Abstract
Purpose: Loco-regional hyperthermia combined with mitomycin C is used for treatment of nonmuscle invasive bladder cancer (NMIBC). Air pockets may be present in the bladder during treatment. The aim of this study is to quantify the effect of air pockets on the thermal dose of the bladder.
Methods: We analysed 16 patients treated for NMIBC. Loco-regional hyperthermia was performed with the in-house developed 70 MHz AMC-4 hyperthermia device. We simulated treatments with the clinically applied device settings using Plan2Heat (developed in-house) including the air pockets delineated on CT scans made following treatment, and with the same volume filled with urine. Temperature distributions simulated with and without air pockets were compared.
Results: The average air and fluid volumes in the bladder were 6.0 ml (range 0.8 − 19.3 ml) and 183 ml (range 47–322 ml), respectively. The effect of these air pockets varied strongly between patients. Averaged over all patients, the median bladder wall temperature (T50) remained unchanged when an air pocket was present. Temperature changes exceeded ±0.2 °C in, on average, 23% of the bladder wall volume (range 1.3–59%), in 6.0% (range 0.6–20%) changes exceeded ±0.5 °C and in 3.2% (range 0.0–7.4%) changes exceeded ±1.0 °C. There was no correlation between the differences in temperature and the air pocket or bladder volume. There was a positive correlation between air pocket surface and temperature heterogeneity.
Conclusion: Presence of air causes more heterogeneous bladder wall temperatures and lower T90, particularly for larger air pockets. The size of air pockets must therefore be minimized during bladder hyperthermia treatments.
Introduction
Hyperthermia is a well-established (neo)-adjuvant modality in the treatment of nonmuscle invasive bladder cancer (NMIBC) [Citation1–4]. In the treatment of NMIBC, hyperthermia is combined with chemotherapeutic instillations, most commonly mitomycin C (MMC). By adding hyperthermia to MMC instillations, 10-year disease free response has been reported to increase from 17 to 54% [Citation5]. The combination of hyperthermia and MMC yields clinical results just as good or even exceeding those of Bacillus Calmette-Guérin (BCG), the present standard of care for intermediate and high-risk NMIBC [Citation6]. Hyperthermia may be delivered using various techniques [Citation7], including external radiofrequency (RF) antennas, using the BSD-2000 (Pyrexar, Salt Lake City, UT) [Citation8] or the in-house developed AMC-4/8 hyperthermia device [Citation9,Citation10] as is done in our institute, or its successor, the ALBA-4D (Medlogix, Rome, Italy).
When the chemotherapeutic solution is instilled, there is frequently some air inserted in the bladder. This results in air pockets which may have an effect on the temperature distribution in the treatment target, i.e. the tumor and the urothelial layer of the bladder wall, and hence on treatment quality. Several authors demonstrated the existence of a temperature dose–effect relationship, also when combining hyperthermia with MMC [Citation11–14]. A too low temperature may lead to a reduced treatment effect, a too high temperature may lead to adverse effects, e.g. thermal damage.
When using external RF antennas, there are two opposing effects that can be expected to occur. On the one hand, the air pocket may act as a reflective shield against the electromagnetic (EM) radiation from the antenna(s) closest to the bladder, due to the air’s low electric conductivity. Consequently, the top part of the bladder in the direct neighborhood of the air pocket may absorb more EM power leading to a higher local temperature, while the remainder of the bladder receives less EM power resulting in a lower temperature in the remainder of the bladder. On the other hand, the air pocket may act as an insulating layer, trapping the heat accumulated in the bladder contents. In particular, the heat transported by convective currents to the upper part of the fluid will not be in contact with the top part of the bladder wall, leading to a lower temperature in the top part of the bladder above the fluid and a higher temperature in the remainder of the bladder. An elegant method of addressing this issue is performing simulations for patients where the anatomy can be kept completely identical except for the presence or absence of such an air pocket, thus eliminating confounding factors.
