of 6 months (); 18 (35%) of masses in the size of 1–2 cm were impalpable at the follow-up of 6 months and 22 (42%) of masses were impalpable at the follow-up of 12 months (); 24 (62%) of masses in the size of ≥2 cm were impalpable at the follow-up of 18 months and 5 masses remained were still palpable at the follow-up of 24 months (). In the results of cosmetic satisfaction, furthermore, 51 (91%) patients reported excellent scale and 5 (9%) patients reported good scale. These results suggested that the good cosmetic outcomes were achieved in nearly all the masses after ablation 24 months.
Figure 5. Palpability outcomes during the follow-up. (A) Variance trends of palpable masses. ***p < .001 by χ2 test. (B–D) Number of impalpable masses by physical examination in different sizes of tumors at follow-up of 3, 6, 12, 18 and 24 months, respectively.
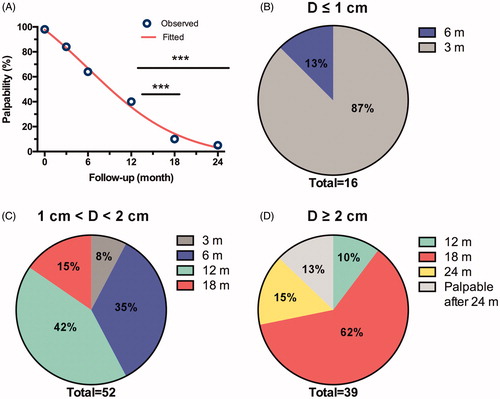
Table 3. Palpability outcomes during follow-up.
Discussion
In order to improve cosmetic outcomes in the treatment of breast benign tumors for women, image-guided ablative technique as a minimally invasive therapy is going to be one of the promising strategies in the clinic [Citation24]. But different from other ablative options such as RFA, laser and cryoablation [Citation12,Citation25,Citation26], the rationale of MWA is based on the rotation of dipole molecules in the surrounding tissue, which could produce higher intratumoral heating, larger volume of coagulative necrosis, less ablation duration, and improved stability on thermal conduction profile [Citation27–30]. Therefore, MWA is now receiving more and more attentions in the use of treatment of different tumors. Currently, several studies reported the successful experience of MWA for small breast cancer treatment but limited focus on breast benign tumors [Citation16,Citation31–34]. To the best of our knowledge, our study is the first time to explore the MWA performance in the minimum 12-month follow-up for the treatment of benign tumors in the breast.
Owning to the superficial location of breast and seldom vascularity in the tumor, MWA under US guidance is preferred to accurate guidance, portable operability and effective surveillance. Thereby, the perfect ablation of breast tumors without damage of any surrounding normal tissues will be achievement by an experienced doctor in theory. Ablative experience as a critical factor for accurate ablation has been recently analysed by Zhou and Wang [Citation35]. In comparison, the common use of vacuum-assisted biopsy inevitably deal with hemorrhage risk and unnecessary excision of normal tissue. In this study, a total of 56 patients with 107 breast benign tumors were successfully ablated by using US-guided MWA. Ablative procedure was well accepted in all patients except for one case with severe pain. All tumors showed technical success through the enhanced imaging examinations (CEUS or CEMR). During the relative long-term follow-up, both VRRs and palpabilities of ablated masses were remarkably descended and cosmetic satisfaction induced in good performance as well. These findings suggest that MWA, as a feasible and safe ablative technique, exhibits dramatic clinical outcomes in treatment of patients with breast benign tumors.
As to the relative research on MWA of breast tumors by Zhou et al, the ratio of complete ablation was 97.5% in 41 tumors [Citation16]. While, 100% of technical success in all tumors (a total of 107) was achieved in this study, consistent with that in our previous reported [Citation18]. The optimized therapeutic efficiency may attribute to the carefully planning before ablation and the use of pull-back techniques, which have been frequently adopted in the procedure of thyroid ablation [Citation11,Citation36]. Moreover, after using the hydro-dissection technique, no injuries were occurred on the skin and chest wall even tumor sites adjacent to skin, pectoralis and areola less than 0.2 cm, consistent with previous studies [Citation16,Citation18], indicating the extensive feasibility for MWA in breast tumors.
Since one concern of thermal ablation under local anesthesia is the pain for treatment of breast tumors [Citation14,Citation33,Citation34], local anesthesia for the combination of regional block and local infiltration in the ablation zone was adopted instead of lidocaine injection only to overcome this challenge. Finding was that only 2% patient (one case) was occurred in severe pain during the procedure, compared with previous report of ∼5% [Citation16], demonstrating a significant relief and valid control on pain. The underlying reason for the one case with severe pain is the unsuitable position of anesthetics injection. Importantly, after additional local anesthesia was performed again in this patient, technical success with slight pain was achieved in the end of the procedure.
Another primary issue in the ablative treatment is the extensive existence of palpable masses formed by coagulative necrosis at the MWA site, which may cause discomfort and anxiety to patients [Citation26]. Previous studies were only focused on the follow-up of less than 12 months. However, data on mass palpability with longer follow-up is not known. Compared with 40–60% of masses reported by ours and others was non-palpability at the follow-up of 12 months, the number of masses with non-palpability was detected to be 90%, 95% at the follow-up of 18, 24 months. The underlying factors related to the resorption of ablated masses were not very clear because of limited studies. Except for the previously reported, tumor sizes may be a key point. More studies are needed for systemic elaboration of the resorption mechanism.
