Abstract
Purpose: It is unclear which kind of interventional therapies is the best when treating early-stage hepatocellular carcinoma (HCC). We conducted Bayesian network meta-analyses to compare local tumor progression (LTP), total tumor recurrence and survival rates and to rank the best intervention arm.
Materials and methods: A literature search of Pubmed, Embase, Cochrane library and Clinicaltrials.gov was conducted and randomized controlled trials (RCTs) comparing the outcomes of interventional therapies on early-stage HCC were enrolled. The quality assessment was conducted using Cochrane Collaboration’s tool, while the outcome synthesis of the network meta-analysis was conducted using R-3.3.4 software.
Results: A total of 35 RCTs were enrolled for further analysis. Using network meta-analysis, it was demonstrated that radiofrequency ablation (RFA) plus adjuvant therapies achieved the best performance in decreasing the LTP rate in early-stage HCC, while hepatic resection ranked as the best arm among all the interventional techniques for LTP at 3 years. Meanwhile, hepatic resection and RFA plus adjuvant therapies were the top two best arms in decreasing total recurrence. Furthermore, RFA plus adjuvant therapeutics ranked the best in achieving overall survival outcome, followed by hepatic resection. For disease-free survival, hepatic resection was the best, while for LTP-free survival, the difference among the included treatments was not significant.
Conclusions: Our network meta-analysis showed that RFA-based adjuvant therapies might be the most effective interventions in achieving the best outcomes, while hepatic resection exhibited the best performance in several situations in treating early-stage HCC. More RCTs are needed to draw more solid conclusions.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and ranks fifth in incidence and is the second leading cause of death among all kinds of cancer worldwide with a 5-year survival of <12% [Citation1,Citation2]. With the development of diagnostics techniques, patients are more frequently be diagnosed as early-stage HCC (single HCC, ≤5 cm; or up to 3 nodules, ≤3 cm). Currently, the most commonly used treatment strategies for early-stage HCC are liver transplantation, hepatic resection and local ablative techniques [Citation3,Citation4]. However, the high cost and a donor shortage limit the availability of liver transplantation, and it was reported that only 15–20% of HCCs are resectable at diagnosis [Citation3].
Over the past two decades, with the development of medical engineering and interventional technology, local ablative therapeutics, such as radiofrequency ablation (RFA), microwave ablation (MWA), percutaneous ethanol injection (PEI), laser ablation, and cryoablation, were also used as important treatments for early-stage HCC patients who were not eligible for hepatic resection treatments or as a bridge to transplantation, according to guidelines from both the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. Furthermore, other adjuvant therapies, such as concomitant agents, combination therapies with transcatheter arterial chemoembolization (TACE), or other local ablative techniques, have also been applied extensively. The mechanisms of these interventions mainly induce thermal coagulation, rapid freezing, cell dehydration, and killing cells by chemotherapies with different postablative effects, of which little is known to most doctors [Citation5–8].
According to the latest studies and the National Comprehensive Cancer Network (NCCN) guidelines, the treatment strategies containing hepatic resection, TACE, RFA and PEI were effective and recommended for the early-stage HCC [Citation9,Citation10]. However, the problem surrounding the determination of which is the best choice is always conflicting and controversial. This has been especially true in recent years, during which time several molecular studies have further indicated that HCC has the characteristics of easy for recurrence and a resistance to chemotherapy [Citation11,Citation12]. Therefore, additional investigation into the best the treatment therapeutics for early-stage HCC is needed. Although several meta-analyses have been conducted to determine the best choice for early-stage HCC, some were not up-to-date [Citation8] and may not be comprehensive [Citation13,Citation14]. In this meta-analysis, we searched the main database and used R software to compare the efficacy of different interventional techniques on the outcomes of local tumor progression (LTP), total recurrence, overall survival, disease-free survival and LTP-free survival.
Methods
Data sources and searching strategies
An electronic literature searching was conducted to identify candidate articles published through June 2018. This search was based on the published articles in Pubmed (MEDLINE), EMBASE, Cochrane Library and unpublished data from the clinical trials website (www.clinicaltrials.gov). Medical subject heading (MESH) terms and other terms were used for literature searching using different combinations. The terms used included ‘carcinoma, hepatocellular’, ‘hepatocellular carcinoma’, ‘liver cell carcinoma’, ‘liver cancer’, ‘ablation techniques’, ‘microwave ablation’, ‘radiofrequency ablation’, ‘percutaneous ethanol injection’, ‘cryosurgery’, ‘cryoablation’, ‘laser ablation’, ‘laser therapy’, ‘external beam radiotherapy’, ‘high-intensity focussed ultrasound ablation’, ‘transcatheter arterial chemoembolization’ and ‘early stage’. The search was limited to randomised clinical studies in humans, but there were no language restrictions. Two authors (Y.Q. Lin and Q. Wen) conducted the literature searching independently and a third investigator (Z.X. Sun) resolved any discrepancies. The final searching strategy was reached and conducted.
