Abstract
Background: The Indian HIPEC registry is a self-funded registry instituted by a group of Indian surgeons for patients with peritoneal metastases (PM) undergoing surgical treatment. This work was performed to
• Evaluate outcomes of cytoreductive surgery ± HIPEC in patients enrolled in the registry.
• Identify operational problems.
Methods: A retrospective analysis of patients enrolled in the registry from March 2016 to September 2017 was performed. An online survey was performed to study the surgeons’ attitudes and existing practices pertaining to the registry and identify operational problems.
Results: During the study period, 332 patients were enrolled in 8 participating centres. The common indication was ovarian cancer for three centres and pseudomyxoma peritonei for three others. The median PCI ranged from 3 to 23. A CC-0/1 resection was obtained in 94.7%. There was no significant difference in the morbidity (p = .25) and mortality (p = .19) rates between different centres. There was a high rate of failure-to-rescue (19.3%) patients with complications and the survival in patients with colorectal PM was inferior. A lack of dedicated personnel for data collection and entry was the main reason for only 10/43 surgeons contributing data. The other problem was the lack of complete electronic medical record systems at all centres.
Conclusions: These results validate existing practices and identify country-specific problems that need to be addressed. Despite operational problems, the registry is an invaluable tool for audit and research. It shows the feasibility of fruitful collaboration between surgeons in the absence of any regulatory body or funding for the project.
Introduction
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is widely accepted as a potentially curative treatment option for selected patients with peritoneal metastases (PM) [Citation1–3]. Over the years, due to increasing experience, standardisation of the procedure and development of clinical pathways by pioneering centres, the overall morbidity and mortality of the procedure has reduced and favourable results have been reported by other centres as well [Citation4]. In the last 5 years, the number of centres offering this treatment in India has increased rapidly [Citation5]. Introducing such complex surgeries in a different healthcare system requires an integrated effort from different medical specialties, investment on infrastructure and developing disease-specific treatment strategies [Citation6]. Currently, the combined procedures are performed largely in the private hospitals in India and are an out-of-pocket expenditure for patients even in public hospitals. An analysis of results is vital to determine the perioperative outcomes and the oncological benefit of this complex procedure.
The Indian HIPEC registry (www.indianhipecregistry.com) was set up in March 2016 by a group of Indian surgeons with a special interest in peritoneal surface malignancies to have an organised system of data collection pertaining to the treatment of PM, analyse individual and pooled data and to collaborate on clinical research. Though initially the registry was instated to enroll only patients undergoing CRS and HIPEC, in due course, the scope was expanded to include patients undergoing any kind of surgical treatment. The current inclusion criteria for enrolment in the registry is patients with PM undergoing any kind of surgical treatment. The name ‘Indian HIPEC registry’ was still retained as it had become popular among surgical oncologists. Surgeons/centres from neighbouring countries are also enrolled in the registry. The importance of such a registry [Citation7] has been demonstrated by the scientific contributions made by two national registries: the RENAPE [Citation8] and BIG-RENAPE [Citation9] in France and the Netherlands cancer registry [Citation10] in the Netherlands.
Running such a registry in India is fraught with a different set of challenges. The healthcare system is run by the private and public sectors both with a vast difference in administrative policies, availability of technology, financial resources and the patient profile [Citation11]. The availability of electronic medical record systems is variable and if present, record only selective data. Eighteen months after instituting the registry, we performed this study with two main goals
To identify the operational problems in running such a registry successfully in India
To evaluate the treatment outcomes in patients enrolled in the registry.
Methods
The functioning of the registry has been described elsewhere [Citation8]. Briefly, the registry enrolls patients with PM who have undergone palliative or potentially curative surgical treatment for PM. Entries can be prospective or retrospective. Data capturing is disease specific comprising 150 data elements, including common prognostic variables used in peritoneal surface oncology [Citation12]. A uniform system for grading of complications based on the common toxicity criteria for adverse events (CTCAE) classification is used by all surgeons [Citation13]. Scanned copies of the histopathology report and hospital discharge summary are uploaded for each patient as a quality control measure and to ensure authenticity of the data without which it was not accepted. An exception is made for public institutions that do not permit sharing of reports with any external organisation. At the time of analysis, follow up of all patients is obtained either by the registry coordinator or the treating surgeon.
