Abstract
Purpose: Localized adult high-grade soft tissue sarcomas (STS) usually require multimodality treatment including surgery, radiotherapy, chemotherapy and hyperthermia. If maximal preoperative tumor-shrinkage is envisaged, neoadjuvant chemotherapy + radiation (CRT) is often applied, however at the expense of relatively high toxicities and increased postoperative complication rates. This study aims to compare preoperative CRT with neoadjuvant chemotherapy + regional hyperthermia (HCT) regarding histopathological response, toxicity and outcome.
Methods: In this retrospective analysis, 61 consecutive high-grade STS patients treated between 2009 and 2016 were included. All patients were treated within a prospective treatment protocol. 28 patients received neoadjuvant CRT 33 patients HCT. CRT consisted of four cycles doxorubicin/ifosfamide and two cycles ifosfamide concomitant to 50.4 Gray external beam radiotherapy. HCT consisted of 4–6 cycles doxorubicin/ifosfamide with deep regional hyperthermia administered bi-weekly during each cycle. Association of treatment modality with overall survival (OS), local control (LC) and freedom from distant metastases (FFDM) was evaluated by Kaplan–Meier and log-rank analyses.
Results: The overall patient characteristics were well balanced. Histopathological tumor response did not differ significantly between both groups (p = .67), neither did higher-grade toxicities during neoadjuvant treatment. Wound dehiscence (p = .018) and surgical hospital re-admissions (p < .001) were both significantly more frequent in the CRT group. Two-year OS, LC and FFDM rates of all patients were 93, 85 and 71% with no significant differences between CRT and HCT.
Conclusion: Compared to CRT, HCT seems equally efficient and appears to bear less surgical complications. Interpretation should be cautious due to the low number of patients and the retrospective nature of this study.
Introduction
High-grade soft tissue sarcomas (STS) represent a rare and heterogeneous group of diseases, including more than 70 histological distinct tumor entities. Despite recent advances in treatment, the long-term outcome remains unsatisfactory. Although aggressive multimodal treatment approaches have been established, disease-related deaths occur in around 50% of patients [Citation1]. The mainstay of curation is radical surgery. After limb-sparing/non mutilating surgery additional radiotherapy is usually indicated, based on the practice changing study from Rosenberg and colleagues [Citation2]. Even with modern surgical approaches radiotherapy significantly decreases the risk of local recurrences. A small prospective study and a large contemporary population based analysis on adjuvant external beam radiotherapy emphasise the important role of radiotherapy [Citation3,Citation4]. Historically, radiotherapy was limited to STS of the extremities. Due to the improved sparing of organs at risk by modern radiation techniques, it is increasingly and efficiently used in other tumor locations particularly retroperitoneal sarcomas [Citation5,Citation6].
The role of chemotherapy for the neoadjuvant or adjuvant treatment of high-grade STS is not well defined by phase 3 evidence. Nevertheless, chemotherapy regimens as doxorubicin/ifosfamide had shown considerable response rates in a palliative setting [Citation7]. For curative intent, a meta-analysis revealed a significant, however moderate, reduction of local as well as distant failures with consecutive improved overall survival (OS) for doxorubicin/ifosfamide based adjuvant chemotherapy [Citation8]. A randomized Italian study compared adjuvant doxorubicin and ifosfamide to no adjuvant chemotherapy. The trial was prematurely closed due to a substantial OS benefit in the chemotherapy arm [Citation9]. Therefore, the use of doxorubicin and ifosfamide combined with radiotherapy seems to be a promising and relatively well-tolerable approach, that is currently investigated by the German IAWS1/2 study [Citation10]. Even with older chemotherapy regimens, high-grade STS seem to benefit from perioperative chemotherapy in respect of metastases free and OS [Citation11]. Chemotherapy can be integrated in a neoadjuvant approach, sometimes additionally combined with radiotherapy if the goal of neoadjuvant treatment is a maximal local effect such as tumor shrinkage [Citation12,Citation13]. However STS require relatively high doses of chemotherapy. Therefore, the combination of chemo- and radiotherapy (CRT) can be associated with considerable acute side effects. Additionally, increased postoperative morbidity has been described after CRT [Citation14]. As an alternative mean to increase local efficacy of chemotherapy, regional hyperthermia can be used as a chemosensitizer in combination with neoadjuvant chemotherapy (HCT). The addition of regional hyperthermia in conjunction with neoadjuvant chemotherapy was shown to be superior compared to neoadjuvant chemotherapy alone in a multicenter Phase-3 trial [Citation15]. In this trial, chemotherapy consisted of four cycles neoadjuvant and four cycles adjuvant etoposide, ifosfamide and doxorubicin. Regional hyperthermia was performed on days 1 and 4 of each cycle. The addition of either chemotherapy or/and hyperthermia to neoadjuvant radiotherapy seems to be feasible and potentially associated with favorable patient outcome compared to radiotherapy alone in a small retrospective cohort of patients [Citation16].