The aim of this study is to quantify, by means of simulations, the effect of air pockets in the bladder on the temperature of the bladder contents and the bladder wall by analysing temperature distributions in NMIBC patients treated at the AMC.
Materials and methods
Patients and treatment
A consecutive cohort of eighteen NMIBC patients who received a loco-regional hyperthermia treatment in our institute between March 2009 and November 2011 in the framework of our pilot study [Citation10] were studied retrospectively; of these, sixteen were included in the analysis (patient #17 was treated with a different device, and there were technical problems for patient #14). After diagnosis with intermediate- or high-risk NMIBC, the visible tumor was removed by transurethral resection. Patients then received six weekly MMC + HT instillations and after that four maintenance instillations at 3, 6, 9, and 12 months.
At the start of treatment, the bladder was emptied and subsequently filled with 40 mg mitomycin C in 50 ml 0.9% saline, using a three-way catheter with a fixation balloon. A multisensor type T thermocouple (Ella-CS, Hradec Kralove) with 14 sensors 0.5 cm apart was also inserted through the same catheter. The tumor area and bladder wall were then heated aiming at 43 °C using the 70 MHz AMC-4 hyperthermia device. Treatment consisted of a warming up phase, lasting until half of the thermocouple sensors in the bladder reached 41 °C or 30 min had passed (whichever occurred first), followed by a treatment phase lasting 60 min, during which time the temperature in the bladder was kept as close to 43 °C as possible, while minimizing patient discomfort. Initial phase and amplitude settings are based on protocol and delta T measurements [Citation15], and adjusted during treatment based on temperature measurements, patient feedback and operator experience.
Electromagnetic and thermal simulations
As part of the treatment protocol, a CT scan (GE LightSpeed RT16, resolution 1.0 × 1.0 × 2.5 mm3) was made after one of the hyperthermia treatment sessions (usually the first), with the catheters containing the temperature sensors still in place. On this scan, a physician delineated the bladder and the air pocket in the bladder was delineated by one of the researchers. Other tissues were automatically segmented into the tissue types muscle, fat, bone and air based on Hounsfield units. For further calculations, the scan was down-sampled to 2.5 × 2.5 × 2.5 mm3 resolution in order to speed up the computations and meet the system's memory constraints, and in correspondence with the clinical workflow. The delineated bladder was completed by adding a 2 voxel (5 mm) thick bladder wall with muscle properties; the rest of the volume was assigned ‘bladder content’ (urine) properties. The cells in the 'bladder contents' volume were subdivided into six equal pyramids by adding an additional vertex to the center of the cell. Tissue properties (listed in ) were based on literature values, using an increased perfusion value for muscle valid under hyperthermic conditions [Citation23,Citation24].
Table 1. Dielectric and thermal parameter values at 70 MHz for the various tissue types used in the simulations: electrical conductivity (σ [S m−1]), relative permittivity (εr [-]), density (ρ [kg m − 3]), specific heat capacity (Ct [J kg−1 K−1]), thermal conductivity (k [W m−1 K−1]) and perfusion (ω Cb [J m−3 s−1 K−1])
Using this segmentation, the hyperthermia treatment was simulated using our in-house developed hyperthermia treatment planning system Plan2Heat [Citation25], which follows a two-step procedure. It first computes the EM field and resulting power absorption using a finite difference time domain solver, with the antenna settings that were clinically applied towards the end of the treatment session. Next, it calculates the temperature distribution, using Pennes’ bioheat equation for the solid tissues and a finite element fluid solver, including natural convection, for the bladder contents. Heat exchange between bladder contents and bladder wall is governed by an effective heat transfer coefficient. The reader is referred to [Citation24,Citation26] for full details. Subsequently, the air pocket was removed by assigning ‘bladder content’ tissue properties to the corresponding voxels, and the treatment was simulated again.