Institutional review board approval is taken by each surgeon as required. Patient consent, explicit or implied, for sharing of de-identified data is mandatory. This study was divided into two main parts:
Assessment of operational problems
Clinical outcomes in patients enrolled into the registry
Operational issues
The professional background and training of participating surgeons and the annual expenses incurred for maintenance were reviewed. An online survey was performed to study the surgeons’ attitudes and existing practices pertaining to the registry (supplementary material Table S1). The number of contributing surgeons, the proportion of their cases enrolled and the type of data (prospective/retrospective) was looked into. We also reviewed the system of data collection at each hospital and the personnel deployed for it.
Clinical outcomes
To assess the clinical outcomes, a retrospective analysis of data entered into the registry from March 2016 to September 2017 was performed. The demographic data, clinical history, investigation results, perioperative outcomes and follow-up were analysed.
The records were analysed for completeness of data elements. Categorical data were described as number (%). Abnormally distributed continuous data were expressed as the median and range. Categorical data were compared with the χ2 test. Univariate Cox proportional hazard regression was used to describe the association between individual risk factors and morbidity and disease-free survival (DFS) and overall survival (OS) both, in terms of hazard ratio and its 95% confidence interval (CI). Multivariate Cox regression was used to assess the impact of risk factors on survival. SPSS Version 20 (SPSS Inc., Chicago, IL) and MedCalc Version 12.2 were used for analysis. A p value of <.05 was considered statistically significant.
Survival was calculated from the date of the surgery for PM for all patients. Only patients undergoing potentially curative procedures (CRS alone or combined with intraperitoneal chemotherapy) were included in the survival analysis. The outcomes between surgeons who had enrolled more than 25 cases were compared.
Results
From March 2016, 43 surgeons were enrolled in the registry (9 from public and 34 from private sector). Of these, only 10 surgeons from 8 centres contributed the data of 332 patients till September 2017. These patients were treated between January 2011 and July 2017.
Operational issues
Participating surgeons
At the time of performing this study, 43 surgeons had been enrolled of which 2 were from Pakistan and 1 from Bangladesh. In the first 12 months there were only 10 surgeons. Further details are provided in .
Table 1. Surgeons participating in the registry and results of the online survey.
Maintenance of the registry
The initial cost of instituting the registry was 6000 US dollars (USD). The annual expenditure was 500 USD for maintenance and 1800 USD for the coordinator.
Results of the survey
Of 43 surgeons, 11 participated in the online survey and 10 enrolled patients.
Prospective data
All consecutive patients undergoing CRS with/without HIPEC were enrolled by 8/10 surgeons after the institution of the registry.
Retrospective data
Two surgeons could not enroll all their retrospective patients. They had changed their institution and patient records and contact details were not available to them.
Overall, six surgeons enrolled 100% of their patients. Two enrolled <10% of their cases and two others could not enroll all retrospective patients.
The main problems with entering data were a lack of time and research assistants/dedicated personnel (10/11 surgeons) and 5/11 surgeons had a problem in obtaining patient consent to participate in the registry ().
Electronic medical records and data entry practices
None of the participating centres had a complete electronic medical record system. Some records are available in the hospital database like laboratory investigations, radiology and histopathology reports. Clinically findings are documented manually and only hard copies are available.
Clinical outcomes
The patient and disease characteristics, operative findings and perioperative outcomes are described in . The clinical outcomes at centres that have contributed more than 25 cases are described individually. CRS and HIPEC was performed for 274 (89.6%) patients, CRS and EPIC for 29 patients and 29 patients had CRS alone. HIPEC was not performed in 13 patients who had incomplete cytoreduction; for 13 others undergoing primary CRS for ovarian cancer, it was deferred due to lack of evidence and in 5 for other reasons.
