Abstract
Background: It is believed that the oncologic behavior of mucinous colorectal adenocarcinoma (MC) is different from non-mucinous adenocarcinoma (NMC). The aim of the study is to compare long-term survivals between patients with MC and those with NMC following cytoreductive surgery (CRS) and intraperitoneal chemotherapy (IPC).
Methods: This was a retrospective study of prospectively collected data of patients with peritoneal metastases of colorectal origin following CRS and IPC. Group I included patients with MC which was defined as being composed of >50% extracellular mucin. Group II included those with NMC. Subgroup analysis was performed according to the location of primary tumor.
Results: A total of 213 patients were included in this study. The two groups had similar hospital mortality, high dependency unit stay. MC group had a significantly longer mean intensive care unit (ICU) stay (p = .037) and total hospital stay (p = .037). There was no significant difference in overall survival (OS) and disease-free survival (DFS) between two groups (p = .657 and p = .938, respectively). Multivariate analysis showed that the presence of mucin was not an independent negative prognostic factor for OS (p = .190).
Conclusion: In summary, patients with MC had a similar long-term survival outcome with those with NMC following CRS and IPC.
Introduction
Mucinous colorectal adenocarcinoma (MC) is a histologic variant of colorectal cancer and accounts for 3.9–19% of colorectal cancers [Citation1]. It is characterized by abundant extracellular mucin of more than 50% of tumor volume [Citation2]. It is believed that the oncologic behavior of MC is different from non-mucinous adenocarcinoma (NMC) [Citation1]. MC is suggested to be more common in younger patients and proximal colon. It seems more aggressive and associated with villous adenomas and poor prognosis [Citation2].
Cytoreductive surgery (CRS) combined with intraperitoneal chemotherapy (IPC) has significantly improved the survival for those with peritoneal metastases of colorectal origin (PM) and has become the gold standard therapy for it in last few decades [Citation3]. CRS involves surgical resection of macroscopic disease to minimize residual tumor burden within the abdomen. After CRS, IPC administers a heated chemotherapy into the abdomen to achieve a high local concentration of chemotherapy drug locally, targeting at microscopic residual diseases [Citation4]. A multicentric French study has shown an encouraging median survival of 30.1 months with a 1-year, 3-year and 5-year survival of 81%, 41% and 27%, respectively for PM following CRS and IPC [Citation5,Citation6].
The current hypothesis in the literature is that MC is a distinct subtype as compared to NMC. However, the evidence is still limited. These two groups of patients receive the same first-line treatment at present [Citation7]. The aim of this study is to compare long-term survivals between patients with MC and those with NMC following CRS and IPC.
Materials and methods
Settings
This is a retrospective study of prospectively collected data of patients with peritoneal metastases of colorectal origin, who underwent CRS and IPC by the same surgical team at St George hospital, Sydney, Australia between January 1996 and Jan 2018. All the clinical and treatment-related data were collected and entered into a computerized database to evaluate the perioperative outcomes of these patients. A signed informed consent was obtained from all patients.
Patients
Patients had a good performance status (World Health Organization Performance Status ≤2), and had a histological diagnosis of PM. All patients were managed by a standard treatment protocol combining CRS and PIC. Suitability to undergo CRS and PIC was evaluated during a regular weekly meeting attended by a multidisciplinary team (MDT) including surgical oncologists, medical oncologists, radiologists, cancer care nurses and research staff. Exclusion criteria include synchronous liver metastases at the time of operation, debulking surgery (i.e., no PIC was given) or incomplete cytoreduction.
Patients were divided into two groups. Group I included patients with MC which was defined as being composed of >50% extracellular mucin. Group II included those with NMC. Subgroup analysis was performed according to the location of primary tumor.
Preoperative management
All patients underwent standard preoperative investigations which included physical examination; double contrast-enhanced computed tomography (CT) scans of the chest, abdomen and pelvis; and CT portography or primovist magnetic reasonance imaging of the liver. Positron emission tomography was performed in all patients in addition to the stating laparoscopy to assess the PCI if the scans showed borderline results.
