Abstract
Introduction: Several techniques can be used to treat intravesical chemohyperthermia (ChHT). We compared radiofrequency-induced hyperthermia (RF-HT) with conductive hyperthermia (C-HT) for their ability to induce bladder wall temperatures of >40.5 °C, the target temperature for ChHT.
Materials and Methods: Fresh porcine bladders (n = 12) were placed in a temperature-controlled saline bath to simulate body temperature and circulation. HT was induced with RF-HT (43 °C) or C-HT (inflow temperature 44 and 46 °C) using a custom-made device. In two additional bladders, we varied intravesical solution and volume. Temperatures were recorded with a three-way catheter containing three mucosal and two urethral thermocouples (TCs) and a 915 MHz RF antenna, and with external TCs in the bladder wall at three different levels and three different locations.
Results: Target temperature (40.5 °C) was reached in the submucosa at all locations by both techniques. In the detrusor, target temperature was reached by RF-HT at the bladder neck and side wall. C-HT46 reached significantly higher submucosal temperatures at the side wall. The bladder dome seemed best heated by C-HT, although a high inflow temperature (46 vs. 44 °C) was required (ns). Intravesical saline resulted in higher temperatures than sterile water for RF-HT. A volume of 100 mL resulted in higher bladder dome temperatures for RF-HT, and higher bladder neck with lower dome temperatures for C-HT.
Conclusion: Our results indicate a slightly superior heating capacity for RF-HT compared to C-HT, whereas for the bladder dome, the reverse seems true. Comparative studies are warranted to evaluate whether HT efficacy differs between both techniques, with emphasis on tumor location.
Introduction
Bladder cancer is a commonly occurring disease, predominantly found in Caucasian men [Citation1–3]. At first diagnosis, about 75% of patients present with non-muscle invasive bladder cancer (NMIBC). It has a recurrence rate of up to 52% after 5 years, and a chance of progression to muscle invasive disease (MIBC) of up to 20% [Citation4]. Current treatment guidelines advise a transurethral resection of the bladder tumor, followed by adjuvant intravesical chemotherapy, typically using cold instillations of Mitomycin C (MMC), or immunotherapy with Bacille Calmette-Guérin (BCG). The exact type and scheme of adjuvant instillations depends on the patient’s risk category for recurrence and progression [Citation5].
A currently available alternative treatment option for NMIBC is intravesical chemohyperthermia (ChHT). A promising and effective technique appears to be radiofrequency (RF)-induced ChHT (RF-ChHT), in which typically MMC is used [Citation6,Citation7]. It compares favorably to standard MMC instillations, with 59% less recurrences after RF-ChHT when compared to cold MMC alone [Citation6], and to BCG immunotherapy, with 2-year recurrence free survival (RFS) rates in intermediate-high risk NMIBC patients of 82% vs. 65%, respectively (p = .02) [Citation8].
In another technique for intravesical ChHT known as conductive ChHT (C-ChHT), the drug solution is heated extracorporeally before intravesical recirculation. Although this technique seems less complex than RF-ChHT, current evidence for its efficacy is limited. Only five explorative studies using two different devices have been published, showing a 2-year RFS rate in intermediate-high risk NMIBC patients of up to 88% in the adjuvant setting [Citation9–13]. No comparison has been made with MMC or BCG therapy.
For any kind of intravesical ChHT to be effective a critical temperature of 40.5 °C or higher must be reached at the mucosa, submucosa and, for potential use in MIBC, detrusor level of the bladder wall [Citation14,Citation15]. In the absence of comparative clinical studies, we aimed to compare the capacity to heat the bladder wall at several depths using RF-induced hyperthermia (RF-HT) vs. conductive hyperthermia (C-HT) in a pig post-mortem bladder model.
Materials and methods
The post-mortem bladder model
Bladders were taken from 12 female pigs weighing 80–100 kg from a commercial slaughterhouse within 3 h of sacrifice. They were placed in a temperature-controlled 0.9% NaCl bath to simulate body temperature and heat dissipation (flow organ bath 2.0 L/min, volume 10 L). The temperature of the organ bath was kept constant at 37 °C to mimic that of humans. A three-way 20-French catheter containing three mucosal and two urethral thermocouples (TCs) and a 915 MHz RF antenna was placed intravesically. Additional TCs were surgically placed at different tissue levels (). A solution of 50 mL of NaCl 0.9% was used intravesically.