Table 2. Patient and disease characteristics and operative findings and perioperative outcomes in 332 patients.
Patient and disease characteristics
The primary tumour site was colorectal in 62 patients (18.6%), ovarian in 127(38.2%), pseudomyxoma peritonei (PMP) of appendiceal origin in 90 (27.1%), mesothelioma in 18 (5.4%), and rare primaries and uncommon indications (supplementary material Table S2) in 33(9.9%). The common indication was PMP of appendiceal origin for three institutions and ovarian cancer for three others. Other parameters like age (p < .001), sex (p < .001), prior surgery (p < .001), prior chemotherapy (p < .001) also varied significantly among institutions (). The median PCI was 9 for patients with colorectal PM (range 3–35); 5 (range 1–31) for primary CRS, 8 (range 1–29) for interval and 7 (range 1–26) for salvage CRS for ovarian cancer; 28 (range 1–39) for PMP from appendiceal tumours, 27.5 (range 5–35) for mesothelioma and 8 (range 1–31) for uncommon indications. A complete cytoreduction (CC-0/1) was obtained in 96.7% patients with colorectal cancer, 100% undergoing primary and interval CRS for ovarian cancer, 98.1% patients undergoing salvage CRS for recurrent ovarian cancer, 92.2% in patients with PMP, 83.4% with mesothelioma and 77.4% for uncommon indications. The median follow-up was 14 months (range 3–72 months)
Morbidity and mortality
Grade 3–4 complications occurred in 76 (22.8%) patients within 30 days of surgery and in 98 patients (29.5%) within 90 days of surgery (). The common complications were pulmonary in 23 patients (6.9%), intraperitoneal haemorrhage in 15 (4.5%), urinary fistulas in 13 (3.9%), and enteric leaks and fistulas in 12 (3.6%). A PCI score >10 (p = .03), duration of surgery >8 h (p < .01) and resection of 4 or more organs (p = .04) were independent predictors of grade 3–4 complications. Urinary fistulas were from the bladder in two cases, lower end of the ureter in 2, break down of the uretero-ureteral anastomosis in 2 patients and the uretero-cystostomy in 2 patients. Two patients had delayed fistulas occurring more than 30 days after surgery and in three the site was not specified. On univariate analysis, the 90-day grade 3–4 morbidity was higher in patients undergoing HIPEC (p = .028), having >3 organ resections (p = .039) and those who were treated before 2014 (p = .05). On multivariate analysis, surgery before 2014 was the only independent predictor (p = .038).
Table 3. Cox-regression analysis showing the impact of various factors on survival outcomes according to the primary tumour site.
In-hospital deaths (within 30 days of surgery) occurred in 15 (4.5%) patients and 19 (5.7%) patients died within 90 days. The morbidity and mortality rates did not vary among surgeons in the private and public centres (p = .69 for grade 3–4 morbidity and p = .23 for in-hospital mortality). The rate of failure-to-rescue was 19.3% (19 patients with grade 3–4 complications died because of these complications). The underlying complication was spontaneous small bowel perforation (perforations occurring in areas of the bowel without a suture line) in five patients, acute respiratory distress syndrome in three, respiratory failure in three, systemic sepsis in three and myocardial infarction in one. All five patients who developed anastomotic leaks were rescued. Following discharge, there were two patients who developed intra-abdominal abscess without a breach in bowel continuity and two others with a urinary fistula could not be rescued. A higher rate of failure-to-rescue was seen in patients with a PCI >20 (p = .02), pulmonary complications (p = .03), spontaneous bowel perforations (p = .005), systemic sepsis (p = .001). The only significant factor on multivariate analysis was systemic sepsis (p = .001).
Disease-specific outcomes
A comparison of the DFS and OS between various primary sites is provided in .
Figure 1. Kaplan Meier survival analysis for disease free survival (A) and overall survival (B) according to the primary tumour site.