Analysis
For each patient, we determined the volume of the air pocket and of the liquid bladder contents, and the part of the bladder wall surface in contact with the air pocket, using the segmented and down-sampled patient scan. Then, the specific absorption rate (SAR) and equilibrium temperature distribution were compared for the simulations with and without air pocket. First, we compared the power deposition (represented by the SAR) in the bladder contents and the bladder wall. Second, we compared the temperature difference in the bladder contents and the bladder wall. For both SAR and temperature, we computed the change in median value, standard deviation, 10th percentile (T90), and 90th percentile (T10). Subsequently, we computed the volume in which noticeable changes in SAR (>5%) and temperature (>0.5 °C) occurred.
We then combined the data for all patients and determined the best fit linear correlation between the effect size and the amount of air (both by volume and by contact surface), and between the effect size and the bladder volume. We finally determined the heterogeneity coefficient (HC) for each patient, which is defined as HC = (T10 − T90)/(T10 − 37 °C) (adapted from van der Koijk et al. [Citation27]).
Results
Bladder and air pocket size
The main results are summarized in , showing the volumes of air and liquid (chemotherapeutic instillation with a varying amount of urine) present in the patient bladders, the contact surface area, the differences in SAR, and the differences in temperature for both the bladder contents and the bladder wall.
Table 2. Overview of the volume of liquid in the bladder at the end of treatment, the volume of air, and the area of the contact surface between the air pocket and the bladder wall, both in absolute and relative measures.
Table 3. Effect of the presence of an air pocket in the bladder on the SAR.
Table 4. Effect of the presence of an air pocket in the bladder on the temperature distribution.
The liquid bladder contents averaged 183 ± 72 ml (range 47−322 ml). There was on average 6.0 ± 4.4 ml (range 0.8−19.3 ml) air in the bladder, making up on average 3.7 ± 2.8% (range 0.6−13.0%) of the bladder contents. The bladder volume as it was delineated by the physician, i.e. including air pocket and bladder wall, was on average 253 ml (range 93 − 452 ml).
Effect of air pockets on SAR
The median (over the population) of the mean ± standard deviation (per patient) of the simulated SAR in the bladder contents was 17.6 ± 6.3 W kg−1 (range 7.1−72.8 W kg−1) with an air pocket in the bladder, and 18.4 ± 5.7 W kg−1 (range 7.2−72.7 W kg−1) without air pocket. The median change in mean SAR was −0.3 W kg−1 (−2%). The largest change was seen in a patient, for whom the average SAR increased from 37.8 to 71.1 W kg−1 (88.4%). In addition to the above-mentioned patient, there were two other patients for whom the relative change in SAR exceeded 5%, with changes of 14.4 and −11.8%.
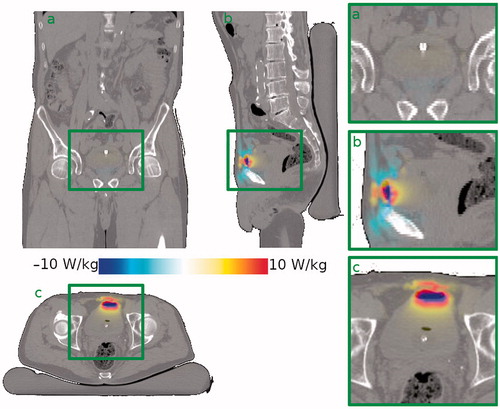
The median simulated mean SAR in the bladder wall was 18.4 ± 15.8 W kg−1 (range 7.9–40.6 W kg−1) with an air pocket in the bladder, and 17.1 ± 15.7 W kg−1 (range 7.0–39.2 W kg−1) without air pocket. The median change in mean SAR was −0.6 W kg−1 (−3.2%). For three patients, the relative change in SAR was smaller than −5%, and for three patients the relative change was larger than +5%. The effect of an air pocket on the SAR distribution in a typical patient is shown in . It can be seen that the change in SAR is focused in a relatively small area around the air pocket.
