Abstract
Purpose: To retrospectively evaluate the suitability of MRgHIFU for osteoid osteomas (OOs) and bone metastases in patients who underwent minimally-invasive percutaneous thermal ablation.
Materials and methods: One hundred and sixty-seven lesions (115 metastases and 52 OOs) treated percutaneously between October 2014 and June 2017 were retrospectively analyzed. Tumors were located in the spine or sacrum (54), pelvis (43), limbs (50), ribs (17) and sternum (3). Tumor volume, matrix, anatomical environment and need for protection of surrounding structures or consolidation were assessed. Cases were classified into three categories: (a) lesions suitable for MRgHIFU therapy alone; (b) lesions suitable for MRgHIFU if protection of surrounding structures and/or bone consolidation is performed; (c) lesions not suitable for MRgHIFU.
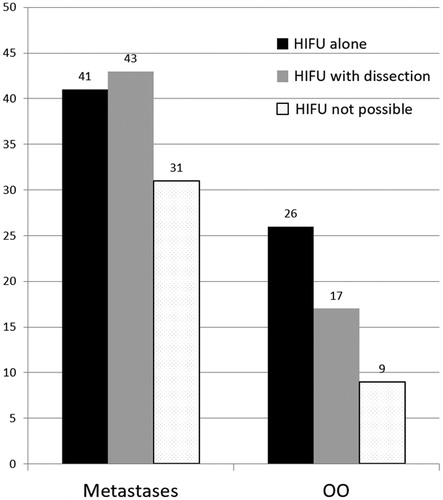
Results: Twenty-six (50%) of OOs were classified as suitable for MRgHIFU alone and 17 (32.7%) as suitable for MRgHIFU with hydro-dissection. Matrix of treatable OOs was sclerotic (19), lytic (15) or mixed (9), with mean volume 0.56 cm3. Forty-one (35.7%) of metastases were classified as suitable for MRgHIFU alone and 43 (37.4%) as suitable with hydro-dissection and/or consolidation. Matrix of metastases was sclerotic (13), lytic (37) or mixed (34), with mean volume 71.9 cm3. Mean depth of targetable lesions was 50.9 ± 28.4 mm. 97.7% of pelvic lesions and 94% of peripheral bone lesions were targetable by HIFU. 66.6% of spinal or sacral lesions were considered untreatable.
Conclusion: MRgHIFU cannot be systematically performed non-invasively on bone tumors. Combination with minimally-invasive thermo-protective techniques may increase the number of eligible cases.
Introduction
The most frequent cancer-related pain is due to metastatic bone lesions [Citation1]. The origin of metastatic bone pain, which greatly compromises quality of life, is multifactorial and includes stimulation of nociceptive intraosseous and periosteal nerves by inflammation, raised intraosseous pressure due to tumor mass effect, and pathological fractures [Citation2]. Pain management is multidisciplinary and effective treatment may be achieved using external beam radiotherapy (EBRT), analgesics, bisphosphonates, chemotherapy and minimally-invasive percutaneous ablative therapy [Citation3]. Osteoid osteomas (OOs) are benign bone lesions, constituted by a nidus which may be surrounded by reactive sclerosis. OOs predominantly affect young people, with 85% of patients aged between 5 and 24 years [Citation4]. The pain is thought to be related to inflammation resulting from prostaglandin release, and stimulation of both unmyelinated nerve fibers surrounding the nidus and axonal fibers crossing the nidus [Citation5–7]. Pain is typically nocturnal and well controlled with NSAIDS.
Several percutaneous image-guided methods are currently used in clinical practice to treat painful bone lesions effectively and durably, with palliative intent for metastases and curative intent for OOs [Citation8]. Percutaneous image-guided thermoablation is the treatment of choice to destroy the nidus in OOs, with excellent clinical results [Citation9–11], even in spinal locations where thermal protection may be necessary to avoid damage to adjacent neurological structures [Citation12].
Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) is a non-invasive thermal ablation technique currently used in various oncological lesions [Citation13]. Several clinical studies have reported the efficacy and safety of HIFU for the treatment of painful bone metastases [Citation14–23] and OOs [Citation24–30]. The efficacy of HIFU in treating unresectable locally recurrent osteosarcoma has also been reported [Citation31]. One major advantage of MRgHIFU is the possibility of treating lesions non-invasively. However, as a method of thermal ablation, the same precautions as for minimally-invasive thermo-ablative techniques may be required: namely, protection of surrounding structures and consolidation to avoid impending fractures of osteolytic metastases.
The objective of this study is to evaluate retrospectively whether MRgHIFU would have been suitable for treating osteoid osteomas and bone metastases in patients who underwent minimally-invasive percutaneous thermal ablation in our Department of Interventional Radiology. The need for adjunctive thermo-protection and bone consolidation to avoid pathological/post-ablation fracture is also considered. It must be noted that HIFU and minimally-invasive ablation heat bone tissue differently, with the heating-zone located on the periosteum for HIFU and at the center of the lesion for percutaneous ablation; heat distribution further depends on differences in thermal conductivity of the surrounding bone [Citation32–34]. However, to obtain equivalent therapeutic effects, the global thermal dose used with MRgHIFU should be similar to that applied during minimally-invasive ablation. This study therefore hypothesizes that the need to protect surrounding structures is similar for MRgHIFU and minimally-invasive ablations.
Materials and methods
All cases of bone lesions treated percutaneously between October 1, 2014 and June 1, 2017 in the University Hospital Department of Interventional Radiology (25 years’ experience in minimally-invasive bone lesion ablation) were included in the study and retrospectively analyzed by the main author. Local IRB approval was obtained to use medical data of the patients treated in our Unit, and all patients consented to the use of their images. Patient anonymity was preserved and the principles of the Declaration of Helsinki were followed.
In our Department, all OOs (generally referred by rheumatologists) are percutaneously treated. Patients with bone metastases are referred by oncologists for three major indications:
Palliative treatment of one painful metastasis refractory to analgesic medication or EBRT (radiosensitive lesions only): cementoplasty is proposed.
Potentially curative treatment of a solitary metastasis or oligometaststic disease (painful or painless): ablation with curative intent is planned.
Osteolytic metastasis at risk of pathological fracture: ablation is performed with either curative or palliative (cytoreductive) intent, and the lesion is consolidated via cementoplasty.
Patients who received EBRT but were not referred to our department may have been too frail to undergo minimally-invasive ablation, or presented with too many lesions to target for local therapy. Patients with spinal lesions and neurological impairment or instability were referred directly to the department of surgery for decompression with or without stabilization. These patients were ineligible for minimally-invasive ablation, and would also be ineligible for HIFU treatment for the same reasons.
One hundred and seventy-one bone lesions (158 patients) were treated using minimally-invasive percutaneous thermal ablation procedures. Only osteoid osteomas (n = 52) and bone metastases (n = 115) were included in this study: four patients with chondroblastoma (1), osteoblastoma (1), fibrous solitary tumor (1) and non-specific lesion without tumoral cells (1) were excluded. A total of 167 lesions (154 patients) were therefore analyzed. Tumors were located in the spine or sacrum (54), pelvis (43), limbs (50), ribs (17) and sternum (3). The type, matrix, volume, location and anatomic environment of lesions; the need for thermo-protection of surrounding structures (nerves, joints); the need for osseous consolidation and the presence of any physical obstruction to the ultrasound beam (e.g. bone, air) were evaluated.
Assessing whether MRgHIFU would have been therapeutically effective for the study population is not possible. However, it is possible to evaluate retrospectively whether MRgHIFU would have been a suitable therapy, based on location and anatomical environment of the percutaneously treated lesions. This may have significant implications for treatment planning. The feasibility of HIFU depends on the acoustic properties of the transducer (central frequency, aperture, focal depth). A transducer of 10 cm in aperture and 10 cm in focal depth was considered to illustrate the ultrasonic beam path and transducer location relative to the treatment-zone (). Due to technical challenges related to deep-lying lesions [Citation35], lesions with depth greater than 12 cm were considered non-targetable.