Eligibility criteria
Clinical trials were included in this meta-analysis if they met the following criteria: (i) Participants: adults older than 18 and diagnosed with small HCC or early-stage HCC (single HCC, ≤5 cm; or up to 3 nodules, ≤3 cm); HCC patients with BCLC stage 0-A; or patients within the Milan Criteria, with hepatic function Child-Pugh-Turcotte classification A or B and without vascular invasion, extrahepatic metastasis and history of treatment; (ii) Intervention: the treatment strategies of the studies contained at least two of the following intervention techniques: RFA, microwave ablation, hepatic resection, PEI, laser ablation, cryoablation, external beam radiotherapy, TACE and different combination of these interventions; (iii) Comparisons: studies that compared the outcomes of different intervention techniques on early-stage HCC; (iv) Outcomes: LTP, total recurrence rates, overall survival rates and complications with a follow-up period of the included studies longer than 1 year and (v) Study design: only RCTs were included for further quality assessment and data synthesis.
The studies were excluded if: (i) they were published in the forms of case reports, reviews, editorials, conference abstracts, observational clinical studies, case-control studies, or retrospective or prospective cohort studies; (ii) they were not written in English or Chinese; (iii) they focused on large HCC or small HCC with vascular invasion, recurrence or extrahepatic metastasis of Child-Pugh classification as C grade; (iv) there was a lack of the important outcomes in the clinical trials or (v) it include duplicate data because a recent study had been included.
Data extraction and quality assessment
After ascertaining the RCTs enrolled in this study, data were extracted by two of the researchers. The data extracted mainly contained (i) Study information: first author, publication date, journal, study size and design; (ii) Patient-related information: HCC stage, tumor stage, basic characteristics, intervention, follow-up, etc.; and (iii) Outcome information: LTP, total recurrence rate, overall survival and complications. The quality assessment was conducted using Cochrane Collaboration’s tool according to the Cochrane Handbook.
Outcome measures and statistical analysis
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation15]. The main outcomes in this meta-analysis were LTP, total tumor recurrence and overall survival. LTP is defined as the appearance of tumor foci at the edge of the ablation zone after at least one contrast-enhanced follow-up study has documented adequate ablation and an absence of viable tissue in the target tumor and surrounding ablation margin by using imaging criteria [Citation16]. The definition of total recurrence rate was the incidence of all types of tumor recurrence including local, intrahepatic and extrahepatic regions during the follow-up period [Citation17]. Survival time was defined as the interval between the first treatment and either death or the last follow-up visit [Citation17]. For the network meta-analysis, R-3.3.4 software (R Foundation for Statistical Computing, Vienna, Austria) was used to perform a Bayesian network meta-analysis was performed using the ‘gemtc’ and ‘rjags’ packages of R-3.3.4 software with the random effect [Citation18]. The consistency of network meta-analysis was assessed using the node-splitting models to detect whether the results of direct and indirect comparison agreed within treatment loops [Citation19], and the risk ratio (RR) values and 95% credible intervals (CrIs) were used to show the results of indirect comparisons. For the network analysis of survival data, hazard ratios (HR) were pooled for synthesis and to compare the effects of different interventions on the overall survival, disease-free survival and LTP-free survival rates. For studies that did not provided the HR values, Engauge Digitizer (version 4.1, M Mitchell, http://markummitchell.github.io/engauge-digitiser/) software was used to extract data from the Kaplan–Meier plot.