Figure 1. Effect of the presence of an air pocket on the SAR distribution in a typical patient (patient #6) compared to the situation without air pocket. The effect is mostly localised around the air pocket. Shown are (a) a coronal view, (b) a sagittal view and (c) a transversal view through the bladder’s center of gravity. The right-hand column shows zoomed in versions of the bladder region.
Effect of air pockets on temperature
The mean simulated median temperature in the bladder contents was 42.3 ± 2.4 °C (range 37.8 − 48.4 °C) with an air pocket in the bladder, and 42.2 ± 2.3 °C (range 38.3 − 48.3 °C) without air pocket. The mean change (situation with air present minus situation with no air present) in median temperature was 0.1 ± 0.3 °C (range −0.7 to +0.6 °C). For the bladder wall, these numbers were, respectively, 41.0 ± 1.5 °C (range 38.3 − 44.4 °C) and 41.0 ± 1.4 °C (range 38.8 − 44.4 °C), with a mean change of −0.0 ± 0.2 °C (range −0.6 to +0.3 °C). Whereas the effect on the median bladder temperature is thus limited (both for the bladder contents and the bladder wall), local temperature changes can be more significant. On average 23% (range 1.3–59%) of the bladder wall, changes exceeded ±0.2 °C, in 6.0% (range 0.6–20%) changes exceeded ±0.5 °C, and in 3.2% (range 0.0–7.4%) changes exceeded ±1.0 °C. The effect of an air pocket on the temperature distribution in a typical patient is shown in . shows typical temperature profiles through the bladder’s center of gravity for the same patient. The effect on the temperature extends through a much larger region than the effect on the SAR. This is partially due to the efficient heat transport by the convective currents inside the bladder.
Figure 2. Effect of the presence of an air pocket on the steady state temperature distribution in a typical patient (patient #6) compared to the situation without air pocket. Since the air pocket acts as a thermally insulating layer, the liquid inside the bladder loses less heat to the upper part of the bladder wall. This leads to a higher temperature in the bladder contents, a slightly higher temperature in the upper part of the bladder wall, still in contact with the liquid bladder contents, and a lower temperature in the part of the bladder wall in immediate contact with the air pocket. Shown are (a) a coronal view, (b) a sagittal view and (c) a transversal view through the bladder’s center of gravity. The right-hand column shows zoomed in versions of the bladder region. Gravity points into the paper, to the left, and to the bottom, respectively.
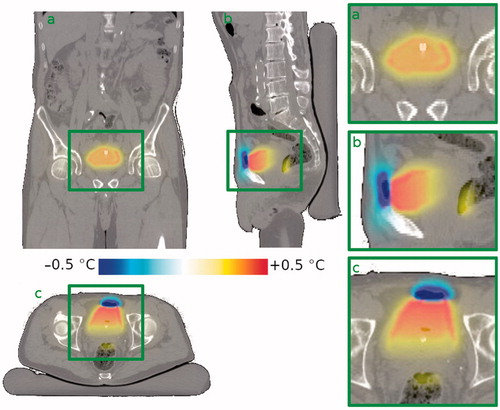
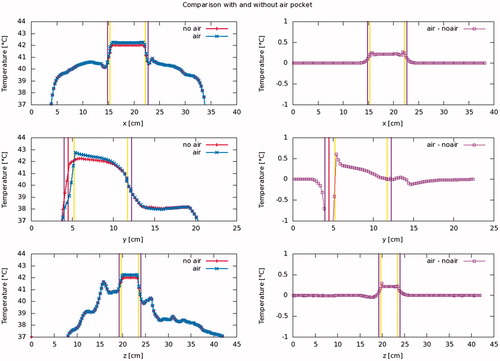
Figure 3. Steady state temperature profile along the cardinal axes, through the bladder’s center of gravity, for a typical patient (patient #6). Vertical purple lines show the outside of the bladder wall, yellow lines the interface between the bladder contents and the bladder wall and the red line the interface between the bladder contents and the air pocket. It is observed that the air pocket causes the bladder contents, and hence the bladder wall in contact therewith, to be slightly warmer, the effect increasing towards the air pocket. The air pocket itself, and the part of the bladder wall in contact therewith, however, are cooler than they would be in the absence of an air pocket. Shown are profiles for the patient’s left-to-right (top), anterior-posterior (middle) and caudal-cranial (bottom) directions.