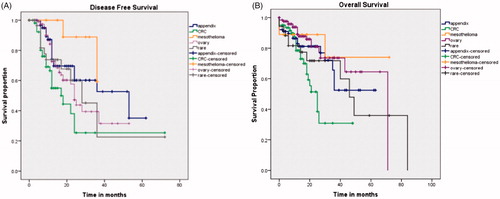
Colorectal cancer—The histology was mucinous in 15 patients (24.1%) and signet ring cell in 10 (16.1%) (>50% with signet ring cell tumours). The median OS was 25 months (95% CI: 19.0–30.9 months) and the median DFS 17 months (95% CI: 11.7–22.2 months). A PCI of <20 was an independent predictor of a better DFS and OS both (p < .01 for both). The OS was significantly shorter in patients with grade 3–4 morbidity (p < .01). There was no difference in survival between patients with synchronous (n = 28) or metachronous (n = 27) PM (p = .8), right- and left-sided tumours (p = .71) or between colonic (44) and rectal (n = 12) primary tumours (p = .76) ().
Ovarian cancer—Of 127 patients, 88 underwent HIPEC, 4 HIPEC and EPIC both, 25 had EPIC alone. The median OS was not reached in patients undergoing primary (n = 24) and interval CRS (n = 48) and was 71 months for salvage CRS (n = 55) with no difference between the 3 indications (p = .11). The median DFS was 24 months following primary and salvage CRS and 28 months following interval CRS (p = .24). Patient PCI <10 (p = .02) had a superior DFS. There was no difference in both DFS (p = .89) and OS (p = .53) in patients who received HIPEC and those who did not ().
Appendiceal tumours, peritoneal mesothelioma and uncommon indications
The median OS was not reached and the median DFS was 53 months (95% CI: 17.3–88.6 months) for PMP. The median DFS and OS were not reached for patients with mesothelioma. The median DFS for uncommon indications was 25 months (95% CI: 19.0–30.9 months) and the median OS 49 months (95% CI: 29.6–68.3 months). In this group, the DFS and OS was inferior (all p values <.05) in patients with lymph node involvement, 90-day major morbidity and high grade tumours on multivariate analysis. Patients undergoing HIPEC had a longer DFS (p = .007).
Discussion
In a short span of 18 months, 43 surgeons were enrolled in the registry and 10 contributed data. Looking at the clinical outcomes, our results fall short of the standards set by experienced centres in certain areas and match well with them in others. Moreover, among different centres within the country, there is a significant variation in the patient population and indications for the procedure but the overall morbidity and mortality are similar.
Operational issues
Only 10 of the 43 surgeons enrolled patients largely due to the lack of dedicated personnel for data collection and entry into the database. None of the centres had a complete electronic medical record system and where available only selective data was recorded. All the data elements have to be manually assimilated and then entered into the database. Support staff are not available at most centres and this is done by the senior or junior surgeons themselves. This was the main reason for a lack of contribution from many members of the registry. To address this problem, we have created exhaustive data collection forms which can be put into the hospital records and submitted to the registry coordinator for data entry into the registry.
All cases were enrolled by six surgeons only. Two surgeons could not enter retrospective data since they had changed their institution and the patients’ treatment records and contact information were not accessible to them. This problem is addressed by the registry that maintains the contact information of each patient.
Though we invited all centres performing more than 15 procedures a year to participate, it is not possible to determine the exact number of HIPEC centres in the country and therefore, the proportion participating in the registry.
Compared to Germany, where it is mandatory for all centres to contribute to the registry [Citation14], in India there is no such regulation; the participation is voluntary. Talking about the registry, increased surgeon enrolment with 33 members joining the registry at international [Citation15] and national meetings was noticed in the last year.
The Indian HIPEC registry has received no funding from either the government or the industry in contrast to the RENAPE and Netherlands cancer registry that are government recognised and funded. A curative approach to PM is a relatively new concept in the country and it will be long before it becomes a priority on the agenda of national policy makers [Citation16,Citation17]. As the scope of the registry increases, dedicated personnel will be needed thereby increasing the annual expenditure. Self-funding is a simple and viable option given the large number of participating surgeons.