Results
Study selection
After the literature search, a total of 1202 studies were identified. After removal of the duplicates and those that did not meet the inclusion criteria, a total of 36 randomized clinical trials were enrolled for data analysis and further synthesis. Those contained 11 treatment arms, namely, RFA, MWA, hepatic resection, PEI, laser ablation, cryoablation, RFA plus radiotherapy, RFA plus PEI, RFA plus TACE, RFA plus sorafenib and PEI plus TACE (). Among them, five studies compared the efficacy of RFA with MWA [Citation20–24], seven studies compared the efficacy of RFA with hepatic resection [Citation25–31], six RCTs compared the effects of RFA with PEI [Citation32–37], three RCTs compared the effects of RFA versus laser ablation [Citation7,Citation38,Citation39], only one study compared the effects of RFA versus cryoablation [Citation6], two studies compared the effects of combined therapy of RFA plus 131I radiotherapy versus RFA treatment alone [Citation17,Citation40], three studies compared the effects of RFA with RFA plus PEI [Citation32,Citation41,Citation42] and four RCTs were enrolled that compared the effects of RFA combined with TACE to RFA alone [Citation43–46]. Meanwhile, Huang et al. compared the efficacy between hepatic resection and PEI [Citation47], one study compared the effect of hepatic resection with RFA plus sorafenib [Citation48] and one study compared hepatic resection with RFA plus TACE [Citation49]. Furthermore, two studies compared the efficacy of PEI plus TACE versus PEI alone [Citation50,Citation51] (). Among them, the sample sizes ranged from 20 to 403, with the follow-up duration ranging from 1 year to 10 years. All the studies concentrated on early stage or small HCC (). For quality assessment, the Cochrane Collaboration Tool was used according to the guidance of the Cochrane Handbook. As a whole, the randomization and the follow-up procedures were conducted well in most of the enrolled studies; however, due to the specificity of hepatic surgeries and interventional treatment, it is quite difficult to maintain double-blindness. In several studies with better double-blindness [Citation23,Citation39,Citation40], the intervention techniques were very similar and were both percutaneous. For studies that compared the efficacy of RFA and HR, double-blindness seemed quite impossible. As a result, for these comparisons, blindness was only available for the statisticians ().
Figure 1. PRISMA-NMA diagram of the literature search evaluating interventional RCTs for early-stage HCC through a selection process. Studies were identified from PubMed, Embase, Cochrane Library and Clinicaltrial.gov databases. There were 1202 references identified from the databases and a total of 35 RCTs were included in this study.
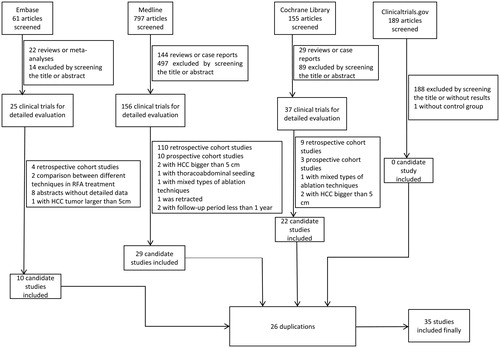
Figure 2. Network plot of enrolled studies. The number labeled beside each line is the number of studies comparing these two intervention therapies.
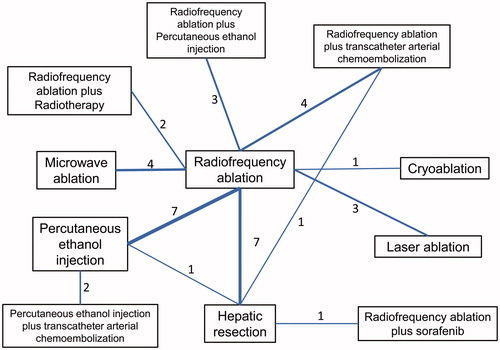
Figure 3. Quality assessment of included studies using Cochrane Collaboration’s tool. (A) Overall and (B) study-level risk of bias were assessed in these seven aspects.
Table 1. Baseline characteristics of included studies.
Network meta-analysis
The network meta-analyses of interventional techniques for early-stage HCC using R-software were presented in –Citation6. The main results are listed as follows.
Figure 4. Network meta-analysis for analysing the rate of different interventional treatments on LTP at 1, 2, 3 and longer than 3 years. (A) Network plots of the included studies and the connections among them. The four panels indicate the subgroup analysis of LTP at 1, 2, 3 and longer than 3 years. Abbreviations: RFA: radiofrequency ablation; MWA: microwave ablation; PEI: percutaneous ethanol injection; TACE: transcatheter arterial chemoembolization. (B) The relative forest plots of different interventional arms on LTP compared with RFA, using risk ratio (RR) values and 95% credible intervals (CrIs). (C) The results of treatment ranks using R-software. The abbreviations were the same as in (A). The dark bars indicate the high probability of being the best interventional arms, whereas the light bars suggest being the worst arms in this outcome.