Figure 1. Experimental set-up. The heat-exchanger was used for C-HT, the radiofrequency source for RF-HT. Temperatures were measured at several locations. C-HT: conductive hyperthermia; RF-HT: radiofrequency induced hyperthermia; TC: thermocouple.
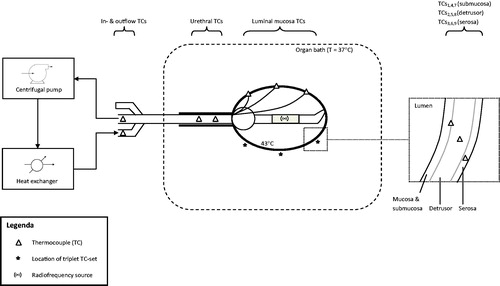
Subsequently, hyperthermia (HT) of 43 °C at luminal mucosa level was induced using either RF-HT or C-HT. We used a custom-made, non-commercially available system consisting of a heat-exchanger (Medtronic MYOtherm XP®, Minneapolis, MN, USA) and a centrifugal pump (Medtronic BP-50 Bio-Pump®) to fine-tune inflow temperature and flow. We continuously and actively measured the flow (mL/min) and fluid temperature at three levels: catheter connector, urethra and luminal mucosa. The latter was fine-tuned to keep temperature stable at 43 °C. Flow was measured using the EmTec Sono TT™ (New York, NY, USA) ultrasonic FlowComputer. For C-HT, a flow of 180 ± 20 mL/min and an inflow temperature of 46 ± 0.8 °C was used (C-HT46). Additionally, we studied an inflow temperature of 44 °C for C-HT (C-HT44) to evaluate the effect of a lower inflow temperature as in clinical use, 46 °C may cause potential urethral burning or pain. The Synergo® device (Medical Enterprises Ltd., Amsterdam, The Netherlands) [Citation7] was used for RF-HT with a flow of 5–10 mL/min and an inflow temperature of 31 ± 2 °C. Here again, the flow and inflow temperature were fine-tuned using the custom-made circulation system.
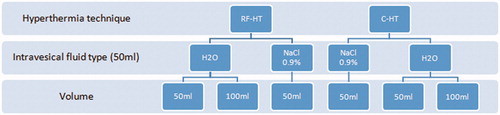
In addition to studying the different HT techniques, we evaluated the effect of different intravesical solutions (sterile water vs. saline) and intravesical volumes (50 mL vs. 100 mL) in two additional bladders to study effects on heating capacity of a volume increase due to urine production ().
Figure 2. Flow diagram of experimental groups. C-HT: conductive hyperthermia; H2O: sterile water; NaCl: sodium chloride (i.e., saline); RF-HT: radiofrequency induced hyperthermia.
We varied the experimental order to prevent any influence on the temperature measurements resulting from the sequence of experiments within the ex-vivo bladders. Comparative experiments were performed within the same bladders, thus minimizing inter-individual differences that might obscure differences between the techniques tested. By doing so, each bladder functioned as its own control. No approval of any ethical committee was required.
Temperature mapping
A schematic illustration of the experimental set-up is given in .
Luminal and urethral temperatures were monitored using the Synergo® system and facilitated temperature stabilisation. Additional external T-type (constantan-cupper) TCs with a 0.6 ± 0.1 mm tip length were surgically placed in the bladder wall at three different locations, the bladder neck (TC1–TC3), side wall (TC4–TC6), and bladder dome (TC7–TC9), resulting in a total of nine TCs located in the bladder wall. At each location, the TCs were placed at different levels in ascending order (submucosa, mid-detrusor and serosa) and fixed using tissue glue (Derma + Flex®, Chemence Medical Products, Inc., Alpharetta, GA, USA) and 6–0 polypropylene sutures (Prolene, Ethicon, LLC., Somerville, NJ, USA). Organ bath temperatures were monitored with two separate TCs (TC10 and TC11). We additionally measured the inflow (TC12) and outflow (TC13) temperatures at about 60 cm from the catheter tip as this is similar to where the inflow temperature is monitored in one of the currently available C-HT techniques [Citation9]. Although the extra-corporeal heating of fluid in our study was performed by a custom-made system rather than a commercially available system, the inflow temperature was measured at a similar distance from the catheter tip. Bladder wall, inflow, outflow and organ bath temperatures were recorded every minute for 10 min using a Keithley Multiplexer device (CN Rood, Zoetermeer, The Netherlands) containing an internal cold junction. The TCs were calibrated using ice water as 0 °C and had a ±0.1 °C accuracy. Bladder wall temperatures were averaged and compared between the different techniques and circumstances.
