Abstract
Background: To evaluate the efficacy and safety of microwave ablation (MWA) followed by immediate biopsy in the treatment of non-small cell lung cancer (NSCLC) and to clarify whether pathology changes can predict treatment responses and patient survival.
Methods: Patients with pathologically confirmed NSCLC pre-ablation were treated with MWA, and immediate biopsy was carried out right after ablation in one procedure. Pathology changes were categorized according to the pre- and postablation pathology: Group A, same histology type; Group B, paired histology type with burning degeneration; Group C, no definite histology type; Group D, no definite cancer cells. The internal correlations between pathology changes and baseline characteristics, responses to MWA and survival were evaluated.
Results: A total of 68 patients were enrolled in the study, of which 19, 28, 11 and 10 patients were classified into Group A, Group B, Group C and Group D, respectively. In total, 85.3 and 69.1% patients were diagnosed with malignant tumors and the same pathology type, respectively. No significant difference in clinical-pathologic characteristics or response to MWA between the groups was observed. Upon combining Groups A, B and C, Group D exhibited longer progression-free survival (PFS) (Groups A + B + C versus Group D, 11.7 months, 95% CI 9.6–13.7 versus 26.6 months, 95% CI 19.0–34.2, p = .253) and overall survival (OS) (15.9 months, 95% CI 14.2–17.5, versus 29.8 months, 95% CI, 24.3–35.3, p = .395), although no significant differences were observed. Complications were identified in 63 (92.6%), of which 17 (25.0%) patients had major complications.
Conclusions: Immediate biopsy post-MWA can distinguish cancer cells or histology types in most cases of NSCLC. However, pathology changes pre- and postablation could not predict the response to MWA and patient survival.
Introduction
Lung cancer remains the leading cause of mortality and morbidity among malignant cancers [Citation1]. Non-small cell lung cancer (NSCLC) accounts for nearly 85–90% of all cases of lung cancer.
Radical surgery, especially lobectomy, is the standard treatment for early-stage NSCLC. However, due to poor pulmonary or cardiovascular function, about 25% of patients, who are more than 65 years old is unable to undergo any type of surgical excision [Citation2]. Sub-lobar resection or stereotactic radiotherapy (SBRT) is an option for these patients. An alternative to radical surgery is thermal ablation, such as radiofrequency ablation (RFA) or microwave ablation (MWA) [Citation3–9]. For advanced NSCLC patients, MWA can also be used in combination with chemotherapy [Citation10–12]. Our previous study revealed that patients with advanced NSCLC had superior survival when treated with a combination of MWA and chemotherapy [Citation11].
Unlike surgery, patients treated with thermal ablation need to undergo a biopsy of the tumors to confirm the definite histology types and to obtain tissues for biomarker assessment [such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS1 or programmed death receptor 1 (PD-L1)]. Biopsy can be achieved in a different day before ablation as a separate procedure, or postablation on the same day in one procedure. However, preablation in a different day means an extra procedure. Hasegawa et al. [Citation13,Citation14] reported that immediate biopsy post-RFA could verify the previous histological diagnosis. For colorectal cancer patients or lung cancer patients treated with thermal ablation, cancer cells in the postablation biopsy were able to predict a higher incidence of local tumor progression (LTP) [Citation15–17].
Nonetheless, no studies to date have explored immediate biopsy after MWA in NSCLC patients. If biopsy performed immediately after MWA results in the same diagnosis as biopsy performed before MWA, then MWA and biopsy can be performed during one procedure. Therefore, we conducted this retrospective study to clarify whether postablation biopsy could verify the preablation histological diagnosis and to determinate whether pre- and postablation pathology changes could predict the response to MWA or patient survival.