Key limitations still existed in this study. First, albeit the minimum 12-month follow-up was achieved in all patients, patients with a routine long-term follow-up period of 3–5 years were warranted. Second, the number of tumors with larger than 3 cm in diameter was relatively minority. Further studies are needed to investigate the volume reduction and cosmetic satisfaction on the breast tumors in large size. Third, though the palpable masses became small and soft during follow-up, the unclear mechanisms and related factors should be systemically explored in the future. Given the above concerns, caution should be detected in the extrapolation of the results to large sample clinical trials.
Conclusions
Through the minimum 12-month follow-up in the prospective study, MWA under US guidance exhibits prominent outcomes on therapeutic efficiency, tumor volume reduction and cosmetic satisfaction for treatment of benign tumors in breast. This approach should be a feasible, safe, and effective strategy for minimally invasive therapy of breast benign tumors in clinic. More clinical trials with multiple centre data and large samples are still investigated to observe the treatment efficacy and compare the results with those of other therapeutic options.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lakoma A, Kim ES. Minimally invasive surgical management of benign breast lesions. Gland Surg. 2014;3:142–148.
- Hahn M, Krainick-Strobel U, Toellner T, et al. Interdisciplinary consensus recommendations for the use of vacuum-assisted breast biopsy under sonographic guidance: first update 2012. Ultraschall Med. 2012;33:366–371.
- Waldherr C, Berclaz G, Altermatt HJ, et al. Tomosynthesis-guided vacuum-assisted breast biopsy: a feasibility study. Eur Radiol. 2016;26:1582–1589.
- Fine RE, Staren ED. Percutaneous radiofrequency-assisted excision of fibroadenomas. Am J Surg. 2006;192:545–547.
- Sosin M, Pulcrano M, Feldman ED, et al. Giant juvenile fibroadenoma: a systematic review with diagnostic and treatment recommendations. Gland Surg. 2015;4:312–321.
- March DE, Coughlin BF, Barham RB, et al. Breast masses: removal of all US evidence during biopsy by using a handheld vacuum-assisted device-initial experience. Radiology. 2003;227:549–555.
- Berna-Serna JD, Guzman-Aroca F, Berna-Mestre JD, et al. A new method for the prevention of skin laceration during vacuum-assisted breast biopsy. BJR. 2017;90:20160866.
- Luo HJ, Chen X, Tu G, et al. Therapeutic application of ultrasound-guided 8-gauge Mammotome system in presumed benign breast lesions. Breast J. 2011;17:490–497.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23:1044–1049.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). International Journal of Hyperthermia. 2017;33:295–299.
- Mauri G, Sconfienza LM. (2016) Percutaneous ablation holds the potential to substitute for surgery as first choice treatment for symptomatic benign thyroid nodules. Int J Hyperthermia. 1–2.
- Peek MCL, Douek M. Ablative techniques for the treatment of benign and malignant breast tumours. J Ther Ultrasound. 2017;5:18.
- Waaijer L, Kreb DL, Fernandez Gallardo MA, et al. Radiofrequency ablation of small breast tumours: evaluation of a novel bipolar cool-tip application. Eur J Surg Oncol. 2014;40:1222–1229.
- Zhou W, Wang R, Liu X, et al. (2016) Ultrasound-guided microwave ablation: a promising tool in management of benign breast tumours. Int J Hyperthermia:1–8.
- Mauri G, Sconfienza LM, Pescatori LC, et al. Technical success, technique efficacy and complications of minimally-invasive imaging-guided percutaneous ablation procedures of breast cancer: A systematic review and meta-analysis. Eur Radiol. 2017;27:3199–3210.
- Yu J, Chen BH, Zhang J, et al. Ultrasound guided percutaneous microwave ablation of benign breast lesions. Oncotarget. 2017;8:79376–79386.
- Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209–216.
- Zhang W, Li JM, He W, et al. Ultrasound-guided percutaneous microwave ablation for benign breast lesions: evaluated by contrast-enhanced ultrasound combined with magnetic resonance imaging. J Thorac Dis. 2017;9:4767–4773.
- Xu J, Cao Y, Xu C, et al. (2016) Combination of microbubbles and diagnostic ultrasound at a high mechanical index for the synergistic microwave ablation of tumours. Int J Hyperthermia:1–25.
- Ahmed M, Solbiati L, Brace CL, et al. image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202.
- Sag AA, Maybody M, Comstock C, et al. Percutaneous image-guided ablation of breast tumors: an overview. Semin Intervent Radiol. 2014;31:193–202.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20:11–22.
- Kaufman CS, Bachman B, Littrup PJ, et al. Cryoablation treatment of benign breast lesions with 12-month follow-up. Am J Surg. 2004;188:340–348.
- Katrina F, Chu DED. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79:124–130.
- Liang P, Yu J, Lu MD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19:5430–5438.
- Lubner MG, Hinshaw JL, Andreano A, et al. High-powered microwave ablation with a small-gauge, gas-cooled antenna: initial ex vivo and in vivo results. J Vasc Interv Radiol. 2012;23:405–411.
- Zhou W, Jiang Y, Chen L, et al. Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur J Radiol. 2014;83:1771–1777.
- Zhou W, Zha X, Liu X, et al. US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology. 2012;263:364–373.
- Fleming MM, Holbrook AI, Newell MS. Update on image-guided percutaneous ablation of breast cancer. Am J Roentgenol. 2017;208:267–274.
- Fornage BD, Hwang RF. Current status of imaging-guided percutaneous ablation of breast cancer. Am J Roentgenol. 2014;203:442–448.
- Li C, Li C, Ge H, et al. Technical analysis of US imaging for precise microwave ablation for benign breast tumours. Int J Hyperthermia. 2018;6:1–7.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276:909–918.