Data analysis and statistics
Temperatures were compared by box plot and analysis of variance (ANOVA) for comparison of the multiple means recorded for the different techniques and locations. Additionally, the values depicting the temperature above which 90% and 50% of the observed temperatures were observed (T90 and T50) and the cumulative equivalent minutes of T90 above 43 °C (CEM43T90) were calculated as a measure for the thermal dose applied.
All analyses were performed using R statistical package version 3.2.4 Revised (2016, The R Foundation for Statistical Computing, Vienna, Austria).
A temperature of 40.5 °C or higher was regarded an effective hyperthermic temperature [Citation14,Citation15].
Results
Twelve different bladders were used for the heating efficacy analysis. Thickness of the bladder wall varied from 3 to 8 mm. The mucosa as measured at the posterior bladder wall was 140–230 µm thick. The RF energy needed to heat the mucosa to a temperature of 43 °C varied between 14–37 W. The average luminal mucosa temperatures were 43.1 ± 0.22 °C, 44.0 ± 0.92 °C and 42.5 ± 0.73 °C for RF-HT, C-HT46 and C-HT44, respectively.
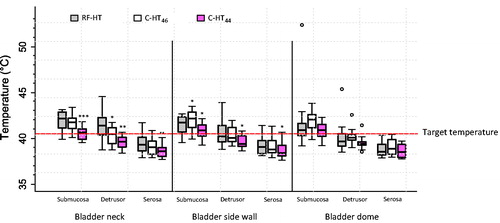
The temperature descriptives (T90, T50, CEM43T90 and mean) per location and technique are presented in . Mean bladder wall temperatures differed significantly between the three groups (ANOVA p < .05 for all), although this was mainly due to differences between RF-HT and the C-HT44 group. Mean urethral temperatures were 37.4 ± 3.0 °C, 45.4 ± 0.4 °C and 43.5 ± 0.4 °C for RF-HT, C-HT46 and C-HT44, respectively. Environmental temperatures varied from 21.5 to 24.3 °C.
Table 1. Bladder wall temperatures per location and technique.
All techniques (RF-HT, C-HT46 and C-HT44) reached the target temperature at the submucosa level. However, significantly higher mean bladder wall temperatures were seen after RF-HT compared to C-HT44 at the bladder neck and side wall (). At the bladder neck, this difference was noted even when the inflow temperature for C-HT was increased from 44 to 46 °C, although the difference was only significant at the detrusor level (p < .05). Importantly, RF-HT reached the critical effective mild hyperthermic temperature of 40.5 °C in the detrusor, whereas C-HT did not (), reflecting a deeper heat penetration into the bladder wall for RF-HT. At the bladder side wall, C-HT46 reached a significantly higher mean temperature at the submucosa (p < .05), although the target temperature was reached by both techniques. At the detrusor level, RF-HT again reached a mean temperature of 40.5 °C, whereas C-HT did not; here the difference between the temperature measurements was not significant (p = .54). C-HT reached higher temperatures at the bladder dome compared to RF-HT, although the target temperature was still reached by RF-HT (p = ns). The heating capacity and its relation between RF-HT and C-HT is additionally well depicted by the CEM43T90 values (), supporting the relationships described above. In one pig bladder, extremely high submucosal temperatures at the bladder dome were measured during RF-HT (submucosal temperature of 52.39 °C), explaining the outlier in . This was probably due to the close proximity of the TC to the RF-antenna during RF-HT. In one other bladder, one with a thinner bladder wall (4–5 mm), a reverse pattern with C-HT outperforming RF-HT at all locations was observed (data not shown).