Patients
Inclusion and exclusion criteria
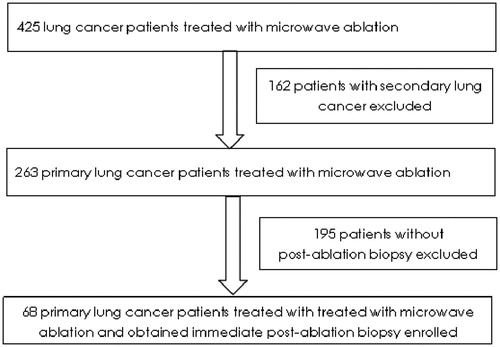
Treatment-naive patients with pathologically verified NSCLC were enrolled. Other inclusion criteria were as follows: (1) age ≥18 years old; (2) Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0–1; (3) ablative tumors located in the lung periphery; (3) stages I–IIIA patients who were poor candidates for radical surgery or refused lobectomy, sublobar surgery, or SBRT; (4) stages IIA–IV patients in whom MWA was conducted at the primary tumor site only and at least one measurable tumor site other than the ablative sites; (5) a biopsy performed immediately after MWA; (6) adequate formalin-fixed and paraffin-embedded (FFPE) tissues (at least 70% tumor tissue) for pathological analysis; (7) adequate bone marrow, hepatic function and renal function.
Patients meeting the following criteria were excluded: (1) other primary tumors over the past 5 years; (2) NSCLC in combination with SCLC; (3) secondary lung cancer treated with MWA; (4) central primary lung cancer or tumors adjacent to major vessels or the trachea.
MWA procedure
MWA was conducted under computed tomography (CT) guidance. The MWA procedure was described in detail in our previous studies [Citation6–9]. Two antennae were applied for patients with a tumor size larger than 3.0 cm; one antenna was applied for those with a tumor size no larger than 3.0 cm. Ground-glass opacity (GGO) of 0.5–1.0 cm larger than the tumor was considered technical success.
Biopsy procedure
A 16-gauge or 18-gauge biopsy core needle (PRECISA finecore needle; H.S. Hospital Service S.P.A, Aprilia, Italy) was inserted into the centre of the tumor through a coaxial cannula before initiating MWA. Upon completing the ablation, biopsy was conducted to obtain three specimens from one core needle, after which the microwave antennae were withdrawn from the puncture pathway. The tissues were preserved in 10% formalin and cut into 4-µm-thick slices. The tissues were pathologically examined after H&E staining.
Pathology changes after MWA
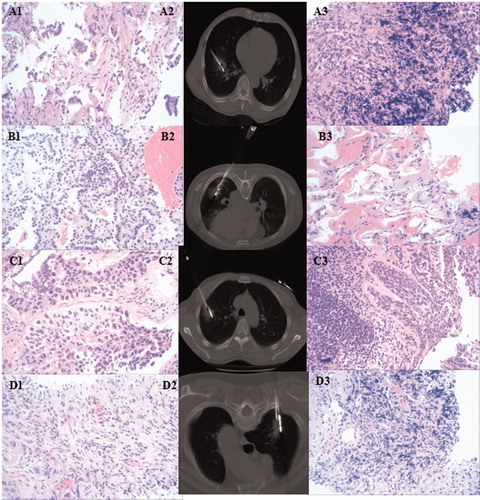
Preablation biopsy was conducted on a different day. Postablation biopsy was conducted once the ablation procedure was finished. Postablation pathology changes were classified into four groups: Group A: preablation definite histology type, postablation the same definite histology type; Group B: preablation definite histology type, postablation the same definite histology type with burning generation; Group C: preablation definite histology type, postablation definite cancer cells, no definite histology type; Group D: preablation definite histology type, postablation no definite cancer cells.
Postablation treatments
No postablation treatments were performed on stage I patients. Platinum-based doublet chemotherapy was administered to stages IIIB–IV patients without EGFR-sensitive mutations or ALK gene fusion and to stages II–IIIA patients regardless of EGFR and ALK status. For stages IIIB–IV patients with sensitive mutations, EGFR tyrosine kinase inhibitors (TKIs) or ALK inhibitors were recommended.