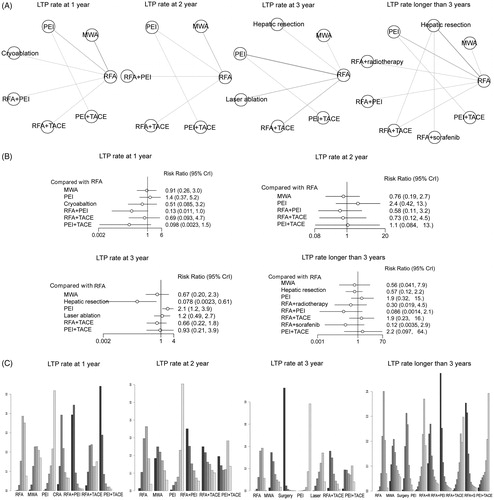
LTP rate
LTP represents the appearance of tumor foci at the edge of the ablation zone. Most of the included studies reported the results of LTP rates. In this meta-analysis, the LTP rates of 1, 2, 3, and longer than 3 years were calculated, respectively, and displayed in . As to LTP rate at 1 year, nine studies involving seven intervention arms were included for the network meta-analysis (). The results showed that local ablative techniques (MWA, PEI) and RFA plus TACE had similar LTP rates when treating early-stage HCC, whereas other RFA plus adjuvant therapies (RFA plus PEI and PEI plus TACE) were superior in decreasing the LTP incidence, exhibiting the best performance compared with most of the other treatments in this subgroup (). Furthermore, using rank probabilities tests, it showed that PEI plus TACE and RFA plus PEI ranked the first and second best treatment arms on the parameter LTP at 1 year (). For LTP at 2 years, a total of six studies involving six intervention arms were enrolled (). It showed that the intervention techniques had similar performance in the outcome of LTP rate at 2 years (). The rank probability test indicated that RFA plus adjuvant therapies (PEI, TACE) and PEI plus TACE showed better efficacy (). Due to the limited number of studies enrolled, the LTP at 2 years conferred limited information. Meanwhile, for the LTP rate at 3 years, 11 studies containing seven treatment arms were enrolled (). The pooled RR estimates showed that the intervention of hepatic resection was superior (RR 0.078, 95% CrI 0.0023, 0.61) and PEI was inferior (RR 2.1, 95% CrI 1.2, 3.9), compared with RFA intervention (). However, local ablative techniques (MWA, laser ablation) and RFA or PEI plus adjuvant therapies (TACE) had almost the same efficacy (). Using the rank probability test, it showed that hepatic resection had the highest probability of being the best treatment arm ().
Studies reporting the LTP rate longer than 3 years mainly contained the data of LTP at 5 years; a total of 12 studies including 10 treatment arms were enrolled for data synthesis (). The results showed that RFA plus adjuvant therapies (radiotherapy, PEI and sorafenib) had a lower LTP rate compared with RFA alone, whereas other interventions showed similar efficacy in decreasing the LTP (). The rank probability results showed that RFA plus adjuvant therapies are the best treatment arms, with RFA plus PEI being the best, followed by RFA plus sorafenib and RFA plus radiotherapy (). Therefore, the results of LTP showed that RFA plus adjuvant therapy and hepatic resection had the highest probability to be the best treatment arms. In detail, for studies with less than 3 years of follow-up data, the intervention of hepatic resection may decrease the LTP incidence most efficiently, while RFA plus adjuvant therapies may become the most effective measures for longer than 3 years.
Total recurrence
Concerning the outcome synthesis of total recurrence rate, the comparison was also divided into the 1-, 2-, 3-, and longer-than-3 year subgroups for further analysis. For the outcome of the total recurrence rate at 1 year, seven studies containing five intervention arms were enrolled for data synthesis (). It showed that the interventions of hepatic resection and RFA plus adjuvant therapies (RFA plus radiotherapy and RFA plus TACE) had decreased risk of total recurrence rate at 1 year (). The rank probability test results were consistent with the forest plot, showing that RFA plus adjuvant therapies (radiotherapy and TACE) also had the highest probability to be the best treatment arm, followed by hepatic resection ().