Figure 1. (a) Sagittal CT image of an OO located in the neck of femur (circle). The nidus is sclerotic. There is no need to protect any surrounding structures during ablation (group (a)). L corresponds to the diameter of the transducer (L/2 = 50 mm) and d to the distance between the focal point and the skin (d = 61 mm). The plane perpendicular to d and containing AB is shown in the bottom right of figure (a): in this case, 12% of the US beam would be obstructed by the great trochanter. (b) Axial CT image illustrating laser ablation of the OO. A 14-gauge penetration set was used to drill the nidus. The laser fiber was inserted coaxially in an 18-gauge spinal needle (arrow).
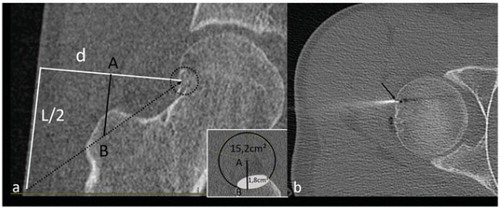
Figure 2. (a) CT-scan showing a mixed metastasis of the scapula (group (a)). L (100 mm) corresponds to the diameter of the transducer and d to the distance between the focal point and the skin (d = 18 mm). (b, c) Sagittal (b) and coronal (c) CT reconstructions showing placement of two cryoprobes used to freeze the lesion. The hypoattenuation surrounding the lesion corresponds to the ice-ball.
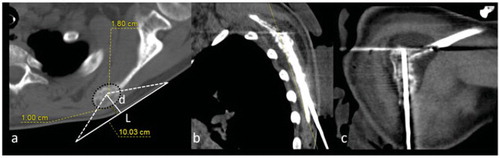
Figure 3. (a) CT-scan of an OO located in the right articular process of L5 (group (b)). The nidus is sclerotic. This case requires protection of the nerve root in the foramen (F for foraminal dissection) and the lumbar canal (E for epidural dissection). As the aim is to heat the nidus to 60 °C, foraminal and medullary canal temperatures may increase up to 45 °C, which may injure the nerve roots. The trajectory of the spinal needles for dissection are represented by dotted arrows. L corresponds to the diameter of the transducer (100 mm) and d to the distance between the focal point and skin (d = 66 mm). (b, c) CT-scan showing insertion of needles for ablation of the OO. A cryoprobe inserted coaxially in a 15-gauge needle is used to access the nidus (b, arrow) and 18-gauge needles are placed for epidural (b) and foraminal (c) dissections (dotted arrows).
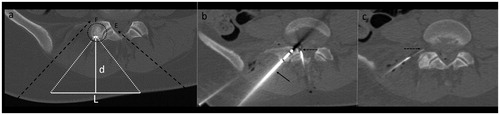
Figure 4. (a) CT-scan of a sclerotic metastasis (group (b)) of the ilium (dotted circle). L corresponds to the diameter of the transducer (100 mm) and d the distance between the focal point and the skin (d = 100 mm). (b, c) CT-scan showing cryoablation of the lesion performed with a 17-gauge cryoprobe inserted coaxially in a 14-gauge penetration set (b, arrow), associated with dissection (b, dotted arrow) of the sciatic nerve (b, star) and coxofemoral articulation (c, dotted arrow). Dissection fluid contains iodinated contrast (+) to optimize CT visibility.
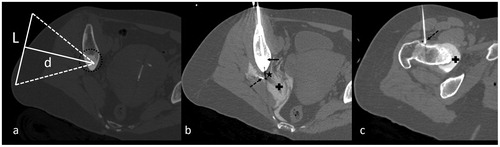
Figure 5. (a) Axial CT image showing an 8-mm OO in the left pedicle of C4. The vertical white line (L = 100 mm) represents optimal transducer placement. The white line (d = 30 mm) corresponds to the depth of the OO. In this case, cooling of the C5 foraminal space is mandatory but impossible due to transducer bulk. This case was considered ineligible for HIFU (group (c)). (b, c) Axial CT image demonstrating ablation: an 18-gauge spinal needle with a coaxially inserted thermocouple for temperature monitoring was positioned in the C4–C5 foramen, in contact with the C5 nerve root (b, arrow). The laser fiber was then inserted coaxially in an 18-gauge needle, with the tip in the center of the nidus (c, arrow).