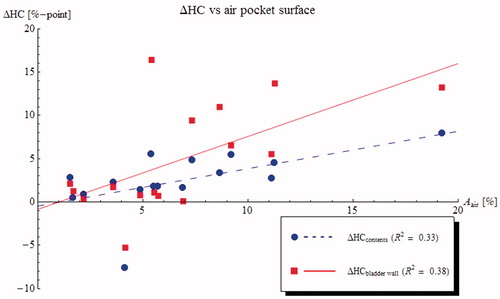
The changes in T50 are plotted against air pocket volume in (results for correlations against relative air volume or contact surface were similar). shows the differences in T10 and T90 for the bladder wall. Results show a moderate effect on T50 but only very weak correlations between temperature differences and the amount of air. The divergent changes in T10 and T90 indicate an increasing temperature heterogeneity. This can be seen more clearly in , showing the effect of the air pocket contact area on the temperature HC. The change in HC is much stronger for the bladder wall than for the bladder contents, due to the efficient heat transport by the convective currents inside the bladder.
Figure 4. Predicted changes in median temperature (T50) of the bladder content (blue circles) and bladder wall (red squares) plotted against air pocket volume for 16 NMIBC patients. On average, the median temperature in the bladder contents is slightly (0.1 °C) higher if there is an air pocket present in the bladder, and the temperature appears to increase slightly with increasing air pocket volume. The median bladder wall temperature is slightly (0.1 °C) lower in the presence of an air pocket, but there is no correlation with air pocket size.
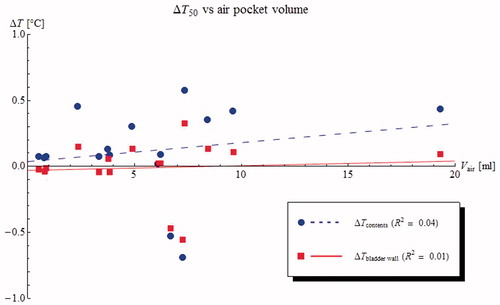
Figure 5. Predicted changes in T90 (blue circles) and T10 (red squares) bladder wall plotted against air pocket contact surface for 16 NMIBC patients. The presence of an air pocket in the bladder has the undesirable effect of making the bladder wall temperature less homogeneous, increasing the T10 and decreasing the T90.
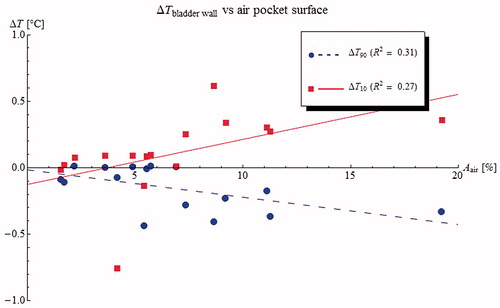
Figure 6. Predicted changes in heterogeneity coefficient (HC = (T10 − T90)/(T10 − 37 °C)) for the bladder contents (blue circles) and the bladder wall (red squares) plotted against air pocket contact surface for 16 NMIBC patients. The presence of an air pocket in the bladder increases the heterogeneity of the bladder wall temperatures, but has less effect on the heterogeneity of the bladder contents.