Figure 5. Network meta-analysis for analysing the rate of different interventional treatments on total recurrence at 1, 2, 3 and longer than 3 years. (A) Network plots of the included studies and the connections among them. The four panels indicate the subgroup analysis of total recurrence at 1, 2, 3 and longer than 3 years. Abbreviations: MWA: microwave ablation; PEI: percutaneous ethanol injection; RFA: radiofrequency ablation; TACE: transcatheter arterial chemoembolization. (B) The relative forest plots of different interventional arms on total recurrence rates compared with RFA, using risk ratio (RR) values and 95% credible intervals (CrIs). (C) The results of treatment ranks using R-software. The abbreviations were the same in (A).
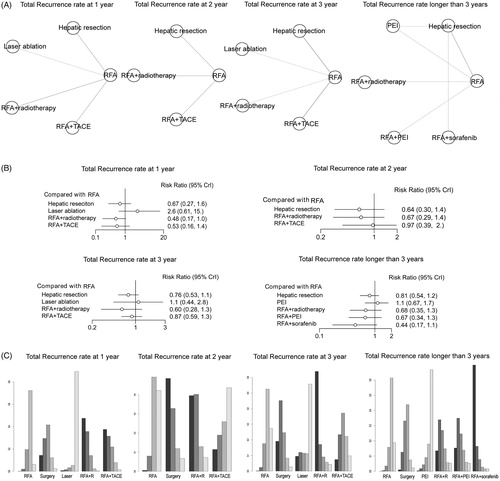
For the subgroup analysis of the total recurrence rate at 2 years, six studies involving four treatment arms were enrolled for network meta-analysis (). It showed that the treatments of hepatic resection and RFA plus radiotherapy had a lower risk of total recurrence at 2 years (). Using a rank probability test, the results were consistent with the forest plot, suggesting that hepatic resection and RFA plus radiotherapy had the high probability of being the best treatment arms ().
For the subgroup analysis of total recurrence rate at 3 years, eight studies containing 5 treatment arms were included (). The relative forest plot showed that the treatments of hepatic resection and RFA plus radiotherapy decreased the total recurrence rate compared with other treatment arms (). The rank probability test showed that RFA plus radiotherapy had the highest probability of becoming the best treatment arm, followed by hepatic resection ().
For the subgroup analysis of total recurrence longer than 3 years, seven studies containing six treatment arms were included (). The network meta-analysis showed that the treatments of hepatic resection and RFA plus adjuvant therapies had decreased total recurrence compared with RFA (). The rank probability test also showed that RFA plus sorafenib, RFA plus PEI and RFA plus radiotherapy had the highest probability of being the best arms (). In this outcome analysis, it still suggested that the treatment of hepatic resection was the most effective for decreasing total recurrence at 1 or 2 years, whereas RFA plus adjuvant therapies help to decrease total recurrence for 3 or more than 3 years.
Overall survival rates
For the analysis of survival rates after different interventions, the HR and its corresponding 95% CtrI values were calculated. The outcome of survival analysis was divided into the subgroup analysis of overall survival (OS), disease-free survival and LTP-free survival. For the data synthesis of OS, a total of 24 studies containing 11 specific interventional arms were included (). It showed that RFA plus adjuvant therapies (radiotherapy, TACE and sorafenib) and hepatic resection were the best interventions to achieve the better OS rates when compared with local ablative techniques. Among the local ablative techniques, MWA and cryoablation had better efficacy, whereas PEI and laser ablation were inferior compared with RFA in OS comparisons (). In addition, the rank probability test showed that RFA plus adjuvant therapies (radiotherapy, sorafenib and TACE) ranked the best treatment arm in achieving the highest OS rate ().
Figure 6. Network meta-analysis for analysing the rate of different interventional treatments on overall, disease-free and LTP-free survival rates. (A) Network plots of the included studies and the connections among them in the comparison of survival rates. The three panels indicate the analyses of overall survival rates at 1, 2, 3 and longer than 3 years. Abbreviations: MWA: microwave ablation; PEI: percutaneous ethanol injection; RFA: radiofrequency ablation; TACE: transcatheter arterial chemoembolization. (B) The relative forest plots of different interventional arms on overall survival, disease-free survival and LTP-free survival rates compared with RFA, using HR values and 95% credible intervals (CrIs). (C) The results of treatment ranks using R-software. The abbreviations were the same in (A).