In medically fit patients without severe co-morbidities, neoadjuvant CRT can be applied with tolerable toxicity and promising OS rates. Albeit this approach is not based on randomized trials and chemotherapy regimens used for CRT differ considerably [Citation17]. Careful selection of patients seems to be an important issue as this approach can lead to severe toxicities. The RTOG 9514 trial which combined neoadjuvant polychemotherapy consisting of modified mesna, doxorubicin, ifosfamide and dacarbazine and split course radiotherapy to a total dose of 44 Gy reported a rate of 83% grade 4 toxicities and 5% grade 5 toxicities [Citation18].
No comparison between neoadjuvant CRT and HCT have been published so far. Neoadjuvant HCT may be associated with less pronounced side effects and surgical complications. Additionally it bears the advantage to individually tailor adjuvant (radio-)therapy based on the pathological response to HCT (regarding dose, topographical distribution of boost dose and potentially omission of radiotherapy in carefully selected patients). We therefore retrospectively evaluated histopathological response and patient outcome after preoperative CRT and HCT.
Materials and methods
Patients
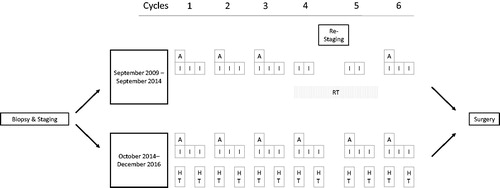
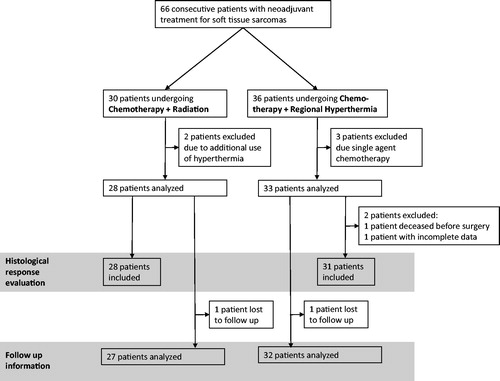
This is a retrospective analysis of consecutive high-grade STS patients treated between September 2009 and December 2016 at our institution. All treatment decisions were based on the votes of an interdisciplinary tumor board including surgeons, oncologists, radiation oncologists, pathologists and radiologists specialized in the treatment of sarcomas. Inclusion criteria for this analysis were: Histologically confirmed high-grade STS undergoing neoadjuvant multimodality treatment (CRT or HCT), exclusion criteria were concomitant use of all three modalities (chemotherapy, hyperthermia and radiotherapy) or medically unfit patients, initially not planned to receive duplet chemotherapy or with palliative treatment intent or evidence of distant metastases. shows a CONSORT diagram of all screened and analysed patients. Until September 2014, the routinely prescribed treatment for all consecutive patients was neoadjuvant CRT. Starting October 2014, due to the availability of a regional hyperthermia device, in-house practice was changed and all patients with high-risk STSs received neoadjuvant HCT without concomitant radiotherapy. Both CRT and HCT were performed according to a standardized treatment protocol (for details see below).
Figure 1. Flow diagram of the study design showing all 66 consecutive patients with neoadjuvant treatment for soft tissue sarcomas and displaying exclusion criteria for the respective endpoints: Histological response evaluation and follow up information.