Response of MWA
Enhanced chest contrast CT was performed within 15 days before treatment, at 24–48 h, and at 1, 3, 6, 12, 18, 24 and 36 months after MWA. The evaluation of response to MWA was done at 1 month after MWA. The response to chemotherapy was evaluated every 6 weeks during the treatment, and the response to targeted therapy was evaluated every 2 months during follow-up. A tumor that was treated according to protocol and covered completely as determined at the time of the procedure was deemed a ‘technical success’. ‘Technique efficacy’ refers to a defined prospective time point, when ‘complete ablation’ of macroscopic tumor, as evidenced by imaging follow-up was achieved [Citation18]. Complete ablation, indicated by lesion disappearance, complete cavitation formation, fibrotic progression or scar, solid nodule involution or no change and/or atelectasis, presenting no contrast-enhanced signs on CT images, was considered technical efficacy. Incomplete ablation was indicated by the following: incomplete cavernous formation with some remaining solid or liquid components; partial fibrosis or fibrotic lesions with solid residues; and/or solid nodules with unchanged or increased size displaying irregular peripheral or internal enhancement signs on CT images [Citation19].
Complications of MWA
Complications were assessed according to the standards of the International Working Group on Image-Guided Tumor Ablation [Citation18]. The Society of Interventional Radiology (SIR) classification system for complications by outcome was applied. Minor complications included (A) no therapy, no consequence and (B) nominal therapy, no consequence, including overnight admission for observation only. Major complications included the following conditions: (C) requiring therapy or minor hospitalisation (<48 h); (D) requiring major therapy, an unplanned increase in the level of care, or prolonged hospitalisation (>48 h); (E) permanent adverse sequelae; and (F) death. Adverse events referred to pain, postablation syndrome, and asymptomatic minor bleeding or fluid accumulation as detected by CT. Postablation syndrome was transient, self-limiting symptoms/signs of low-grade fever, nausea, vomiting and/or general malaise [Citation19].
Statistical analyses
SPSS 17.0 was used for analyses. All tests were two-sided, and a p values less than .05 was considered significant. Progression-free survival was calculated from the date of MWA to the date of disease progression, including LTP or death. Overall survival was calculated from the date of MWA to death. Time to local progression (TTLP) was calculated from the date of MWA to the date of LTP. Correlations between clinicopathological characteristics and pathology changes pre- and postablation were assessed by the chi-squared test. The correlation between changes in pathology and survival was evaluated with the Kaplan–Mirer method using the log-rank test.
Results
Baseline characteristics
From January 2015 to June 2017, 68 patients were enrolled according to the inclusion and exclusion criteria (). Among them, 39 patients (57.4%) were male, and 42 (61.8%) were ≥65 years old. Thirty-four patients had a history of smoking, and 59 (86.8%) had an ECOG performance of 1. The common pathology was adenocarcinoma, which occurred in 55 patients (80.9%). Thirty-three patients (48.5%) were at stages IIIB–IV; 35 patients (51.5%) were in the early stage but refused lobectomy (details of the baseline characteristics of the 68 enrolled patients are shown in ).
Table 1. Baseline characteristics of 68 enrolled patients.
Postablation pathology changes
Immediate biopsy after MWA was conducted for all patients. Patients were classified into four groups according to pathology changes: Group A, Group B, Group C and Group D contained 19 (27.9%), 28 (41.2%), 11 (16.2%) and 10 (14.7%) patients, respectively (). In total, 58 (85.3%) of the patients were diagnosed with malignant tumors, and the same definite pathology type was determined for 47 (69.1%) of the patients.
No significant differences in baseline characteristics or ablation parameters were observed among the four groups ().
Table 2. Baseline characteristics of four groups.
Specimens from postablation biopsy of 47 patients belonging to Groups A, B or C with adenocarcinoma were used for the detection of EGFR mutations, ALK and ROS1 refusion genes ().
Figure 2. Pathology changes in the four groups. Group A: A1 (adenocarcinoma before MWA), A2 (MWA followed by immediate biopsy), A3 (adenocarcinoma after MWA); Group B: B1 (adenocarcinoma before MWA), B2 (MWA followed by immediate biopsy), B3 (adenocarcinoma after MWA, with burning degeneration). Group C: C1 (squamous cell carcinoma before MWA), C2 (MWA followed by immediate biopsy), C3 (definite cancer cells, no definite histology type); Group D: D1 (squamous cell carcinoma before MWA), D2 (MWA followed by immediate biopsy), D3 (no definite cancer cell).