Clinical outcomes
Of the 332 patients enrolled, 10.4% did not undergo HIPEC due to various reasons. There was variability among surgeons in terms of primary tumour site. The median PCI ranged from 3 to 23. The 30 and 90-day morbidity rates compare favourably with recently published reports. But mortality of 4.5% and 5.7% at 30 and 90 days, respectively, is high compared with other published reports from experienced centres [Citation18,Citation19]. The failure to rescue rate of 19.3% is much higher than that reported by an expert centre (4.4%) [Citation20]. These rates were not different among the private and public sectors or among the individual surgeons. The most common cause of failure-to-rescue was sepsis and respiratory complications. Complications like bowel perforations lead to systemic sepsis and subsequently failure-to-rescue. Mortality did not occur in most patients who did not develop systemic sepsis. Another problem was the relatively high incidence of urological complications. A detailed analysis into the underlying causes is needed with adoption of safer techniques of anastomosis [Citation21]. Similarly, the incidence of spontaneous bowel perforation was high. Such perforations occur due to unidentified thermal injuries when HIPEC is performed by the open abdominal technique and surgeons have to be cautious to avoid such complications.
The DFS and OS are at par with reported results for each disease site except colorectal cancer [Citation19,Citation20]. The median DFS and OS of 17 and 25 months, respectively, is inferior to results published by expert centres [Citation22,Citation23]. The histology was mucinous in 24.1% of these patients and 16% had signet ring cell tumours (>50% signet ring cells). Over 90% of the patients received 6 months of perioperative chemotherapy. The high proportion of mucinous tumours which are less chemosensitive may represent a selection bias rather than a disease trend since surgeons are more likely to take up patients with a high PCI for surgery [Citation24]. Notably, <15% of these patients had received targeted therapy which may be another contributor to the inferior survival. Further evaluation of the underlying causes is needed with use of validated prognostic scores for selecting patients for the procedure [Citation25]. In contrast, the median DFS and OS in patients undergoing CRS with and without HIPEC at different time points in the history of ovarian cancer, compares favourably with published results though this is a very selected group of patients [Citation26,Citation27]. The same surgeons treated patients with ovarian and colorectal cancer.
The median PCI [Citation28] was significantly higher for PMP arising from appendiceal primary tumours and the median DFS of 53 months in these patients compares favourably with published results [Citation18].
The above results reflect a good patient selection and surgical skill as shown by the median PCI and the high rate of CC-0/1 resections.
However, they need to be viewed in light of the fact that not all patients have been enrolled by each surgeon in the registry and thus, they represent outcomes in a random group of patients. The median follow-up was also short (14 months) which is another limitation of this work.
They also highlight the need to have a quality of life and cost–benefit analysis for the procedure which is an out-of-pocket expenditure for Indian patients and devise cost-effective treatment strategies [Citation28].
Benefit of having the registry
In comparison to our previous study in which 42.7% of the patients were lost to follow up, we had a complete follow-up in 98.5%, which is a significant improvement [Citation5]. The data capturing was non-uniform before the registry was instituted, for example, most surgeons used the Clavien-Dindo classification and systemic toxicity and 90-day morbidity was not recorded [Citation5,Citation29]. The registry thus provided an effective solution for complete and uniform data collection.
Fifty-three percentage of the surgeons were enrolled in the first year of starting a peritoneal surface oncology programme. The registry can be useful for evaluating individual results, especially since there are no regulatory norms or resources for audit at a national level or even at regional levels.