All patients had histological confirmed high-grade STS and were defined as FNCLCC (Fédération Nationale des Centres de Lutte Contre le Cancer) grades 2 or 3, diameter larger than 5 cm and deep seated. Staging included a computed tomography (CT) scan of the thorax and abdomen and magnetic resonance imaging (MRI) of the tumor region. If distant metastases were suspected, additional 18F-fluorodesoxyglcuose (FDG) positron emission tomography (PET) was performed and/or biopsies of suspected distant lesions were taken. During neoadjuvant treatment, re-staging CT or MRI was commonly performed after the second or third and the fifth cycle of chemotherapy.
Chemotherapy plus radiation and chemotherapy plus regional hyperthermia
Chemotherapy and radiation in both groups (CRT and HCT) followed the german IAWS protocol [Citation10] on duplet neoadjuvant chemo(radio-) therapy containing doxorubicin and ifosfamide, except for the additional use of regional hyperthermia on days 1 and 3 in the HCT group. The combination of ifosfamide with radiotherapy is similar like in the recently published Phase-III trial from Palassini and colleagues [Citation19]. If doxorubicin/ifosfamide could not be administered due to patient´s general health condition, restricted kidney function, age or patient’s refusal, either epirubicin + ifosfamide or doxorubicin + dacarbazine was given.
Chemotherapy plus radiation: The CRT protocol consisted of six cycles chemotherapy: three cycles duplet therapy with doxorubicin 60 mg per m2 body surface on day 1 and ifosfamide 3000 mg per m2 body surface on days 1, 2 and 3. After completion of the third cycle, radiotherapy was initiated with two cycles of concomitant ifosfamide 3000 mg on days 1 and 2. After completion of radiotherapy, the sixth cycle full dose duplet chemotherapy (doxorubicin and ifosfamide) was administered. Concomitant radiotherapy was applied as intensity modulated radiotherapy using either a volumetric arc approach or helical tomotherapy. Planning target volume (PTV) comprised the gross tumor volume (GTV) as defined by planning CT and fused pre-therapeutic MRI and additional safety margins of at least 3 cm accounting for microscopic tumor spread (clinical target volume = CTV) and accelerator-dependent additional safety margins of 0.2–1.0 cm. Single dose was 1.8 Gy given each workday to a total dose of 50.4 Gy. Preoperative as well as postoperative delineation of treatment volumes was according to published recommendations [Citation20].
Chemotherapy plus regional hyperthermia (HCT): Regional hyperthermia was applied using a BSD-2000 hyperthermia system and specific applicators according to tumor localization (Pyrexar Medical, formerly BSD Medical Corporation, Salt Lake City, UT). After a 30 min warming up period, 60 min therapeutic hyperthermia with a target temperature of 42 °C within the PTV was given. Thermometry was performed in surrounding tissues, depending on tumor location. Chemotherapy consisted of 4–6 cycles of the same duplet therapy used in CRT, i.e., doxorubicin 60 mg per m2 body surface on day 1 and ifosfamide 3000 mg per m2 body surface on days 1, 2 and 3. Hyperthermia was usually performed at days 1 and 3 of chemotherapy with ifosfamide application during or immediate after regional hyperthermia. depicts the treatment schedule of both regimes. Regional hyperthermia and thermal mapping of surrounding tissues were done according to the ESHO guidelines for quality and safety assurance [Citation21,Citation22].
Response evaluation, side effects, surgery and follow up
Toxicity was scored at least weekly during concomitant CRT and HCT. The toxicity scoring was based on Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Re-hospitalization due to surgical complications within 3 months after surgery was also assessed. Radiographic response was assessed according to RECIST-criteria after four cycles of chemotherapy. For histopathological response evaluation, the Salzer–Kuntschik regression score was used. This score defines complete pathological remission without microscopic tumor cells as grade 1 and completely vital tumors without any sign of regression as grade 6 [Citation23]. This score was originally used for osteosarcomas but also showed a prognostic impact in STSs in a recent publication [Citation24]. Resection status was defined as follows: Complete resection (R0) in case of clear pathological margins of at least 10 mm, close margin in case of 1–9 mm, R1 in case of 0 mm, and R2 if macroscopic residual tumor remained incompletely resected [Citation25,Citation26]. Follow-up examinations were commonly performed every three to six months, usually including a clinical examination and laboratory evaluation, MRI of the former tumor region and CT of the thorax/upper abdomen.