Figure 3. Boxplot of temperatures in the bladder wall after RF-HT, C-HT46, and C-HT44. Dashed horizontal line represents the target temperature of 40.5 °C. Bold horizontal bars represent the median value. C-HT44: conductive hyperthermia with an inflow temperature of 44 °C; C-HT46: conductive hyperthermia with an inflow temperature of 46 °C; RF-HT: radiofrequency induced hyperthermia. *p < .05 compared to RF-HT. **p < .01 compared to RF-HT. ***p < .001 compared to RF-HT.
Because saline and sterile water are used for bladder irrigation in the clinic, we compared the effect of using both these intravesical fluids. For both techniques saline resulted in higher temperatures compared to sterile water at all locations, although this effect was less pronounced for C-HT (Supplementary Figure 1).
Lastly, the installation volume was varied, as larger volumes (and a more extended bladder) might alter the temperature distribution. The use of either 50 mL or 100 mL intravesical volume did not result in different bladder wall temperatures at the bladder neck and side wall for RF-HT (Supplementary Table 1). With a volume of 100 mL, higher temperatures were reached at the bladder dome, although the critical temperature of 40.5 °C was not reached. For C-HT, a volume of 100 mL was beneficial for bladder neck heating, whereas 50 mL resulted in higher temperatures of the side wall and dome (Supplementary Figure 2). However, the differences were small and too few bladders (n = 2) were tested to draw reliable conclusions.
Discussion
The combination of intravesical chemotherapy and RF-HT proved more effective in preventing recurrences of NMIBC than the current standard therapies (MMC alone or BCG immunotherapy) for intermediate to high risk NMIBC [Citation7–13]. HT, however, can be induced by several techniques, and for intravesical use, C-HT and RF-HT are available. We hypothesized that a difference in heating capacity between these two most commonly used techniques of inducing hyperthermia might exist, and we compared both methods in an ex-vivo porcine bladder model for its ability to heat the bladder wall to a minimum temperature of 40.5 °C. Our results show that using a custom-made device, slight differences in the capacity to heat between RF-HT and C-HT exist, in which bladder wall temperatures differ per region and per individual bladder. The minimum effective mild hyperthermic temperature of 40.5 °C was generally reached in the submucosa at all locations using either technique. However, in the detrusor, the target temperature was only reached at the bladder neck using RF-HT (statistically different from C-HT), and at the side wall by RF-HT (no statistical difference with C-HT46). Thus, the bladder neck can best be heated by RF-HT. This is supported by the calculated CEM43T90 values. The bladder dome, however, seems best heated by C-HT, although a high inflow temperature of 46 °C is needed and the difference with RF-HT was not statistically different. Given that only 14–18% of high grade NMIBC tumors are located at the dome, the potential clinical relevance is restricted to those tumors [Citation16]. At the bladder side wall, the bladder submucosa was significantly better heated by C-HT46 compared to RF-HT, but C-HT46 did not reach the minimum effective hyperthermic temperature at the detrusor level, whereas RF-HT did. The serosa is generally not heated to effective temperatures and if so, after RF-HT at the bladder neck only (data of a single separate bladder, data not shown).
The differences in heating capacity per region of the bladder and per anatomical depth layer was unanticipated, and is important in view of the current clinical use. It should be stressed that treatment of the dome is a challenge in any intravesical therapy, especially ChHT, as both the drug and heat need to be sufficiently transferred to the dome. In our study, RF-HT seemed to penetrate deeper into the different anatomical layers of the bladder wall. With RF-HT, an effective mild hyperthermic temperature in the detrusor layer of the bladder neck and side wall could be more easily reached in most of the bladders, compared with C-HT with an inflow temperature of 46 °C. Achieving effective hyperthermic temperatures at these depths may be beneficial for NMIBC patients if any residual tumor after TURBT is present (implying incomplete TURBT). Additionally, an increased amount of collagen in papillary tumor or sub-epithelial stroma is associated with progression to muscle invasion, which might be better heated by RF-HT [Citation17]. In treatment of minimally muscle-invasive disease, HT at the detrusor level might be of value in experimental bladder-sparing therapy, for example with thermosensitive nanoparticles [Citation18,Citation19]. Thus, whereas a tumor located at the bladder neck or side wall seems to be well-heated by RF-HT, using C-HT for tumors growing at the bladder dome could be considered, as C-HT seemed to achieve higher temperatures there.