Our current selection criteria for consideration of CRS and hyperthermic intraperitoneal chemotherapy (HIPEC) included PCI ≤15, PCI <10 in the presence of liver metastases (maximum of four liver metastases), being able to perform complete cytoreduction, absence of extra-abdominal disease, no evidence of progressive disease in preoperative chemotherapy and no severe comorbidity. In early years, PCI was limited to 20 in patients with colorectal cancer. This was lowered to 15 in 2012. We would also consider repeat CRS and HIPEC if PCI <10 and recurrence after 12 months after primary CRS and IPC.
CRS
An initial assessment of the volume and extent of disease was recorded using PCI. This assessment combines maximal diameter of lesion size (LS) (LS 0: no Macroscopic tumor; LS 1: tumor <0.5 cm; LS 2: tumor 0.5–5 cm; and LS3: tumor >5 cm) with tumor distribution (abdominopelvic region 0–12) to quantify the extent of disease as a numerical score (PCI 0–39). CRS was performed using Sugarbaker’s technique [Citation8].
All sites and volumes of residual disease following CRS were recorded prospectively using CC score (CC0-no Macroscopic residual cancer remained; CC1-no nodule >2.5 mm in diameter remained; CC2-nodules 2.5 mm-2.5 cm in diameter remained; CC3-nodules >2.5 cm in diameter remained) [Citation9]. In patients with colorectal cancer (CRC), only complete cytoreduction (i.e., CC0 or CC-1) is considered appropriate and included in this study. Perioperative complications in all patients were graded I to IV with increasing severity based on the Clavien-Dindo classification (Grade I: no treatment; Grade II: medications only; Grade III: surgical, endoscopic or radiological intervention; Grade IV: life-threatening complications requiring intensive care unit (ICU) admission) [Citation10]. Major morbidity was defined as grade III or grade IV complications.
Hyperthermic intraperitoneal chemotherapy
After complete CRS, but prior to intestinal anastomosis or repair of seromuscular tears, HIPEC was performed by installation of a heated chemoperfusate into the abdomen using the coliseum technique at approximately 42 °C. Oxaliplatin 350 mg/m2 in 500 mL of 5% dextrose was given over 30 min or mitomycin C 12.5 mg/m2 in 3 L of 1.5 dextrose peritoneal dialysis fluid if oxaliplatin in contraindicated.
Early postoperative intraperitoneal chemotherapy
Patients with peritoneal dissemination of low-grade appendiceal mucinous neoplasms (LAMNs) are routinely offered. Early postoperative intraperitoneal chemotherapy (EPIC) is not routinely performed in patients with PM. However, in cases where there was lack of availability of HIPEC (e.g., emergency) or where there may have been contraindication to oxaliplatin or MMC, patients received EPIC. Furthermore, in instances where the macroscopic appearance suggested abundant areas of low-grade appendiceal disease or pseudomyxoma peritonei, patients were consented to receive EPIC. 5-Fluorouracil (5-FU) (650 mg/m2) following CRS/HIPEC was administered either in ICU or high dependency unit (HDU) on postoperative days 2–6. The criteria for EPIC have been previously reported [Citation11].
Follow-up
All patients were followed up at monthly intervals for the first three months and six-monthly intervals thereafter until the last time of contact or death. The follow-up review included clinical examination, measurement of tumor markers and assessment of CT scans with or without PET scans.
Statistical analysis
All statistical analyses were performed by using IBM SPSS for Windows version 22. Comparison of normally distributed variables was performed using analysis of variance (one way-ANOVA) test. Categorical variables were analyzed using the Chi-square test or Fisher’ exact test where appropriate. Perioperative morbidity and mortality were the primary outcomes of this study. Hospital mortality was defined as any death that occurred during the same hospital admission for CRS. Median survival was calculated based on the date of death or last follow-up in the unit of months. Survival analysis was performed using the Kaplan–Meier curves and Log Rank test for comparison. A subgroup analysis was further on presence of liver metastases. Prognostic factors for survival were evaluated using the Cox proportional hazards regression model for the multivariate analysis. A significant difference was defined as p values <.05.
Results
Descriptive characteristics
A total of 319 patients were diagnosed with PM. Eight patients were excluded because of incomplete cytoreduction (i.e., CC2 and CC3). Five patients were excluded from the study due to missing information on details of histopathology. Three hundred and six patients formed the cohort of this study. It includes 213 patients with NMC (69.6%) and 93 patients with MC (30.4%). summarized patients’ background characteristics. 42.5% of patients were males (n = 130). The median age was 57.0 years old (Range 15–84; Mean 55.4, Standard Deviation (SD) = 13.5). The overall mean PCI was 9.6 (SD = 6.5, Median = 9.0, Range 0–35).