Statistical analysis
Categorical tumor and patient characteristics were compared between both treatment arms by chi-squared tests. Differences in continuous parameters were evaluated by Mann–Whitney U tests. The follow up endpoints were OS, local control (LC) and freedom from distant metastases (FFDM). All endpoints were calculated from the first day of neoadjuvant treatment to the date of event or censoring, tumor progression during neoadjuvant therapy impeding radical surgery was accounted as local failure. Corresponding survival curves were estimated by the Kaplan–Meier method. All calculations were performed by SPSS 24 software (IBM Corporation, Armonk, NY). For all analyses, two-sided tests were applied and p values < .05 were considered statistically significant.
Results
61 patients were evaluable for analysis. 28 patients received neoadjuvant CRT and 33 patients neoadjuvant HCT. Median patient age was 54 (18–72) years. Patient characteristics did not differ significantly between both treatment groups: Patient age, maximal pretherapeutic tumor diameter, Ki-67 proliferation index, gender, histological subtype and tumor location were well-balanced between both groups, although patients in the CRT group presented more grade 3 tumors compared to the HCT group (p = .032) and a trend for higher patient age was observed in the HCT group (p = .09). Twenty patients in the CRT group presented extremity, six patients trunk and two patients abdominal/retroperitoneal location. The corresponding numbers were 23, 1 and 9 (extremnity, trunk, abdominal/retroperitoneal) for HCT patients. Only the number of applied cycles of chemotherapy was significantly different, with all CRT patients receiving six cycles and 15 of 33 HCT patients receiving <6 cycles (mostly 5 cycles). Additionally HCT was more often prescribed to patients with locally recurrent disease (7 of 33), compared to only 1 of 28 patients in the CRT group. shows patient and tumor characteristics for both treatment arms and statistical comparisons between both groups.
Table 1. Patient and tumor characteristics.
Acute radiation induced side effects were all ≤ grade 2 (according to CTCAE): With radiation dermatitis being the most frequently reported side effect (>grade 1 in 14 of 28 patients). In the HCT group except discomfort during treatment no acute adverse events were reported. Most severe side effects observed during neoadjuvant treatment (CRT and HCT) were attributable to chemotherapy. Besides thrombocytopenia and leukocytopenia, three patients presented transient psychosis. Two patients in the HCT arm developed severe septic complications after the first cycle of chemotherapy, one developing multiple organ failure with fatal outcome. Regarding late toxicities > grade 2: one patient in the CRT group developed severe wound complications requiring amputation during follow-up. Two patients in the HCT group developed chronic kidney disease grade 4 requiring dialysis, not topographically related to regional hyperthermia (one patient with extremity and one patient with retroperitoneal localization).
About 27 of the CRT and 31 of the HCT patients underwent radical surgery. Histopathological response did not show a statistically significant difference between both treatment arms (p = .67), depicts tumor regression scores after both treatment modalities. Resection margin status was not significantly different between HCT and CRT. Surgical complication rates were more pronounced in the CRT group. Wound dehiscence was the most frequently observed side effect after CRT and significantly more frequent compared to neoadjuvant HCT (p = .018). Hospital readmissions due to surgical complications were more often observed after CRT (p < .001). One CRT patient died from subsequent uncontrollable wound dehiscence and infection. summarises surgical outcomes. One patient died after three cycles HCT due to septic multiple organ failure. Most patients in the HCT group received adjuvant radiation therapy to 60–66 Gy, except if radiation was not feasible due to organs at risk. Additionally, three patients with complete pathologic remission after HCT did not receive adjuvant radiation—mostly due to patient´s preference/refusal.
Figure 3. Radiographic (a) and Histopathological (b) response evaluation after neoadjuvant chemotherapy plus radiation or chemotherapy plus regional hyperthermia. PR: partial remission; SD: stable disease; PD: progressive disease. Histopathological tumor regression is evaluated by the Salzer-Kuntschik regression score. For the detailed score, see methods.