Discussion
This study investigated the effect of air pockets in the bladder on thermal dose during MMC/hyperthermia treatments of NMIBC patients. Results showed that although the effect on the mean temperature of the bladder contents and bladder wall was quite small (about 0.1 °C), regions with significant temperature changes of more than ±1 °C were identified, generally in the top (ventral) part of the bladder wall, close to the air pocket. This is clinically very relevant, as dose-modifying factors for 42 °C and 43 °C heating with MMC ranging from 1.3 to 2.0 and 2.6 to 3.8, respectively, are reported in the literature [Citation12,Citation13]. In two patients, the extra heat conducted from the bladder to the region surrounding the ventral side of the bladder (and close to the top antenna), resulting from the absence of an air pocket, led to the appearance of a small hot spot (∼45.5 °C).
Our study considered an external loco-regional hyperthermia device consisting of multiple 70 MHz antennas, and can easily be extended. However, results would also apply to other RF or microwave external loco-regional hyperthermia devices [Citation8], or when an air pocket occurs when using an intracavitary applicator microwave antenna like the 915 MHz Synergo applicator (Medical Enterprises, Amsterdam, the Netherlands) [Citation5,Citation6].
An air pocket in the bladder has the undesirable effect of making the bladder wall temperature less homogeneous, increasing the T10 and decreasing the T90. This effect increases with the contact surface between the air pocket and the bladder wall. As the T90 is correlated to therapeutic effect, this implies that the treatment may be less effective when an air pocket is present, and in particular in the presence of a large air pocket. A high T10 may lead to pain complaints during treatment, which requires the power deposition to be reduced, resulting in a lower thermal dose. These effects may be partially mitigated by advanced adaptive planning [Citation28,Citation29], but such phase and amplitude settings adjustments to mitigate the effects of an air pocket fall outside the scope of this study. Additionally, the air pocket may have a detrimental effect on the quality of temperature measurements in the bladder [Citation30].
Both these effects may be due to the fact that the bladder wall temperature is for a large part determined by the temperature of the bladder contents. The bladder contents may absorb a large amount of power, due to their high electric conductivity, and this heat is then transported to the bladder wall. As heat conduction to the bladder wall is the only cooling mechanism for the bladder contents, the bladder contents are typically warmer than the bladder wall. If a similar amount of heat is absorbed by the bladder contents, the effect of an air pocket, thermally insulating part of the bladder wall, leads to a similar amount of heat being transferred to a smaller part of the bladder wall. This in turn leads to higher temperatures in the bladder contents and parts of the bladder wall, and lower temperatures in the parts of the bladder wall that are in direct contact with the air pocket.
Due to the geometry of the bladder, air pockets typically manifest as relatively thin layers. As a result, the surface of the air pocket, and hence the surface of the bladder wall in contact with the air pocket, is relatively large. Therefore, even a small amount of air may have a noticeable effect on the temperature distribution in the bladder wall. As the amount of air increases, the main effect is that the layer of air becomes thicker. Consequently, the surface increases only little, and hence, the effect on the bladder wall can be expected to also be relatively insensitive to the amount of air, explaining the weak correlations found in and .
The effect of air pockets in the bladder is clinically much more relevant than the effect of air pockets that may be present elsewhere (for instance in the bowel or rectum) for a number of reasons. First, they coincide with part of the treatment target (the bladder wall), so any local effect will affect the treatment target, whereas small temperature differences in the bowel are not relevant for treatment outcome. Second, air pockets in the bladder remain effectively stationary during the entire 1-h treatment session, and will, therefore, have an effect during the entire treatment, whereas air in the bowel and rectum generally continuously moves around, having only a transient effect.