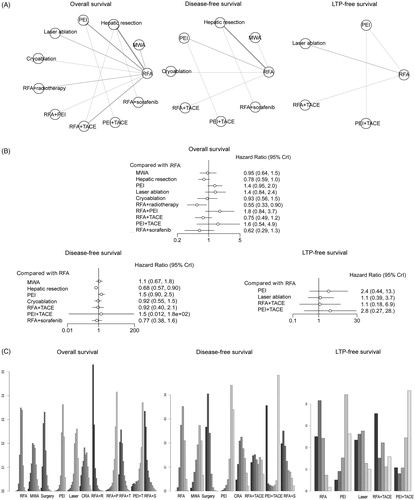
For the analysis of disease-free survival, a total of 13 studies containing 8 specific interventional arms were enrolled (). The forest plot in exhibited similar efficacies in achieving better disease-free survival, with hepatic resection being the best among all the interventional arms (RR 0.68, 95% CrI 0.57, 0.90, compared with RFA). Using the rank probability test, it showed that hepatic resection ranked the best in the comparison of disease-free survival ().
For the analysis of LTP-free survival, five studies containing five specific treatment arms were enrolled, shown in . The forest plot indicated that the efficacy of local ablative techniques (RFA and laser ablation) and RFA plus TACE were better than PEI (). In addition, the rank probability test suggested that RFA plus adjuvant therapy (TACE) ranked the first and it is the best intervention arm for in achieving better LTP-free survival ().
Discussion
To date, several comparisons of different intervention techniques on HCC treatment have been published; however, the network meta-analyses on this topic are still very rare [Citation8,Citation13]. This study collected the latest data and compared the most commonly used interventional techniques for early-stage HCC, which might be the most comprehensive study currently. In this network meta-analysis, we summarized the main interventional techniques for early-stage HCC and compared the rates of LTP and total recurrence, the overall, disease-free and LTP-free survival of different treatment arms on these patients. It was demonstrated that RFA plus adjuvant therapies achieved the best performance in decreasing LTP in early-stage HCC, while for LTP at 3 years, hepatic resection ranked the best of being the best arm among all the interventional techniques, with RR 0.079 and 95% CrI (0.0023, 0.61) when compared to RFA alone. Meanwhile, for the outcome of total recurrence, the results showed that hepatic resection and RFA plus adjuvant therapies were also the two best arms in decreasing total recurrence, with hepatic resection being the best when less than 3 years, while RFA plus adjuvant therapies ranked the best if the follow-up period was longer than 3 years. Furthermore, the survival data was also synthesised, demonstrating that RFA plus adjuvant therapeutics rank the best in achieving the best OS rate, followed by hepatic resection and local ablative techniques. For disease-free survival, hepatic resection was the best while for LTP-free survival, the difference between these treatments was not significant.
Recently, as a result of technological development, the choice for the best treatment strategy became quite difficult, and it mainly depends on the doctor’s experience and the patients’ consent. Hepatic resection is the always the first-line choice in early-stage HCC treatment, but it always brings about a high morbidity of complications, such as infection, bleeding, hepatic failure, etc. Meanwhile, local ablative techniques are also widely used in the treatment of early-stage HCC. Among them, RFA is a kind of commonly used medical procedure that achieves tumor ablation through high-frequency alternation current and has now become the most commonly used local ablation therapeutic. Several studies indicated that RFA was better than PEI [Citation52,Citation53] but was inferior to hepatic resection for early-stage or small HCC [Citation54]. Recent advancement also brings about new techniques such as microwave ablation, laser ablation and cryoablation, which destruct the tumor through multiple mechanisms [Citation14], with the new classification of chemical ablation and energy-based ablation. The effects of different treatments on tumor recurrence and survival are still not clear. For microwave ablation, recent studies have concentrated on the efficacy of microwave ablation compared with RFA and hepatic resection. A recent meta-analysis showed that microwave ablation may be superior to hepatic resection as it is as effective as hepatic resection in terms of overall survival, disease-free survival, tumor recurrence and is associated with fewer complications [Citation55], while microwave ablation is better than RFA in preventing LTP in treating large tumors [Citation5,Citation56]. In our network meta-analysis, we demonstrated that microwave ablation also performed well in regard to LTP. The treatment of microwave ablation had similar efficacy in achieving better survival rates, but limited data was available as to the role of microwave ablation in the outcome of total recurrence. Therefore, more importance should be attached to microwave ablation and more RCTs are needs to compare the efficacy of microwave ablation with other interventions. Retrospective studies have demonstrated that cryoablation had similar treatment successes and complication rates but had increased local recurrence rate compared with RFA [Citation57,Citation58]. However, Li et al. have demonstrated that cryoablation was as effective as hepatic resection in the treatment of solitary, small HCC with a short duration of hospitalisation and fewer complications [Citation59]. In our study, the treatment of cryoablation played a better role in the outcome of LTP at 1 year compared with microwave ablation and PEI and with similar OS and disease-free survival compared with RFA. Furthermore, the treatment of laser ablation has also been studied in recent years with almost similar efficacy compared to RFA, but with fewer complications [Citation7,Citation38,Citation39]. However, the number of studies concerning laser ablation is not very large. It has been shown in our study that laser ablation had inferior total recurrence and overall survival rates compared to RFA, but even so, more RCTs concerning the new techniques such as cryoablation, laser ablation and irreversible electroporation should be conducted. In our network meta-analysis, we showed that hepatic resection is better in all the aspects compared to local ablative techniques such as RFA, PEI, and microwave ablation, indicating that hepatic resection will achieve the most complete efficacy when treating early-stage HCC but with more complications and a longer recovery time.