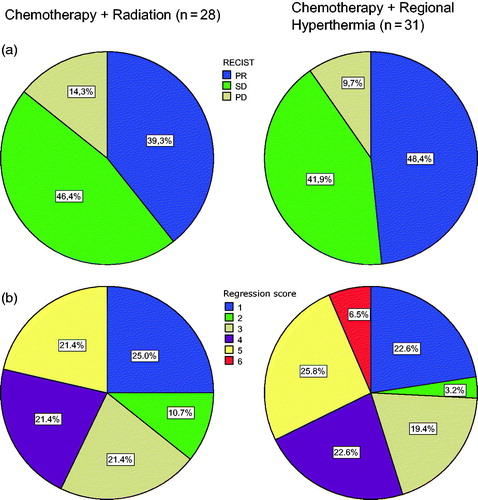
Table 2. Surgical outcome according to neoadjuvant treatment.
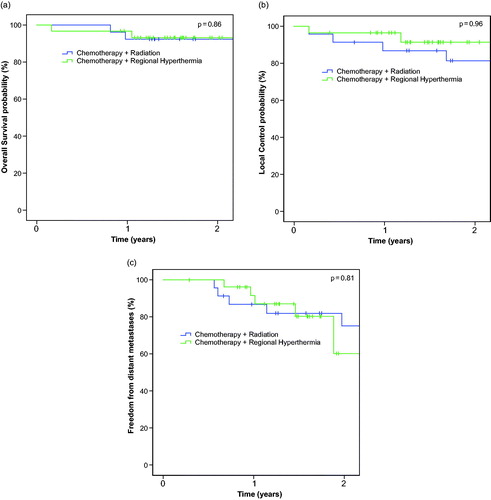
Median follow-up time was 33 months (range: 15 –93 months) in the CRT group and 18 months (range: 11–35 months) in the HCT group. One patient in each group did not undergo radical surgery as initially planned due to local progressive disease, these patients were accounted as local failure. The 2-year OS, LC and FFDM rates of all patients were 93, 85 and 71%. OS, LC and FFDM did not differ significantly between both neoadjuvant treatment approaches (p values: .86 for OS, .96 for LC and .81 for FFDM). depicts Kaplan–Meier estimates for all evaluated endpoints according to neoadjuvant treatment. Additionally neither radiographic response assessment after preoperative therapy (PR versus SD versus PD, p = .44) nor histopathological tumor regression (binarized for less or equal/higher 10% vital cells, p = .267) were significantly associated with improved LC (). Radiographic response did not correlate with histopathological regression (r = 0.17, p = .20).
Discussion
In this retrospective analysis of patients with high-grade STS, we observed very promising results for both neoadjuvant treatment modalities: CRT and HCT. Although limited by its retrospective design and the low number of patients, it is important to note that both approaches may have different strengths and weaknesses, which should be considered for future individualized treatment approaches. While neither radiographic nor histopathological regression scores did show a statistically significant difference between both treatment approaches and rates of complete resections did not differ, surgical complications seem to be more prevalent after CRT. Although these complications mostly resolved without major sequelae, the rate of hospital re-admissions was significantly higher after CRT compared to HCT. An increased incidence of wound complications after preoperative irradiation has been described in a randomized trial, comparing pre- and post-operative radiotherapy [Citation27,Citation28]. However as irreversible long-term side effects tend to be higher after postoperative irradiation [Citation29,Citation30], preoperative radiotherapy is commonly recommended for the majority of STS patients within the USA. However if further treatment intensification is envisaged, e.g. by the additional use of chemotherapy, this could be limited by the high rate of wound complication rates after CRT. Since treatment was non-randomized, the observed difference could be due to selection biases. However, although not significantly, patients tended to be older in the HCT group, additionally prescription of CRT was more restricted to medically very fit patients due to concerns about toxicity. The more restrictive use of CRT compared to HCT is reflected by the increased number of treated patients per year since the establishment of HCT. Additionally a significantly higher amount of patients received a reduced number of chemotherapy cycles in the HCT group, while all CRT patients received full six cycles of chemotherapy.