Table 1. Patient characteristics.
67 patients had liver metastases (21.9%) whereas lymph node involvement was present in 209 patients (72.3%). There were more females who were diagnosed with NMC whereas more males were diagnosed with MC (p = .017). Patients with MC also had a significantly higher mean PCI as compared to those with NMC (p < .001). There were more patients who were found to have signet cells in MC group as compared to those in NMC group (26.2% vs. 1.6%, p < .001). In contrast, more patients in NMC group had liver metastases at the time of surgery as compared to patients with MC (27.1% vs. 8.6%, p < .001). In addition, more patients with MC received HIPEC (p = .035). There was no statistical difference in mean age (p = .776), use of EPIC (p = .757), site of primary tumor (p = .322) and use of preoperative chemotherapy (p = .744).
Perioperative mortality and morbidity
summarized the perioperative mortality and morbidity results. The overall hospital mortality was 0.7% (n = 2) with an overall major morbidity rate of 33% (n = 101). The overall mean ICU, HDU, total hospital stay was 3.2 days (SD = 6.8, median = 2.0, range = 0–101), 2.1 days (SD = 4.1, median = 1.0, range = 0–38) and 22.9 (SD = 21.6, median = 17.0, range = 3–206). Patients with MC had a significantly longer mean ICU stay and total hospital stay (p = .037 and p = .037, respectively, ). There was no difference in hospital mortality (p = .347), major morbidity rate (p = .750) and mean length of HDU stay (p = .820) ().
Table 2. Perioperative mortality and morbidity and survival outcomes.
Survival outcomes
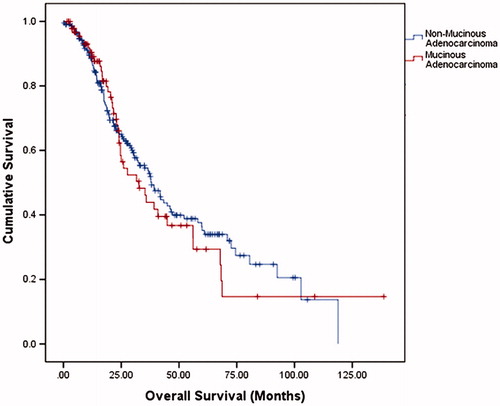
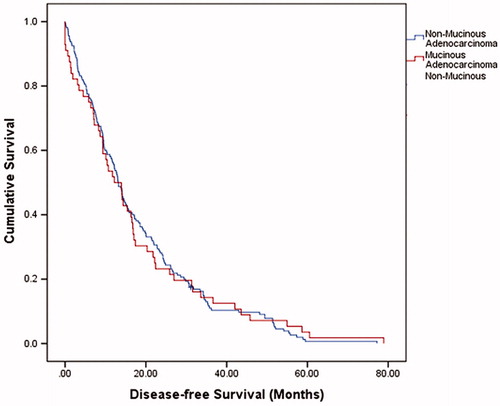
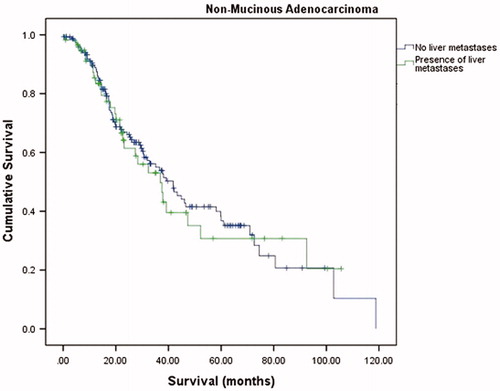
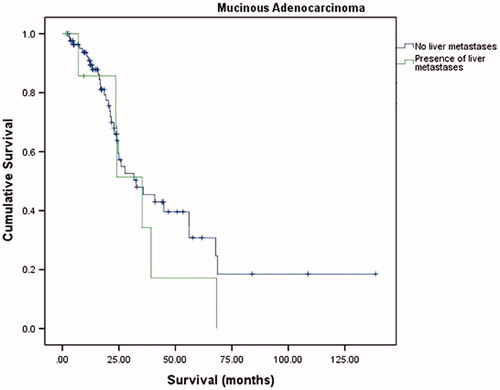
The median overall survival (OS) was 37.5 months (95% confidence interval (CI) = 31.5–43.5) with a 1-year OS, 3-year OS and 5-year OS of 88.6%, 51.8% and 33.7%, respectively. The median disease-free survival (DFS) was 13.1 months (95% CI = 11.4–14.8). summarizes the overall OS, 1-year OS, 3-year OS and 5-year OS of two groups. There was no significant difference in OS and DFS between NMC and MC groups (p = .657 and p = .938, respectively) (, and ). A subgroup analysis was performed on presence of liver metastases. There were no significant differences in the long-term survivals of patients with MC (p = .805, ) and those with NMC (p = .346, ).