Remarkably, in one of the bladders investigated, C-HT was clearly more effective than RF-HT in bladder heating in contrast to the other bladders. This emphasizes inter-individual variation. The wall of this bladder was one of the thinner (maximum 5 mm) bladders studied, possibly explaining this difference. This might imply consequences for women in particular, given that their bladder wall is generally thinner compared to men due to a lower urethral resistance. Alternatively, methodological variation might influence the heating capacity (e.g., catheter positioning) or temperature measurement (e.g., TC positioning). The variation in heating capacity of the two techniques in combination with target temperatures reached in different bladder regions within a single bladder may partially explain variation in the individual response to chemohyperthermic treatment in the clinic, and thus result in a preference for C-HT in women.
Temperatures of 40.5 °C or higher are required to achieve a chemohyperthermic treatment effect [Citation14,Citation15]. However, efficacy increases when higher temperatures are reached [Citation20,Citation21]. In this sense, it is important to note that in most bladders, the highest temperatures were reached using RF-HT, or with our custom-made C-HT device at a 46 °C inflow temperature. C-HT with a 44 °C inflow temperature generally resulted in the lowest (ineffective) bladder wall temperatures. This would indicate that either RF-HT or C-HT with a 46 °C inflow temperature be the best options in clinical use. However, a 46 °C inflow temperature resulted in high urethral temperatures ranging from 44.5 to 46.1 °C, temperatures that are known to produce local adverse events such as pain, bladder spasms and discomfort. This could be solved by designing a dedicated catheter which allows an inlet temperature of 46 °C while assuring that the urethra temperature remains bearable. Thus, a specific catheter which is either well insulated or able to simultaneously cool the urethra is needed to be able to apply these temperatures in patients. A recent conference abstract on a clinically approved device for C-HT has evaluated these potential side-effects in intermediate risk NMIBC patients (n = 307) and found no significant differences between C-HT and normothermic MMC on pain, dysuria, urgency, incontinence, nocturia, urinary tract infection, allergic reactions or urethral strictures [Citation22]. Urinary frequency, hematuria, and bladder spasm were significantly more frequently observed after C-HT. Worthy of note is that an inflow temperature of 43 °C was used, and no data on efficacy have yet been reported.
Intravesical instillation of saline resulted in higher bladder wall temperatures than sterile water; this was most pronounced for RF-HT. The higher heat conductivity to the tissue may be due to the higher number of ions in the saline solution. Specifically, RF waves are known to be partially reflected and absorbed by ion-rich fluids [Citation23–25]. Consequently, RF waves are spread throughout the bladder more evenly, possibly leading to more homogeneous heating compared to sterile water. Additionally, an intravesical volume of 100 mL resulted in higher bladder dome temperatures for RF-HT, whereas 100 mL resulted in higher bladder neck and lower dome temperatures for C-HT. This possibly reflects the different anatomy of the more extended bladder, leading to improved contact with the surface area. Both the results on the intravesical fluid and the volume used for instillation indicate that these factors might influence ChHT efficacy and thus could be adapted to the individual patient if only one technique is available. Moreover, it suggests that a limited amount of urine production during a treatment session does not hamper the heating capacity, although any chemotherapeutic intravesical drug will be diluted by urine production. It needs to be stated that intravesical volume and solution were only studied in two bladders, and therefore these results should be viewed with caution.