Another limitation is the differing time of treatment between both groups. An improvement in OS for STS has been described during the last decades [Citation31]. Additionally, CRT patients have a longer follow-up compared to HCT patients. However both groups were relatively homogeneously treated according to a prospective chemotherapy protocol. Furthermore the investigated period of time is relatively short and can be regarded to reflect contemporary treatment. Neither surgical nor radiation techniques have changed in our institution during that period. All patients received intensity modulated radiotherapy (either as CRT or as adjuvant radiation after HCT). The follow-up results have to be interpreted with caution, as the time of follow-up is too short to detect all potential treatment failures, especially in the HCT group. However, it is astonishing that the local failure rate during the first 2 years seems comparable between both groups, since patients in the HCT group were more often treated for recurrent disease or with retroperitoneal located disease, both factors known for increased risk for local recurrence [Citation32–34]. Additionally three patients with complete remission after HCT did not undergo planned adjuvant radiotherapy and did not present local recurrence so far. Our findings support that both neoadjuvant approaches are equally effective. No significant differences in the resection margins were observed between HCT and CRT. We note that the resection status is considered as crucial prognostic factor associated with improved OS in a population based analysis [Citation35].
While the important role of radiotherapy in STS is supported by various randomized controlled trials, much less evidence is available for the use of chemotherapy or about the optimal combination and timing of chemo- and radiotherapy. The results of the ISG-STS 1001 trial suggest that neoadjuvant chemotherapy alone with ifosfamide and anthracyclines offers an advantage in terms of disease free survival, although in that study the treatment was not compared to omission of adjuvant chemotherapy, but to histologically tailored regimes [Citation36]. Preoperative radiotherapy is supported by a recent database analysis [Citation6], however the indication for preoperative CRT is more controversial [Citation37]. The use of neoadjuvant HCT and additional adjuvant chemotherapy and radiotherapy was shown to be highly effective and improved OS compared to the normothermic application of neoadjuvant chemotherapy in a multicenter phase-3 trial [Citation38]. The use of pre-operative chemotherapy plus regional hyperthermia and individually tailored adjuvant radiation in case of unsatisfactory tumor regression or close margin resection seems to be a promising approach that potentially avoids the high toxicity of neoadjuvant CRT and potentially further reduces toxicity of adjuvant radiotherapy in patients with excellent tumor responses. Contrary to the phase-3 trial etoposide was not administered in this cohort of patients. While earlier studies included etoposide in neoadjuvant regimes, pre-clinical data suggest that the additional benefit of adding etoposide to a multimodal therapy regime including hyperthermia is only small [Citation39,Citation40].
One caveat of additional hyperthermia is a potential increase of toxicity, that could be another reason for the reduction of chemotherapy cycles observed in our study. The EORTC-ESHO phase-3 study reported an increased rate of thrombocytopenia and leucopoenia in the HCT arm compared to chemotherapy only [Citation15]. This effect was not observed in our cohort of patients, which may be due to the retrospective evaluation of toxicity, which is an important limitation to this study. However two patients in the HCT group developed chronic kidney disease compared to none in the RCT group. Unfortunately data on late renal toxicity in adults after ifosfamide is sparse in general, and even less is known for STS patients, as randomized trials did not report long-term toxicity [Citation7,Citation9,Citation41]. Although Issels and colleagues did not report an increase of nephrotoxicity when using hyperthermia, preclinical data suggest that hyperthermia could potentially increase renal toxicity [Citation42]. The low numerical incidence of chronic kidney disease and the missing correlation with tumor localization preclude a conclusive attribution to the concomitant use of hyperthermia, especially as patients in this group were slightly older. Future trials on hyperthermia should nevertheless closely monitor renal function.