Perhaps, the most distinct feature of the registry is the collaboration between the public and private sectors which is seldom the case in this country [Citation30]. There is a dearth of established research networks, which facilitate collaborative studies and high-quality cancer research. Most published reports are single institution experiences [Citation31]. The National cancer registry programme run by the Indian Council of Medical Research (ICMR) has 26 population-based registries and 7 hospital-based registries which cover roughly 15% of the population and collect disease at two time points: diagnosis and death [Citation32]. The Indian HIPEC registry is the only site-specific cancer registry in the country. As the number of participating centres increases, it could provide epidemiological information in addition to the treatment-related information. The surgeons participating in the registry can collaborate and form national consensus guidelines as done by other developing countries [Citation33,Citation34]. With a population of over a billion people, even an uncommon disease will have a large number of affected individuals. The proportion of eligible patients undergoing HIPEC is unknown yet, in terms of numbers alone, the registry can contribute significantly to ongoing international research. Over 300 patients were enrolled in a span of 18 months by 10/43 surgeons. There is a uniform recording of data with all pertinent data elements recorded in the registry including a detailed pathology report which ensures high quality of data.
In the Indian scenario, it is unlikely that a multi-institutional clinical trial will be conducted in the near future. In addition to the registry, we have formed a collaborative group, the Indian Network for DEvelopment of Peritoneal Surface Oncology (INDEPSO) through which prospective studies are being conducted.
This study is retrospective with a short median follow-up and there is bias due to enrolling of selected patients which is the greatest limitation. Nevertheless, it provides a broad overview of outcomes at Indian centres and identifies areas needing improvement.
Conclusions
These results validate existing practices and identify country-specific problems that need to be addressed like the high failure-to-rescue rate and poor survival in patients with colorectal PM. Some old obstacles like selecting the right patients (reflected in the median PCI), obtaining a complete cytoreduction and controlling the morbidity have been overcome. New obstacles like lack of funding and personnel for data collection need to be overcome. Despite operational problems, the registry is an invaluable tool for audit and research. It shows the feasibility of fruitful collaboration between surgeons in the absence of any regulatory body or funding for the project. The registry can contribute to ongoing collaborative international research in peritoneal surface oncology. This model can be adopted by other surgical specialties and other developing countries.
Supplemental Material
Download PDF (92.7 KB)Acknowledgements
The authors thank Prof. Olivier Glehen for his suggestions and inputs on the registry. The authors thank Dr. Aasma Girkar and Dr. Hardik Chavda for helping with data entry into the registry.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chia CS, You B, Decullier E, et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol. 2016;23:1971–1979.
- Goéré D, Malka D, Tzanis D, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257:1065–1071.
- Baratti D, Kusamura S, Cabras AD, et al. Diffuse malignant peritoneal mesothelioma: Long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer. 2013;49:3140–3148.
- Moran BJ. Decision-making and technical factors account for the learning curve in complex surgery. J Public Health (Oxf). 2006;28:375–378.
- Bhatt A, Mehta S, Seshadri RA, et al. The initial Indian experience with cytoreductive surgery and HIPEC in the treatment of peritoneal metastases. Indian J Surg Oncol. 2016;7:160–165.
- Chang KH, Kazanowski M, Staunton O, et al. Mentored experience of establishing a national peritoneal malignancy programme—experience of first 50 operative cases. Eur J Surg Oncol. 2017;43:395–400.
- Verwaal VJ, Rau B, Jamali F, et al. Registries on peritoneal surface malignancies throughout the world, their use and their options. Int J Hyperthermia. 2017;33:528–533.
- Villeneuve L, Passot G, Glehen O, et al. The RENAPE observational registry: rationale and framework of the rare peritoneal tumors French patient registry. Orphanet J Rare Dis. 2017;12:37.
- Maillet M, Glehen O, Lambert J, et al. Early postoperative chemotherapy after complete cytoreduction and hyperthermic intraperitoneal chemo- therapy for isolated peritoneal carcinomatosis of colon cancer: a multicenter study. Ann Surg Oncol. 2016;23:863–869.
- Razenberg LG, van Gestel YR, Creemers GJ, et al. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment 905 of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol. 2015;41:466–471.
- Pramesh CS, Badwe RA, Borthakur BB, et al. Delivery of affordable and equitable cancer care in India. Lancet Oncol. 2014;15:e223–e233.