In two other studies, the bladder tissue temperatures associated with the heating techniques used in this study were examined [Citation26,Citation27]. In a recent conference abstract, the results of the Combat BRS device for C-HT in pigs were described. In this in-vivo study, C-HT was able to heat the bladder lumen to a temperature of 42.9 ± 0.14 °C and generated a transmural gradient of 1.5 °C across the detrusor, resulting in full thickness bladder heating of >41 °C [Citation27]. However, no comparison with RF-HT was made, and no information on the inflow temperature of the C-HT was given. In the other in-vivo study, two sheep were treated with RF-ChHT to study the safety of the hyperthermia treatment [Citation26]. Temperatures at the luminal mucosa, the external bladder level, and the surrounding organs were measured. The authors concluded that RF-HT combined with intravesical chemotherapy was a safe therapeutic option with no irreversible thermal damage to the bladder or adjacent tissues. Similar to our experiments, the variation of temperatures per bladder region and per animal was observed. The median luminal mucosa temperature was 44.7 °C (range 39.9–46.3 °C) and the median extravesical temperature was 39.5 °C (range 37.2–42.6 °C) in the two sheep. Temperatures inside the bladder wall were not evaluated. The heating phase at the beginning of the treatment session to reach effective mucosal temperatures took about 10 min. We only evaluated the equilibrium phase in our study. As our model was ex-vivo and vascularization is an important factor in the heating phase, we found it of insufficient value to evaluate the heating phase in our experiments. To simulate body temperature and perfusion, our abattoir derived porcine bladders were placed in a circulated organ bath. Despite this attempted approximation of reality, vascularization of vital tissue might result in different bladder wall temperatures in pigs. Nonetheless, we were able to adequately assess the heating capacity and temperature distribution of two important clinical hyperthermic techniques in standardized ex-vivo conditions. Moreover, TC placement in the sheep model was restricted to intravesical, extravesical and surrounding visceral TCs [Citation26]. Compared to our study, no information on the intramural temperatures and depth of heat penetration was obtained, nor was C-HT evaluated. Thus, our intramural temperatures significantly add to the current understanding of the bladder wall temperature gradient using either method.
The two different technologies used in our study in combination with the bladder anatomy may explain the observed differences in heating capacity. Ultimately, the goal of hyperthermia is to have a higher treatment efficacy through an increased drug tumor tissue penetration [Citation28]. Therefore, it would be interesting to compare the resulting efficacy of both RF-HT and C-HT in a clinical setting and correlate it to the achieved tissue-drug concentrations and temperatures.
A limitation of our study is the missing bladder perfusion that may have had an impact on the temperature gradients. Moreover, the human situation might be different, even though the anatomy of the pig internal urological tract, including the bladder, closely resembles the human internal urological tract. Nonetheless, our model fits the aim of our study, i.e., to investigate which technique resulted in a better energy transmission in standardized circumstances – irrespective of the commercial system associated with either technique. Another methodology-derived limitation is our small lumen catheter, which may have prevented the higher flow required, compared to RF-HT, for C-HT by our custom-made system. However, this catheter facilitated a flow rate only slightly lower than that of commercially available C-HT systems (180 ± 20 mL/min), and provided the substantial benefit of urethral and luminal temperature measurement. Furthermore, we used a custom-designed, non-clinically approved, recirculation system to heat the fluid extra-corporeally with the ultimate goal of reaching a temperature of 43 °C at the mucosa level. We are aware that direct extrapolation to commercially available C-HT systems is not possible due to use of a custom-made C-HT device in our model, although we made every possible effort to control our system similarly to commercial systems. Thus, our use of an alternative custom-made device did not hamper our comparison of C-HT and RF-HT, as we were able to guarantee stable temperatures of 43 °C at the luminal mucosa level, and a constant flow. Lastly, the exact depth of the TC location could not be measured, but was dependent on the surgeon’s assessment during placement.
Conclusions
Using our custom-designed device, RF-HT has a slightly higher heating capacity for the bladder neck compared to C-HT, resulting in an effective mild hyperthermic temperature of the submucosa and detrusor layer. For the bladder dome, C-HT appears to give a superior performance, suggesting that the intravesical tumour location could be taken into consideration if it is possible to choose a particular technique for inducing HT. Further comparison of both heating capacity and, most importantly, ChHT efficacy, in both preclinical and clinical studies is warranted.
Supplemental Figure 2
Download PDF (309.5 KB)Supplemental Figure 1
Download PDF (312.2 KB)Supplementary table 1
Download PDF (225.1 KB)Acknowledgements
We would like to thank Gerard van Ooijen for his technical support with the thermocouples, and Alex Hanssen for his support during the implementation of the experiment.
Catheters for this study were donated by Medical Enterprises Ltd. (MEL). The Synergo® system was provided by MEL. No further sponsoring was received.
Disclosure statement
J. Alfred Witjes is an Advisor for Sanofi Pasteur, Spectrum, Astellas (not related to this manuscript); is an Advisor for Medical Enterprises Ltd. (Synergo®); has no associated financial interest or conflict of interest. No potential conflict of interest was reported by the remaining authors.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403.
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108.
- Dy GW, Gore JL, Forouzanfar MH, et al. Global burden of urologic cancers, 1990–2013. Eur Urol. 2017;71:437–446.