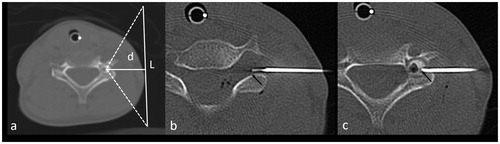
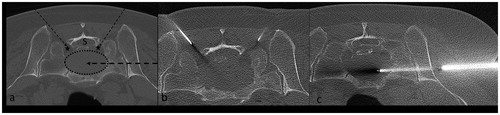
Figure 6. (a) Axial CT image of an osteolytic metastasis (dotted ellipse) in the sacrum. In this case, considering the deep location of the lesion in relation to the sacral canal (S) and sacral foramina (stars), there is no suitable access for the US beam (group (c)). (b, c) CT-scan images showing ablation of the lesion. Manual instillation of 0.9% normal saline in the sacral foramina was performed for thermo-protection (b). The ablative probe was inserted coaxially in a 14-gauge penetration set, introduced laterally into the lesion.
Based on radiological features and anatomical relations, lesions were classified into three categories:
HIFU may be performed alone (). There is no particular risk of injury to critical structures (e.g. neural structures, joints), and there is no significant physical obstruction of the ultrasound beam. No adjunctive hydro-/carbo-dissection or consolidation was performed during minimally-invasive percutaneous ablation.
HIFU may be performed using protection of surrounding structures or bone consolidation (). Hydro- or carbo-dissection was performed during percutaneous ablation, and/or consolidation of the bone was deemed necessary to prevent fracture. The lesion remains accessible for treatment as in group (a), but HIFU may damage adjacent neural structures or joints. Spinal needles (18–22G) must be placed to facilitate protection using hydro-dissection. Cases where dissection fluid/gas diffused within the proposed ultrasound beam path were not included in this category, and were classified as category (c). If necessary, consolidation may be performed following HIFU treatment.
HIFU is not feasible (). The lesion is unsuitable for HIFU if:
• an acoustic barrier (interposition of bone or air between the transducer and target-lesion) significantly obstructs the US beam from focusing on the target;
• the lesion is deeper than 12 cm from the skin surface, without any possibility of compressing overlying soft tissue;
• the lesion is surrounded by a thick bone layer deeper than 12 mm;
• the lesion is located within 1 cm of the skin (risk of skin burn is too high);
• surrounding structures located less than 2 cm from the target-lesion cannot be protected using hydro-dissection due to physical obstruction of needle-access by the HIFU transducer;
• surrounding structures requiring protection lie within the ultrasound beam, even if active thermo-protection is possible.
Cases with reactive sclerosis around OOs or a layer of normal bone surrounding the target-lesion were not systematically excluded. The capacity to heat the nidus of OOs depends on thermal conductivity of the surrounding bone, which is correlated with its density [Citation32]. OOs surrounded by a thick bone layer (<12 mm) were considered treatable, assuming that adequate thermal energy can be delivered by adjusting HIFU parameters such as central frequency [Citation25,Citation36]. Finally, HIFU treatment was considered suitable despite possible partial obstruction (≤20%) of the ultrasound beam due to bone interposition [Citation37], since this situation can be managed by adequate choice of active elements of the transducer.
Results
Of the 167 lesions, 43 were in the pelvis, 50 in the limbs, 54 in the spine or sacrum, 17 in the ribs and three in the sternum (. Overall, 67 (40.1%) lesions would have been eligible for HIFU therapy alone; 60 (35.9%) were eligible with adjunctive hydro-dissection or consolidation and 40 (24%) were not eligible for HIFU therapy. Most of the lesions in the pelvis (42; 97.7%) and peripheral bone (47; 94%) could have been treated with HIFU, whereas HIFU was considered unsuitable in 36 of 54 (66.6%) spinal lesions (. Mean depth of targetable lesions was 50.9 ± 28.4 mm.