Furthermore, TACE, which concentrates chemotherapeutic agents at the tumor site while blocking the primary artery feeding the tumor, was considered to be the most frequent loco-regional treatment for unresectable HCC [Citation3], whereas it is reported that TACE is starting to be used in early-stage HCC and shows a prolonged survival rate. Therefore, combination therapeutics become more and more popular in HCC treatment. In addition to TACE as the combination therapy, adjuvant therapies also develop very quickly with the classification of concomitant agents, combination therapies, and concurrent therapies. A previous meta-analysis also showed that RFA-based combination treatment has the highest probability in achieving the best OS rate [Citation8]. In our network meta-analysis, we showed that the treatments of RFA plus adjuvant therapeutics achieved the best efficacy in almost all the aspects of the outcomes. Therefore, combination therapy is strongly recommended for early-stage HCC.
The methodological quality in this meta-analysis shows that several studies included did not perform well in the procedure of randomization and blindness. It is because in some comparisons, the two treatment arms were totally different, such as RFA versus HR. Therefore, these studies had a high risk of bringing bias. Meanwhile, there were some heterogeneity for some of the outcomes in conventional meta-analysis. For example, the participants in the studies enrolled may have different viral infections, while some were cirrhotic and some were noncirrhotic. Therefore, when interpreting the results of this network meta-analysis, these factors should be taken into consideration.
There were some limitations. First, network meta-analysis has the advantage of theoretically being able to compare technologies/interventions that were not actually compared in any trial. There are many unknowns about this methodology and the results need careful interpretations. Second, we only enrolled the RCTs in this meta-analysis and some high-quality cohort studies were still excluded because this may bring great heterogeneity when synthesising data. Third, we did not detail the composition of chemotherapy because different compositions of chemotherapy were found in the limited number of studies included. Fourth, we did not synthesise the data of complications because different complications were reported, and some were rather subjective.
In conclusion, by using network meta-analysis, it was shown that RFA plus adjuvant therapeutics and hepatic resection achieved the best results in treating early-stage HCC, by decreasing the LTP and the total recurrence rate and by improving the OS, disease-free, and LTP-free survival rates. Nevertheless, more high-quality RCTs are needed to provide more solid evidence.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
- Wallace MC, Preen D, Jeffrey GP, et al. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765–779.
- Bruix J, Sherman M, American Association For The Study Of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022.
- Poon D, Anderson BO, Chen LT, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118.
- Chinnaratha MA, Chuang MY, Fraser RJ, et al. Percutaneous thermal ablation for primary hepatocellular carcinoma: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294–301.
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590.
- Orlacchio A, Bolacchi F, Chegai F, et al. Comparative evaluation of percutaneous laser and radiofrequency ablation in patients with HCC smaller than 4 cm. Radiol Med. 2014;119:298–308.
- Lan T, Chang L, Rahmathullah MN, et al. Comparative efficacy of interventional therapies for early-stage hepatocellular carcinoma: A PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore). 2016;95:e3185.
- Zhang YY, Xia HH. Novel therapeutic approaches for hepatocellulcar carcinoma: fact and fiction. WJG. 2008;14:1641–1642.
- Ramanathan R, Sharma A, Lee DD, et al. Multimodality therapy and liver transplantation for hepatocellular carcinoma: a 14-year prospective analysis of outcomes. Transplantation. 2014;98:100–106.