- Bhatt A, Mehta S, Ramakrishnan AS, et al. Setting up of the Indian HIPEC registry: a registry for Indian patients with peritoneal surface malignancies. Indian J Surg Oncol. 2017;8:527–532.
- Sugarbaker PH, Van der Speeten K, Stuart OA, et al. Patient- and treatment related variables, adverse events and their statistical relationship for treatment of peritoneal metastases. In: Sugarbaker PH, editor. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy: textbook and video atlas. Woodbury: Ciné-Med; 2012.
- Pelz JO. Peritoneal carcinomatosis: registry and centers in Germany. Viszeralmedizin. 2013;29:3–229.
- Sugarbaker PH. The Seven Best from PSOGI 2016. Ann Surg Oncol. 2017;24:870–874.
- Sullivan R, Badwe RA, Rath GK, et al. Cancer research in India: national priorities, global results. Lancet Oncol. 2014;15:e213–e222.
- Bhandare M, Patil P, Pai V, et al. Peritoneal carcinomatosis in colorectal cancers—management perspective needs a change. Clin Colorectal Cancer. 2017;16:e1–e6.
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456.
- Levine EA, Stewart JH, Shen P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 Patients. J Am Coll Surg. 2014;218:573–585.
- Passot G, Vaudoyer D, Villeneuve L, et al. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113:796–803.
- Pinar U, Tremblay JF, Passot G, et al. Reconstruction after ureteral resection during HIPEC surgery: re-implantation with uretero-neocystostomy seems safer than end-to-end anastomosis. J Visc Surg. 2017;154:227–230.
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–685.
- Ihemelandu C, Sugarbaker PH. Management for peritoneal metastasis of colonic origin: role of cytoreductive surgery and perioperative Intraperitoneal chemotherapy: a single Institution’s experience during two decades. Ann Surg Oncol. 2017;24:898–905.
- Brandl A, Weiss S, von Winterfeld M, et al. Predictive value of peritoneal cancer index for survival in patients with mucinous peritoneal malignancies treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a single centre experience. Int J Hyperthermia. 2018;34:512–517.
- Enblad M, Ghanipour L, Cashin PH. Prognostic scores for colorectal cancer with peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2018;1–6.
- Di Giorgio A, De Iaco P, De Simone M, et al. Cytoreduction (peritonectomy procedures) combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in advanced ovarian cancer: retrospective Italian multicenter observational study of 511 cases. Ann Surg Oncol. 2017;24:914–922.
- Arjona-Sanchez A, Rufián-Peña S. Progress in the management of primary and recurrent ovarian carcinomatosis with peritonectomy procedure and HIPEC in a high volume centre. Int J Hyperthermia. 2017;33:554–561.
- Bhatt A, Prabhu R, Sethna K, et al. The “homemade” HIPEC machine—a cost-effective alternative in low-resource countries. Pleura and Peritoneum. 2017;2:163–170.
- Alyami M, Kim BJ, Villeneuve L, et al. Ninety-day post-operative morbidity and mortality using the National Cancer Institute’s common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2018;34: 532–537.
- Pramesh CS, Badwe RA, Sinha RK. The National Cancer Grid of India. Indian J Med Paediatr Oncol. 2014;35:226–227.
- Gupta N, Asif S, Gandhi J, Rajpurohit S, et al. Role of CRS and HIPEC in appendiceal and colorectal malignancies: Indian experience. Indian J Gastroenterol. 2017;36:126–130.
- National Cancer Registry Programme. (2015) WWW page. URL: http://ncrpindia.org. Last accessed 2018 Feb 27.
- Li Y, Zhou Y-F, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. WJG. 2016;22:6906–6916.
- Batista PB, Sarmento BJQ, Loureiro FL, et al. A proposal of Brazilian Society of Surgical Oncology (BSSO/SBCO) for standardizing cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in Brazil: pseudomixoma peritonei, appendiceal tumors and malignant peritoneal mesothelioma. Rev Col Bras Cir. 2017;44:530–544.