The optimal timing of perioperative chemo- and radiotherapy remains unclear. Both neo- or adjuvant concepts or combinations showed similar efficacy [Citation43]. When comparing the pathological tumor response with other published data, HCT achieved a (nearly) complete response rate of 45.2%, defined as less than 10% vital tumor cells after neoadjuvant treatment. This rate compares favorable to more invasive treatment approaches like isolated limb perfusion with tumor necrosis factor α and melphalan with nearly complete response rates between 17 and 47% [Citation44]. Histopathological treatment results after HCT or CRT in our study are comparable to studies investigating CRT: Kraybill et al. [Citation18] reported a rate of 27% complete pathological response, DeLaney et al. [Citation45] reported a complete response rate of only 8% (4 of 48 patients). Surgical wound complications after RCT reported here are relatively high, but similar to those reported by Look Hong, that described a rate of 47% [Citation17]. It has to be noted that complete pathological response after HCT in our study was favourable compared to the data published by Schlemmer and colleagues, who only reported a complete remission in 5% of patients (one pathological complete remission and one clinical w/o consecutive surgery). Furthermore histopathological response after neoadjuvant treatment, measured by tumor necrosis, may not always correlate with patient outcome [Citation46]. However other means of response evaluation, especially radiological re-staging during or shortly after neoadjuvant treatment, are even less reliable. Re-Staging does neither seem to correlate with histopathological response nor with outcome after preoperative HCT or radiotherapy [Citation47–49]. Therefore histological evaluation can still be regarded as the gold standard for response evaluation.
Taken together our data indicate, although limited by the small sample size and retrospective nature, that neoadjuvant HCT can potentially achieve similar local effectiveness compared to neoadjuvant CRT but probably leads to less postoperative morbidity. In addition, HCT bears the advantage to potentially further increase local treatment efforts by the additional use of adjuvant or additional preoperative radiotherapy.
Supplemental Material
Download PDF (28.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. JCO. 2003;21:2719–2725.
- Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315.
- Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203.
- Jebsen NL, Trovik CS, Bauer HCF, et al. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: a Scandinavian sarcoma group study. Int J Radiat Oncol Biol Phys. 2008;71:1196–1203.
- Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77:203–209.
- Nussbaum DP, Rushing CN, Lane WO, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17:966–975.
- Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275.
- Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581.
- Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. JCO. 2001;19:1238–1247.
- Hartmann JT, Schütte J. Neoadjuvante Chemotherapie des lokalisierten Weichteilsarkoms. Onkologe. 2009;15:389–397.
- Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann. Oncol. 2010;21:2436–2441.
- Demetri GD, Antonia S, Benjamin RS, et al. Soft tissue sarcoma. J Natl Compr Canc Netw. 2010;8:630–674.
- Pisters PWT, Ballo MT, Patel SR. Preoperative chemoradiation treatment strategies for localized sarcoma. Ann Surg Oncol. 2002;9:535–542.
- Curtis KK, Ashman JB, Beauchamp CP, et al. Neoadjuvant chemoradiation compared to neoadjuvant radiation alone and surgery alone for Stage II and III soft tissue sarcoma of the extremities. Radiat Oncol. 2011;6:91.
- Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570.
- Eckert F, Gani C, Kluba T, et al. Effect of concurrent chemotherapy and hyperthermia on outcome of preoperative radiotherapy of high-risk soft tissue sarcomas. Strahlenther Onkol. 2013;189:482–485.
- Look Hong NJ, Hornicek FJ, Harmon DC, et al. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: a 10-year single institution retrospective study. Eur J Cancer. 2013;49:875–883.
- Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. JCO. 2006;24:619–625.
- Palassini E, Ferrari S, Verderio P, et al. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian Sarcoma Group/Grupo Español de Investigación en Sarcomas Randomized Clinical Trial: three versus five cycles of full-dose Epirubicin Plus Ifosfamide. JCO. 2015;33:3628–3634.
- Haas RLM, Delaney TF, O'Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where?. Int J Radiat Oncol Biol Phys. 2012;84:572–580.
- Lagendijk JJ, Van Rhoon GC, Hornsleth SN, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia. 1998;14:125–133.
- Bruggmoser G, Bauchowitz S, Canters R, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol. 2011;187:605–610.
- Salzer-Kuntschik M, Delling G, Beron G, et al. Morphological grades of regression in osteosarcoma after polychemotherapy - study COSS 80. J Cancer Res Clin Oncol. 1983;106(Suppl):21–24.
- Andreou D, Werner M, Pink D, et al. Prognostic relevance of the mitotic count and the amount of viable tumour after neoadjuvant chemotherapy for primary, localised, high-grade soft tissue sarcoma. Br J Cancer. 2015;112:455–460.