- Zheng YW, Nie YZ, Taniguchi H. Cellular reprogramming and hepatocellular carcinoma development. WJG. 2013;19:8850–8860.
- Yagci T, Cetin M, Ercin PB. Cancer stem cells in hepatocellular carcinoma. J Gastrointest Cancer. 2017;48: 241–245.
- Majumdar A, Roccarina D, Thorburn D, et al. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;(3):CD011650.
- Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Onc. 2017;15:126.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [Guideline Research Support, Non-U.S. Gov't]. BMJ. 2009;339:b2535.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria a 10-year update. J Vasc Interv Radiol. 2014;25:1706–1705 e4.
- Bian H, Zheng JS, Nan G, et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J Natl Cancer Inst. 2014;106:dju239.
- Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Statist Med. 2002;21:1601–1623.
- van Valkenhoef G, Dias S, Ades AE, et al. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7:80–93.
- Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014;28:3429–3434.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22:1983–1990.
- Tian WS, Kuang M, Lyu M, et al. A randomized comparative trial on liver tumors treated with ultrasound guided percutaneous radiofrequency versus microwave ablation. Chin J Hepatobiliary Surg. 2014;20:119–122.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337.
- Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66:1172–1173.
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328.
- Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:193–200.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802.
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912.
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775–1784.
- Lee HW, Lee JM, Yoon JH, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018;94:74–82.
- Lu MD, Kuang M, Liang LJ, et al. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86:801–805.
- Azab M, Zaki S, El-Shetey AG, et al. Radiofrequency ablation combined with percutaneous ethanol injection in patients with hepatocellular carcinoma. Arab J Gastroenterol. 2011;12:113–118.
- Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735.
- Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res. 2011;31:2291–2295.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240.
- Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156.
- Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130.
- Di Costanzo GG, Tortora R, D'Adamo G, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J Gastroenterol Hepatol. 2015;30:559–565.
- Ferrari FS, Megliola A, Scorzelli A, et al. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med. 2007;112:377–393.
- Chen K, Chen G, Wang H, et al. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J Hepatol. 2014;61:1304–1311.
- Zhang YJ, Liang HH, Chen MS, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology. 2007;244:599–607.
- Chen MS, Zhang YJ, Li JQ, et al. Randomized clinical trial of percutaneous radiofrequency ablation plus absolute ethanol injection compared with radiofrequency ablation alone for small hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 2005;27:623–625.
- Kobayashi M, Ikeda K, Kawamura Y, et al. Randomized controlled trial for the efficacy of hepatic arterial occlusion during radiofrequency ablation for small hepatocellular carcinoma–direct ablative effects and a long-term outcome. Liver Int. 2007;27:353–359.
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432.
- Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913.
- Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460.
- Huang GT, Lee PH, Tsang YM, et al. Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Ann Surg. 2005;242:36–42.
- Yan SY, Zhang Y, Sun C, et al. The clinical effect and relevant mechanism of combined sorafenib and radiofrequency ablation in the treatment of early small hepatocellular carcinoma. Oncol Lett. 2016;12:951–955.
- Liu H, Wang ZG, Fu SY, et al. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg. 2016;103:348–356.
- Koda M, Murawaki Y, Mitsuda A, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer. 2001;92:1516–1524.
- Mizuki A, Tatemichi M, Tsukada N, et al. Addition of transcatheter arterial chemoembolization decreased local recurrence but had no survival benefit to percutaneous ethanol injection therapy for patients with small hepatocellular carcinoma: a multicenter randomized control study. Oncol Lett. 2010;1:855–859.
- Shen A, Zhang H, Tang C, et al. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol. 2013;28:793–800.
- Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380–388.
- Xu XL, Liu XD, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2017;287:461–472.
- Zhang M, Ma H, Zhang J, et al. Comparison of microwave ablation and hepatic resection for hepatocellular carcinoma: a meta-analysis. OTT. 2017;10:4829–4839.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344.
- Adam R, Hagopian EJ, Linhares M, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339.
- Dunne RM, Shyn PB, Sung JC, et al. Percutaneous treatment of hepatocellular carcinoma in patients with cirrhosis: a comparison of the safety of cryoablation and radiofrequency ablation. Eur J Radiol. 2014;83:632–638.
- Li Z, Zhang C, Lou C, et al. Comparison of percutaneous cryosurgery and surgical resection for the treatment of small hepatocellular carcinoma. Oncol Lett. 2013;6:239–245.