Inherent uncertainties in tissue properties and changes in anatomy during treatment may cause uncertainty in the simulated temperature distributions. In a previous study, it was shown that the simulated median temperature was found to be 0.6 °C lower, on average, than the measured median temperature; after correction for errors in SAR, this difference was reduced to −0.2 °C [Citation24]. Future research, including convergence studies, might further improve these results. However, while errors may vary greatly between patients, they will be very similar when the bladder with and without air pocket is compared for the same patient as was done in the present study. The effect size we found will therefore be relatively insensitive to uncertainties in tissue properties. Regarding changes in anatomy during treatment, the CT scan on which our computations were based was made after completion of hyperthermia treatment, which lasts about 90 min. The amount of bladder filling that is visible on the CT scan, and which is used for all computations, thus represents the maximum amount present during the treatment. If the entire volume of air in the bladder seen on the scan is inserted when the catheter and the chemotherapeutic solution are inserted, that is to say, at the start of treatment, then the relative amount of air will decrease during treatment, thus the results we reported may be underestimating the actual effect. Indeed, given that only 50 ml of fluid is inserted, the initial relative amount of air is on average about 12%, exceeding 25% in some patients. While this study found only very weak correlations between the amount of air and effect size, it is to be expected that such large relative amounts of air will have a more noticeable effect.
It seems likely that the presence of an air pocket in the bladder also prevents the chemotherapeutic solution from reaching parts of the bladder wall, which may also have a negative effect on treatment efficacy. That effect is, however, more difficult to quantify and is beyond the scope of this article. Since it was observed that the presence of air causes lower bladder minimum temperatures (T90 decreases), the effects of an air pocket should be mitigated during hyperthermia treatments of bladder cancer patients; or if possible, air pockets should be avoided. A straightforward method to mitigate the possible effects of an air pocket in the bladder, is to rotate the patient halfway during the treatment from supine to prone position, effectively moving the air pocket to the opposite side of the bladder. Apart from the effect on the thermal dose distribution, doing so will also resolve the problem that part of the bladder wall may remain unexposed to the chemotherapeutic instillation when the patient is treated in only one position. If rotating the patient during treatment is not feasible, one may consider to treat the patient alternatively in supine and prone position in subsequent weekly sessions. However, for some patients, treatment in prone position may be more difficult to endure. Possible solutions to avoid air pockets in the bladder are instillation of larger volumes of MMC or combination with recirculation during treatment [Citation31]. This can be expected to further improve the effectiveness of hyperthermia combined with MMC for patients with nonmuscle invasive bladder cancer.
Conclusion
The presence of even relatively small air pockets during loco-regional hyperthermia treatment of NMIBC causes lower temperatures in part of the bladder wall, with potentially detrimental effects on the treatment outcome. The effect of air pockets should therefore be minimized and proposed effective counter measures include change of position during treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liem EIML, Crezee H, de la Rosette JJMCH, et al. Chemohyperthermia in nonmuscle-invasive bladder cancer: an overview of the literature and recommendations. Int J Hyperthermia. 2016;32:363–373.
- Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur Urol. 2017;71:447–461.
- Longo TA, Gopalakrishna A, Tsivian M, et al. A systematic review of regional hyperthermia therapy in bladder cancer. Int J Hyperthermia. 2016;32:381–389.
- Owusu RA, Abern MR, Inman BA. Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. Biomed Res Int. 2013;2013:262313.
- Colombo R, Salonia A, Leib Z, et al. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with Mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int. 2011;107:912–918.
- Arends TJH, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus calmette-guerin for adjuvant treatment of patients with intermediate- and high-risk nonmuscle-invasive bladder cancer. Eur Urol. 2016;69:1046–1052.
- Stauffer PR, van Rhoon GC. Overview of bladder heating technology: matching capabilities with clinical requirements. Int J Hyperthermia. 2016;32:407–416.
- Inman BA, Stauffer PR, Craciunescu OA, et al. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for nonmuscle-invasive bladder cancer. Int J Hyperthermia. 2014;30:171–175.
- van Dijk JD, Schneider C, van Os R, et al. Results of deep body hyperthermia with large waveguide radiators. Adv Exp Med Biol. 1990;267:315–319.