Figure 7. Classification resulting from our suitability study according to lesion location, for metastases and OOs. Categories a, b and c correspond to (a) lesions suitable for MRgHIFU therapy alone; (b) lesions suitable for MRgHIFU if protection of surrounding structures and/or bone consolidation is performed; (c) lesions not suitable for MRgHIFU.
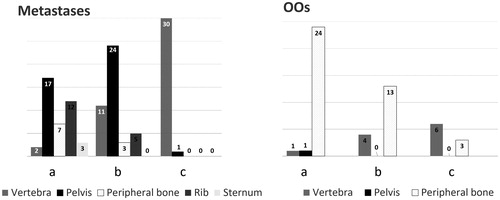
For the OO group (n = 52), 26 (50%) were considered eligible for HIFU therapy alone (group (a)), and 17 (32.7%) were eligible with hydro-dissection (group (b)). Dissection aimed at protecting an articulation (13; 76.4%), a nerve/nerve root (2; 11.8%) or the spinal cord (2; 11.8%). No cases required consolidation (). Mean volume of targetable OOs (groups (a) and (b)) was 0.56 cm3, and matrices were sclerotic (19; 44.2%), lytic (15; 34.9%) or mixed (9; 20.9%) (. Mean thickness of sclerotic or healthy bone overlying treatable lesions was 2.5 mm (range 0–12 mm). Nine lesions were classified as unsuitable for HIFU due to depth >12 mm beneath the cortical surface (3), excessive bone interposition (2), proximity of the spinal cord without any possible means of thermo-protection (3) and location close to the skin surface (1).
Figure 9. Nature of lesions (sclerotic, lytic or mixed) according to lesion type (metastases and OOs).
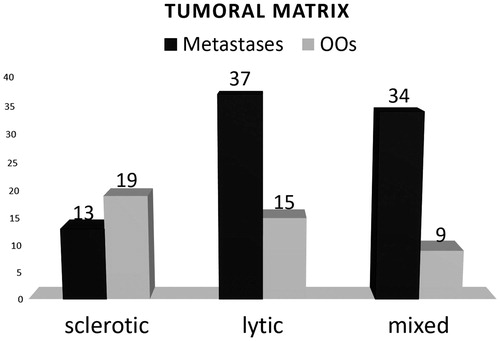
For the metastases group (n = 115), 41 (35.7%) were considered eligible for HIFU therapy alone and 43 (37.4%) were eligible with hydro-dissection and/or consolidation (). Regarding the 43 lesions treated using dissection or consolidation, 34 were dissected only (to protect a nerve/nerve root (26; 76.5%), the spinal cord (5; 14.7%) or an articulation (3; 8.8%)); four underwent cementoplasty; three were treated using percutaneous screw fixation and two underwent articular hydro-dissection and cementoplasty. Mean volume of treatable metastases (groups (a) and (b)) was 71.9 cm3 and matrices were sclerotic (13; 15.5%), lytic (37; 44%) or mixed (34; 40.5%) (. Mean thickness of bone overlying the treatable lesion was 2.1 mm (range 0–25 mm). Four lesions deeper than 12 mm were not excluded as the overlying bone was osteolytic: in these cases, the painful target was a deep portion of the metastasis. Thirty-one cases were considered unsuitable for HIFU due to presence of neural structures located within the US beam which could not be protected (30), and physical obstruction of hydro-dissection needle entry-points by the transducer (1).
Partial obstruction of the US beam by interposed bone was present for eight OOs and six metastases. Eleven cases with <20% beam obstruction and no other contra-indications were considered suitable for MRgHIFU. The three remaining cases demonstrated >20% obstruction (29%, 32% and 50%), but were classified as unsuitable for other reasons. Partial obstruction (e.g. ) is distinguished from total obstruction corresponding to deep lesions where no access is possible (e.g. sacrum/vertebra, ): the latter cases were classified as unsuitable for MRgHIFU.