- Kandel R, Coakley N, Werier J, et al. Surgical margins and handling of soft-tissue sarcoma in extremities: a clinical practice guideline. Curr Oncol. 2013;20:e247–2e254.
- McKee MD, Liu DF, Brooks JJ, et al. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol. 2004;85:68–76.
- O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241.
- Davis AM, O’Sullivan B, Bell RS, et al. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. JCO. 2002;20:4472–4477.
- Davis A, Osullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53.
- Zagars GK, Ballo MT, Pisters PWT, et al. Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys. 2003;56:482–488.
- Jacobs AJ, Michels R, Stein J, et al. Improvement in overall survival from extremity soft tissue sarcoma over twenty years. Sarcoma. 2015;2015:279601.
- Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226.
- Brennan MF, Antonescu CR, Moraco N, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–421. discussion 421–422.
- MacNeill AJ, Miceli R, Strauss DC, et al. Post-relapse outcomes after primary extended resection of retroperitoneal sarcoma: a report from the Trans-Atlantic RPS Working Group. Cancer. 2017;123:1971–1978.
- Gingrich AA, Bateni SB, Monjazeb AM, et al. Neoadjuvant radiotherapy is associated with R0 resection and improved survival for patients with extremity soft tissue sarcoma undergoing surgery: a national cancer database analysis. Ann Surg Oncol. 2017;24:3252–3263.
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18:812–822.
- Fairweather M, Keung E, Raut CP. Neoadjuvant therapy for soft-tissue sarcomas. Oncology (Williston Park, N.Y.). 2016;30:99–106.
- Issels RD, Lindner LH, Verweij J, et al. Neoadjuvant chemotherapy plus regional hyperthermia and long-term outcomes among patients with localized high-risk soft tissue sarcoma. JAMA Oncol. 2018;4:483–410.
- Pfeffer MR, Teicher BA, Holden SA, et al. The interaction of cisplatin plus etoposide with radiation +/- hyperthermia. Int J Radiat Oncol Biol Phys. 1990;19:1439–1447.
- Westermann AM, Wiedemann GJ, Jager E, et al. A systemic hyperthermia oncologic working group trial. Ifosfamide, carboplatin, and etoposide combined with 41.8 degrees C whole-body hyperthermia for metastatic soft tissue sarcoma. Oncology. 2003;64:312–321.
- Farry JK, Flombaum CD, Latcha S. Long term renal toxicity of ifosfamide in adult patients-5 year data. Eur J Cancer. 2012;48:1326–1331.
- Brauer LP, Prieshof B, Wiedemann GJ, et al. Whole-body hyperthermia combined with ifosfamide and carboplatin causes hypotension and nephrotoxicity. J Cancer Res Clin Oncol. 1998;124:549–554.
- Mahmoud O, Tunceroglu A, Chokshi R, et al. Overall survival advantage of chemotherapy and radiotherapy in the perioperative management of large extremity and trunk soft tissue sarcoma; a large database analysis. Radiother Oncol. 2017. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0167814017324805
- Jakob J, Hohenberger P. Role of isolated limb perfusion with recombinant human tumor necrosis factor α and melphalan in locally advanced extremity soft tissue sarcoma. Cancer. 2016;122:2624–2632.
- DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–1127.
- Schaefer I-M, Hornick JL, Barysauskas CM, et al. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: assessment of the european organization for research and treatment of cancer-soft tissue and bone sarcoma group response score. Int J Radiat Oncol Biol Phys. 2017;98:375–383.
- Stahl R, Wang T, Lindner LH, et al. Comparison of radiological and pathohistological response to neoadjuvant chemotherapy combined with regional hyperthermia (RHT) and study of response dependence on the applied thermal parameters in patients with soft tissue sarcomas (STS). Int J Hyperthermia. 2009;25:289–298.
- Canter RJ, Martinez SR, Tamurian RM, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol. 2010;17:2578–2584.
- Egawa S, Tsukiyama I, Kajiura Y, et al. Characteristics of the response of soft tissue sarcoma to hyperthermia: the correlation between temperature distribution, radiological examination and histology. Int J Hyperthermia. 1989;5:23–35.