Correlation between pathology changes and response or survival
Until the last follow-up on November 19, 2017, 28 patients (41.2%) had disease progression, including 6 (8.8%) with LTP, and 14 (20.6%) died, with a mean follow-up time of 11.3 months (range, 5.0–33.0 months). The mean PFS, TTLP and OS were 18.6 months (95% CI, 14.7-22.6), 28.6 months (95% CI, 25.3–32.0) and 26.4 months (95% CI, 23.4–29.4), respectively.
Technical success and technique efficacy were achieved in 68 (100.0%) and 51 (75.0%) patients among the 68 procedures, respectively. Complete ablation was observed in 15 (78.9%), 20 (71.4%), 7 (63.6%) and 9 (90.0%) patients in Group A, Group B, Group C and Group D, respectively (p = .781).
Table 3. Survival between the groups.
As the number of patients with PFS, TTLP and OS events was small, we combined Group A and Group B for comparison with Group C and Group D. The corresponding mean PFS was 10.8 months (95% CI, 8.8–12.7), 11.0 months (95% CI, 6.3–15.8), and 26.6 months (95% CI, 19.0 − 34.2) (p = .519) for Group A + B, Group C and Group D, respectively (). OS for Group A + B versus Group C versus Group D was 14.0 months (95% CI, 12.4–15.6) versus 16.4 months (95% CI, 12.7–20.1) versus 29.8 months (95% CI, 24.3–35.3) (p = 0.632) () ().
Figure 3. The Kaplan–Mirer curve of survival among groups. A: Group A + B versus Group C versus Group D: A1: PFS; A2: OS. B: Group A + B + C versus Group D: B1: PFS; B2: OS.
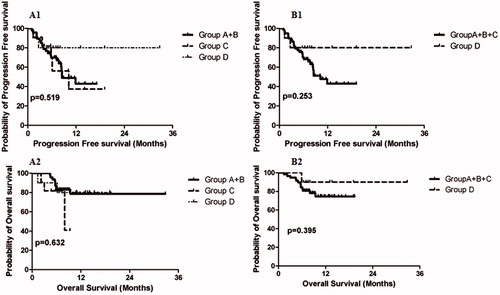
When Group A, Group B and Group C were combined and compared with Group D, longer PFS and OS was noted for patients in Group D, although the difference was not significant. The mean PFS for Group A + B + C versus Group D was 11.7 months (95% CI, 9.6–13.7) vs. 26.6 months (95% CI, 19.0–34.2), (p = .253) (), and the mean OS was 15.9 months (95% CI, 14.2–17.5) versus 29.8 months (95% CI, 24.3–35.3) (p = .395) () ().
Safety of MWA and immediate biopsy
Major complications and minor complications were observed in 17 (25.0%) and 24 (35.3%) patients, respectively. The common major complications included pneumothorax (10 patients), pleural effusion (6 patients), pulmonary infection (2 patients) and chest bleeding (1 patient). All patients with symptomatic pneumothorax and pleural effusion were resolved with insertion of a chest tube with a mean interval of 5 days (range 1–14 days). Two patients with pulmonary infection received anti-inflammatory therapy. In addition, 50 patients (73.5%) experienced adverse events, including 20 patients with pain, 17 with postablation syndrome, 13 patients with asymptomatic minor bleeding or fluid accumulation.
Discussion
This study is the first research to explore immediate biopsy post-MWA in NSCLC treatment. The results showed that 85.3 and 69.1% of patients were diagnosed with malignant tumors and the same pathology type, respectively. However, pathological changes postablation showed no correlation with baseline characteristics or response to MWA. PFS and OS were superior in the patients of Group D than those in Group A + B, Group C and Group A + B + C, although no significant differences were observed.