Table 3. Survival outcomes.
Multivariate analysis using cox-regression model showed presence of mucin was not an independent negative prognostic factor for OS (HR = 1.41, 95%CI = 0.84–2.36, p = .190), adjusted for PCI, presence of liver metastases and lymph node involvement, age, use of HIPEC, tumor grade, use of preoperative chemotherapy and site of primary tumor ().
Table 4. Univariate and multivariate analysis of prognostic factors for OS.
Discussion
The prognostic significance of presence of secretorymucin remains controversial. Some studies have reported poor prognosis with MC [Citation12–15], whereas others failed to demonstrate the difference in survival outcomes between patients with MC and those with NMC [Citation16,Citation17]. A recent meta-analysis performed by Verhulst et al. analyzed 44 studies and suggested that mucinous differentiation results in a 2–8% increased hazard of death after correction for stage. However, there was a significant heterogeneity in included studies [Citation18]. A recent study performed by Yu et al. demonstrated an improvement in cancer-specific survival for patients with stage III and high-risk stage II NMC after receiving oxaliplatin in addition to 5-FU, however, it failed to demonstrate a benefit with addition of oxaliplatin for patients with stage III or stage II MC [Citation19]. It may reflect the oncologic behaviors between different histological subtypes.
Poorer prognosis associated with MC could be explained by several mechanisms. It allows tumor cells to gain access to peritoneal cavity. Also, mucoid material is taken up by regional lymph nodes, facilitating lymphatic spread [Citation20,Citation21]. In addition, mucin may also interfere with inflammatory response and immunological recognition of malignant cells [Citation22]. Another possible reason could be due to poorer responsiveness to chemotherapy [Citation7] One study compared the molecular features between carcinoma with signet ring cell component and carcinoma with mucinous component but no signet cell component. They found similar molecular properties between these two groups, including higher incidence of BRAF mutation, MSI and MLH1 loss [Citation23].
These two groups of patients demonstrated a similar incidence of hospital mortality and major morbidity. However, patients with NMC had a significantly shorter ICU stay and total hospital stay. Our findings in this study did not demonstrate a long-term survival difference between these two groups in both univariate analysis and multivariate analysis. One of the recent studies performed by Park et al. analyzed survival outcomes of 6475 patients with stages I to III who underwent radical surgery. They identified a 5-year OS survival difference (81.3% and 87.4% for patients with MC and those with NMC, respectively p = .005) [Citation1]. Interestingly, a recent study performed by Hugen et al. demonstrated the poor prognosis for MC is only present in rectal cancer. With adjuvant chemotherapy, there was no difference in efficacy of chemotherapy between MC and NMC. The reason is unclear but it could be due to the fact that MCs in the rectum are usually larger and often have a positive margin after resection [Citation24].
In our study, there was no significant difference in OS outcomes between MC and NMC, including rectal subgroup. Thus in patients with PM who underwent CRS and IPC, MC might not necessarily indicate a poor prognosis and at the current time should not be considered a contraindication to treatment. The observation in our sample that synchronous liver metastasis was more likely in the mucinous tumors warrants further investigation. Mucinous tumors may have molecular characteristics that make liver metastasis less likely however our observation could also reflect bias introduced by our local selection criteria for simultaneous liver resection an cytoreduction being four or fewer liver metastasis and PCI less than or equal to 10. Mucinous tumors tended to have higher PCI. Kermanshahi et al. have demonstrated previously that mucinous tumors are less likely to develop liver metastasis and more likely to develop peritoneal disease [Citation25]. A recent study of a Dutch cohort has shown colorectal cancer peritoneal metastases to be enriched for the consensus molecular subtype (CMS) four or the mesenchymal subtype. Eight of twenty-four of these patients had mucinous adenocarcinoma [Citation26]. In a review of clinical, morphological and molecular classification of colorectal cancer, Jass notes that mucinous differentiation is not specific to clinicopathological subtypes [Citation27]. Therefore, given the distribution of adverse molecular prognostic markers, there are many variables that would potentially abrogate any effect of mucinous differentiation.