- Cambier S, Sylvester RJ, Collette L, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus calmette-guerin. Eur Urol. 2016;69:e123–e129.
- Babjuk M, Burger M, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:e173–e161.
- Lammers RJ, Witjes JA, Inman BA, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2011;60:81–93.
- van Valenberg H, Colombo R, Witjes F. Intravesical radiofrequency-induced hyperthermia combined with chemotherapy for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32:351–362.
- Arends TJ, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus calmette-guerin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol. 2016;69:1046–1052.
- Sousa A, Inman BA, Pineiro I, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia. 2014;30:166–170.
- Sousa A, Pineiro I, Rodriguez S, et al. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32:374–380.
- Soria F, Milla P, Fiorito C, et al. Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: a phase I-II study. World J Urol. 2016;34:189–195.
- Ekin RG, Akarken I, Cakmak O, et al. Results of intravesical chemo-hyperthermia in high-risk non-muscle invasive bladder cancer. Asian Pac J Cancer Prev. 2015;16:3241–3245.
- Ekin RG, Akarken I, Zorlu F, et al. Intravesical bacillus calmette-guerin versus chemohyperthermia for high-risk non-muscle-invasive bladder cancer. CUAJ. 2015;9:278–283.
- Overgaard J. Combined adriamycin and hyperthermia treatment of a murine mammary carcinoma in vivo. Cancer Res. 1976;36:3077–3081.
- Rampersaud EN, Vujaskovic Z, Inman BA. Hyperthermia as a treatment for bladder cancer. Oncology (Williston Park, N.Y.). 2010;24:1149–1155.
- Vukomanovic I, Colovic V, Soldatovic I, et al. Prognostic significance of tumor location in high-grade non-muscle-invasive bladder cancer. Med Oncol. 2012;29:1916–1920.
- Brooks M, Mo Q, Krasnow R, et al. Positive association of collagen type I with non-muscle invasive bladder cancer progression. Oncotarget. 2016;7:82609–82619.
- Kneidl B, Peller M, Winter G, et al. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine. 2014;9:4387–4398.
- May JP, Li SD. Hyperthermia-induced drug targeting. Expert Opin Drug Deliv. 2013;10:511–527.
- Wallner KE, Banda M, Li GC. Hyperthermic enhancement of cell killing by mitomycin C in mitomycin C-resistant Chinese hamster ovary cells. Cancer Res. 1987;47:1308–1312.
- Uchibayashi T, Lee SW, Kunimi K, et al. Studies of effects of anticancer agents in combination with/without hyperthermia on metastasized human bladder cancer cells in chick embryos using the polymerase chain reaction technique. Cancer Chemother Pharmacol. 1994;35:S84–S87.
- Tan WS, Palou J JK. Safety and tolerability analysis of hyperthermic intravesical mitomycin to mitomycin alone in HIVEC I and HIVEC II: an interim analysis of 307 patients. Eur Urol Suppl. 2017;16:e1150. Conference Abstract.
- Bruners P, Hodenius M, Gunther RW, et al. Flussigkeitsmodulierte RF-Ablation: In-vitro-Experimente [Fluid-modulated RF ablation: in-vitro experiments]. Rofo. 2007;179:380–386. German.
- Provenzano DA, Watson TW, Somers DL. The interaction between the composition of preinjected fluids and duration of radiofrequency on lesion size. Reg Anesth Pain Med. 2015;40:112–124.
- Lara NC, Haider AA, Ho JC, et al. Water-structuring molecules and nanomaterials enhance radiofrequency heating in biologically relevant solutions. Chem Commun. 2016;52:12630–12633.
- Rath-Wolfson L, Moskovitz B, Dekel Y, et al. Combined intravesical hyperthermia and mitomycin chemotherapy: a preliminary in vivo study. Int J Exp Pathol. 2003;84:145–152.
- Brousell SC, Longo TA, Fantony JJ, et al. Heat-targeted drug delivery using the Combat BRS device for treating bladder cancer. J Urol. 2017;197:e855. Conference Abstract.
- van Valenberg FJP, van der Heijden AG, Lammers RJM, et al. Intravesical radiofrequency induced hyperthermia enhances mitomycin C accumulation in tumour tissue. Int J Hyperthermia. 2017;1–6.