Surgery or biopsy postablation has been applied in several small sample studies. For example, Steinke et al. [Citation20] first reported complete ablation of metastasis in one patient with pulmonary metastasis treated with RFA followed by surgery 3 weeks later, and Scott et al. [Citation21] reported complete ablation in four of five patients (80%) treated with RFA followed by surgery. Nguyen et al. [Citation22] also found that although tumor necrosis was commonly observed in lung cancer patients treated with RFA, complete necrosis was identified in 3 of 8 patients (37.5%) and more than 80% tumor necrosis was observed in four of the five remaining patients (80.0%). Additionally, Ambrogi et al. [Citation23] conducted a study with nine early-stage NSCLC patients treated with RFA and immediate surgery or postablation surgery 15 days later, with complete ablation observed in 6 (66.7%) patients. Belfiore et al. [Citation24] reported that 19 patients with pulmonary tumors were treated with RFA, and biopsy was conducted six months postablation; cytohistologic examinations showed total coagulation necrosis in 7 lesions (36.8%) and partial necrosis in 12 lesions (63.2%). In another study, H&E-stained histologic biopsy specimens at 2 months of follow-up revealed necrosis, fibrosis or both, with no viable cells in 20 of the 33 ablation zones (60.6%) and morphologically residual tumor cells in 13 ablation zones (39.4%) [Citation25].
According to the results of these studies, not all patients treated with RFA experienced complete necrosis. Thus, biopsy or surgery postablation may determine the histology. Only two studies to date have explored pathology changes pre- and postablation. In one, post-RFA biopsy was able to identify the histology type, in accordance with the histology type preablation [Citation13]. In the other, which was a study with a large sample size, post-RFA biopsy was able to identify pathology in most patients [Citation14].
In this study, patients were divided into four groups according to their pre-ablation and postablation pathology changes: Groups A, B, C and D containing 19, 28, 11 and 10 patients, respectively, were identified. Malignancy or even the same definite histology type was distinguished in the majority of patients. Specimens from postablation biopsy were also used for the sensitive mutations test. Most adenocarcinoma patients could undergo the sensitive mutations. Therefore, immediate biopsy postablation may be an alternative for NSCLC patients who must undergo biopsy and MWA on the same day. However, it appears that the necrosis rate in this study was lower than that in previous studies, which may be explained by several factors. First, the interval between thermal ablation and biopsy or surgery varies. As opposed to no interval, previous studies had intervals of 15 days, 3 weeks or even 6 months. In our study, biopsy immediately after ablation was performed in all patients [Citation20,Citation22–26]. Second, tumor sizes and stages differ among studies. Most studies have restricted the tumor size to less than 3.5 cm and clinical stages to stages I–IIA [Citation20–24]. In this study, we enrolled 33 patients (48.5%) with stage IIIB or IV tumors, and a tumor diameter ≥3.0 cm was observed in 33 patients and ≥3.5 cm in 24 patients. Multiple studies have verified that tumor size is significantly associated with the complete necrosis rate. Previous studies on both early-stage and advanced NSCLC patients have reported a tendency of incomplete ablation in patients with a tumor size greater than 3.0 or 3.5 cm compared to those with tumors less than 3.0 or 3.5 cm [Citation7,Citation26].
Regarding baseline characteristics, ablation indices, response to MWA and survival postablation, no significant differences were found among Groups A, B, C and D. Group D had greater PFS and OS compared with Group A + B and Group A + B + C, but no significant differences were found. Sotirchos et al. [Citation15] verified that proof of complete tumor ablation at both the centre of the ablation and minimal ablation margins of at least 5 mm via biopsy are independent predictors of LTP, and they yield the best oncologic outcomes in colorectal cancer tumors with liver metastases. Additionally, Snoeren et al. [Citation16] showed that adherence of viable tumor cells to the needle applicator used for local ablation is an independent risk factor for LTP. Sofocleous et al. [Citation17] also found that the presence of Ki-67+ tumor cells on the electrode after pulmonary tumor RF ablation was an independent predictor of LTP, local tumor PFS and disease-specific survival. It is possible that the small sample size of patients and the limited follow-up duration prevented the observed differences from reaching significance in the current study.