There are several limitations that need to be taken into consideration when interpreting the outcomes of this study. It was a retrospective analysis of a prospectively maintained patient cohort, leading to selection bias. Also, this study was conducted in a center with an experience of more than 1200 patients. The learning curve and volume outcome affects should be considered. Over the time frame of the study patterns of use of systemic therapy including targeted therapies has also evolved.
Conclusions
In summary, patients with MC had a similar long-term survival outcome with those with NMC following CRS and IPC. More studies are warranted to further investigate the survival differences between patients with MC and those with NMC following CRS and IPC.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Park JS, Huh JW, Park YA, et al. Prognostic comparison between mucinous and nonmucinous adenocarcinoma in colorectal cancer. Medicine. 2015;94:e658.
- Papadopoulos V, Michalopoulos A, Netta S, et al. Prognostic significance of mucinous component in colorectal carcinoma. Tech Coloproctol. 2004;8:s123–s125.
- Smeenk R, Verwaal V, Zoetmulder F. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94:1408–1414.
- Chua TC, Yan TD, Saxena A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann Surg. 2009;249:900–907.
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743.
- Debunne H, Ceelen W. Mucinous differentiation in colorectal cancer: molecular, histological and clinical aspects. Acta Chir Belg. 2013;113:385–390.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Peritoneal carcinomatosis: principles of management. Boston, MA: Springer; 1996. 359–374.
- Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205.
- Huang Y, Alzahrani NA, Liauw W, et al. Early postoperative intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasms with Pseudomyxoma peritonei: is it beneficial?. Ann Surg Oncol. 2017;24:176–183.
- Coindre J, Trojani M, Maree D, et al. The prognostic significance of specific histologic features of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1981;153:511–514.
- Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer. 1976;37:1891–1900.
- Green JB, Timmcke AE, Mitchell WT, et al. Mucinous carcinoma-just another colon cancer? Dis Colon Rectum. 1993;36:49–54.
- Bagante F, Spolverato G, Beal E, et al. Impact of histological subtype on the prognosis of patients undergoing surgery for colon cancer. J Surg Oncol. 2018;117:1355–1363.
- Coco C, Magistrelli P, Vecchio F, et al. The prognostic role of anatomo-pathological factors in colorectal cancer: an univariate analysis. Ann Ital Chir. 1991;62:355–362.
- Langner C, Harbaum L, Pollheimer MJ, et al. Mucinous differentiation in colorectal cancer–indicator of poor prognosis? Histopathology. 2012;60:1060–1072.
- Verhulst J, Ferdinande L, Demetter P, et al. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381–388.
- Yu D, Gao P, Song Y, et al. The differences on efficacy of oxaliplatin in locally advanced colon cancer between mucinous and nonmucinous adenocarcinoma. Cancer Med. 2018;7:600–615.
- Numata M, Shiozawa M, Watanabe T, et al. The clinicopathological features of colorectal mucinous adenocarcinoma and a therapeutic strategy for the disease. World J Surg Oncol. 2012;10:109.
- Yamamoto S, Mochizuki H, Hase K, et al. Assessment of clinicopathologic features of colorectal mucinous adenocarcinoma. Am J Surg. 1993;166:257–261.
- Glasgow S, Yu J, Carvalho L, et al. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer. 2005;92:259.
- Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59.
- Hugen N, Verhoeven R, Radema S, et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol. 2013;24:2819–2824.
- Kermanshahi TR, Magge D, Choudry H, et al. Mucinous and signet ring cell differentiation affect patterns of metastasis in colorectal carcinoma and influence survival. Int J Surg Pathol. 2017;25:108–117.
- Ubink I, van Eden W, Snaebjornsson P, et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br J Surg. 2018;105:e204.
- Jass J. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130.