- Geijsen ED, de Reijke TM, Koning CC, et al. Combining mitomycin C and regional 70 MHz hyperthermia in patients with nonmuscle invasive bladder cancer: a pilot study. J Urol. 2015;194:1202–1208.
- Oleson JR, Samulski TV, Leopold KA, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys. 1993;25:289–297.
- van der Heijden AG, Dewhirst MW. Effects of hyperthermia in neutralising mechanisms of drug resistance in non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32:434–445.
- Wallner KE, Banda M, Li GC. Hyperthermic enhancement of cell killing by mitomycin C in mitomycin C-resistant Chinese hamster ovary cells. Cancer Res. 1987;47:1308–1312.
- Teicher BA, Kowal CD, Kennedy KA, et al. Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res. 1981;41:1096–1099.
- Kok HP, Ciampa S, de Kroon-Oldenhof R, et al. Toward online adaptive hyperthermia treatment planning: correlation between measured and simulated specific absorption rate changes caused by phase steering in patients. Int J Radiat Oncol Biol Phys. 2014;90:438–445.
- Balidemaj E, van Lier ALHMW, Crezee H, et al. Feasibility of electric property tomography of pelvic tumors at 3T. Magn Reson Med. 2015;73:1505–1513.
- Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol. 1996;41:2231–2249.
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41:2251–2269.
- Gabriel C, Gabriel S. Compilation of the dielectrical properties of body tissues at RF and microwave frequencies. Report no.: AL/OE-TR-1996-0037. Brooks Air Force Base Texas: Armstrong Laboratory, 1996.
- Rossmann C, Haemmerich D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit Rev Biomed Eng. 2014;42:467–492.
- Klein L, Swift C. An improved model for the dielectric constant of sea water at microwave frequencies. IEEE Trans Antennas Propagat. 1977;25:104–111.
- Sharqawy MH, Lienhard JH, Zubair SM. Thermophysical properties of seawater: a review of existing correlations and data. Desalin Water Treat. 2010;16:354–380.
- Balidemaj E, Kok HP, Schooneveldt G, et al. Hyperthermia treatment planning for cervical cancer patients based on electric conductivity tissue properties acquired in vivo with EPT at 3T MRI. Int J Hyperthermia. 2016;32:558–568.
- Schooneveldt G, Kok HP, Bakker A, et al. Clinical validation of a novel thermophysical bladder model designed to improve the accuracy of hyperthermia treatment planning in the pelvic region. Int J Hyperthermia. 2018. doi:10.1080/02656736.2018.1506164
- Kok HP, Kotte ANTJ, Crezee J. Planning, optimisation and evaluation of hyperthermia treatments. Int J Hyperthermia. 2017;33:593–607.
- Schooneveldt G, Kok HP, Balidemaj E, et al. Improving hyperthermia treatment planning for the pelvis by accurate fluid modeling. Med Phys. 2016;43:5442
- van der Koijk JF, Lagendijk JJ, Crezee J, et al. The influence of vasculature on temperature distributions in MECS interstitial hyperthermia: importance of longitudinal control. Int J Hyperthermia. 1997;13:365–385.
- Kok HP, Korshuize-van Straten L, Bakker A, et al. Online adaptive hyperthermia treatment planning during locoregional heating to suppress treatment-limiting hot spots. Int J Radiat Oncol Biol Phys. 2017;99:1039–1047.
- Kok HP, Korshuize-van Straten L, Bakker A, et al. Feasibility of on-line temperature-based hyperthermia treatment planning to improve tumour temperatures during locoregional hyperthermia. Int J Hyperthermia. 2017;16:1–10.
- Schooneveldt G, Bakker A, Balidemaj E, et al. Thermal dosimetry for bladder hyperthermia treatment. An overview. Int J Hyperthermia. 2016;32:417–433.
- Sousa A, Piñeiro I, Rodríguez S, et al. Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate–high-risk non-muscle-invasive bladder cancer. Int. J Hyperthermia. 2016;32:374–380.