Complications occurred in 63 patients and commonly included pneumothorax, pleural effusion and bleeding. However, major complications were found in only 17 patients and most major complications were pneumothoraxes managed by thoracotomy, indicating that MWA combined with postablation biopsy is safe. However, there were also several limitations in the study: small sample size, lack of tumor cell viability test and short follow-up period.
In conclusion, immediate biopsy after MWA in one procedure is safe and effective for NSCLC patients. Meanwhile, pre- and postablation pathology changes could not predict MWA response and patient survival.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205.
- Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211:68–72.
- Powell JW, Dexter E, Scalzetti EM, et al. Treatment advances for medically inoperable non-small-cell lung cancer: emphasis on prospective trials. Lancet Oncol. 2009;10:885–894.
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9:621–628.
- Yang X, Ye X, Huang G, et al. Repeated percutaneous microwave ablation for local recurrence of inoperable Stage I nonsmall cell lung cancer. J Can Res Therapy. 2017;13:683–688.
- Han X, Yang X, Ye X, et al. Computed tomography-guided percutaneous microwave ablation of patients 75 years of age and older with early-stage nonsmall cell lung cancer. Indian J Cancer. 2015;52:56–60.
- Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34:96.
- Nour-Eldin NA, Exner S, Al-Subhi M, et al. Ablation therapy of non-colorectal cancer lung metastases: retrospective analysis of tumour response post-laser-induced interstitial thermotherapy (LITT), radiofrequency ablation (RFA) and microwave ablation (MWA). Int J Hyperthermia. 2017;33:1–9.
- Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142.
- Wei Z, Ye X, Yang X, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32:464.
- Zhong L, Sun S, Shi J, et al. Clinical analysis on 113 patients with lung cancer treated by percutaneous CT-guided microwave ablation. J Thorac Dis. 2017;9:590–597.
- Hasegawa T, Kondo C, Sato Y, et al. Diagnostic ability of percutaneous needle biopsy immediately after radiofrequency ablation for malignant lung tumors: an initial experience. Cardiovasc Intervent Radiol. 2016;39:1187–1192.
- Hasegawa T, Kondo C, Sato Y, et al. Pathologic diagnosis and genetic analysis of a lung tumor needle biopsy specimen obtained immediately after radiofrequency ablation. Cardiovasc Intervent Radiol. 2018;41:594–602.
- Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: Biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280:949–959.
- Snoeren N, Huiskens J, Rijken AM, et al. Viable tumor tissue adherent to needle applicators after local ablation: a riskfactor for local tumor progression. Ann Surg Oncol. 2011;18:1527–1510.
- Sofocleous CT, Garg SK, Cohen P, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol. 2013;20:676–683.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Ye X, Fan W, Wang H, et al. Expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition). J Cancer Res Ther. 2018;14:730–744.
- Steinke K, Habicht JM, Thomsen S, et al. CT-guided radiofrequency ablation of a pulmonary metastasis followed by surgical resection. Cardiovasc Intervent Radiol. 2002;25:543–546.
- Scott WJ, Young N, Goldberg M, et al. Tumor cell viability following radiofrequency ablation of resectable primary lung cancer: initial results from an ablate and resect study. Lung Cancer. 2003;41:S135.
- Nguyen CL, Scott WJ, Young NA, et al. Radiofrequency ablation of primary lung cancer: results from an ablate and resect pilot study. Chest. 2005;128:3507–3511.
- Ambrogi MC, Fontanini G, Cioni R, et al. Biologic effects of radiofrequency thermal ablation on non–small cell lung cancer: Results of a pilot study. J Thorac Cardiovasc Surg. 2006;131:1002–1006.
- Belfiore G, Moggio G, Tedeschi E, et al. CT-guided radiofrequency ablation: a potential complementary therapy for patients with unresectable primary lung cancer—a preliminary report of 33 patients. AJR. 2004;183:1003–1011.
- Yasui K, Kanazawa S, Sano Y, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231:850–857.
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758–763.