Discussion
A large variety of cancers are currently being treated with MRgHIFU in clinical practice with encouraging results [Citation38,Citation39]. Numerous case series have reported the efficacy and safety of MRgHIFU for treatment of metastatic bone lesions [Citation14–23] and OOs [Citation24–30]. One randomized multi-center phase III trial evaluating HIFU for patients with painful bone metastases has been published [Citation16]. The technique has been approved by the US Food and Drug Administration (FDA) and the European Union for treatment of pain related to bone metastases. Despite these encouraging clinical results, there remains a substantial need for research addressing critical issues such as the optimal acoustic energy for ablation, as well as the question of accurate real-time therapeutic monitoring [Citation40–42]. Moreover, feasibility with respect to tumor morphology and effects on surrounding structures should be thoroughly evaluated.
Successful treatment of OOs requires complete destruction of the nidus, which means that sufficient energy has to penetrate the bone to ensure adequate ablation. Due to its high acoustic absorption, HIFU heats bone tissue with moderate acoustic power and facilitates thermal energy delivery to the nidus [Citation40]; the lesion is then rapidly heated due to its small volume (mean 0.56 cm3 in our series) [Citation21], enabling complete ablation. However, localized backscattering and reflection phenomena may injure adjacent soft-tissues, particularly if poorly perfused [Citation40]. Moreover, OOs may be located next to a nerve, a nerve root ( and ), the spinal cord or an articulation, rendering the ablative procedure particularly hazardous in the absence of adjunctive thermo-protection.
Treatment objectives are different for metastatic bone lesions. HIFU heats the periosteum to 60 °C, resulting in destruction of nociceptive nerves, tumor mass reduction, neuromodulation and consequent pain palliation [Citation36]. Gianfelice et al. have reported ablation of the medullary component of osteolytic metastases using HIFU, suggesting that it may also be possible to achieve entire local tumor destruction [Citation17]. Additionally, HIFU offers the possibility to induce mild hyperthermia (target temperature 40–45 °C), as a means to optimize radiosensitivity [Citation43] and chemotherapy [Citation44], and facilitate local drug delivery [Citation45] even for large-volume lesions [Citation46]. This feature is of significant interest, since radiation therapy is commonly used in clinical practice for pain palliation, and combination of these two non-invasive approaches appears to be particularly promising [Citation47].
Our retrospective study suggests that 50% of OOs and 35.7% of bone metastases could have been treated with HIFU, at least from a planning point of view, without adjunctive hydro-dissection and/or consolidation for metastases. A similar evaluation of targetability has been reported in pediatric sarcomas and neuroblastomas: the authors considered that 64% of sarcomas and 21% of neuroblastomas were targetable at diagnosis [Citation48]. The most targetable tumors were primary lesions in the extremities. Median tumor volume was 173.6 cm3 for sarcomas and 176.7 cm3 for neuroblastomas, approximately 2.5 times greater than the average volume of bone metastases in our series. Lesions within and next to the spine were considered untreatable in this study. The suitability of these locations for HIFU is questionable, since treatment of spinal lesions (excluding the sacrum) has not been reported in clinical studies. However, we did not exclude spinal locations for three reasons: firstly, some spinal and sacral locations (e.g. posterior aspect of lumbar and sacral vertebrae) were considered suitable for HIFU in two recent reviews [Citation27,Citation36], and sacral lesions have been included in one study [Citation16]. Secondly, spinal lesions may be treated percutaneously using thermal protection and/or consolidation, and these measures could be extrapolated for use in HIFU ablation. Thirdly, there is significant variation in spinal lesion location, and although tumors in the vertebral body and pedicle may not be treatable without injuring the spinal cord or nerves, some posterior lesions (e.g. articular process) may be eligible for therapy. In fact, only one-third of the spinal lesions in our study were suitable for HIFU, and most required thermal protection. Interestingly, a preclinical feasibility study on Thiel soft embalmed cadavers has reported the possibility of performing MRgHIFU in the disc and vertebral body without heating the spinal canal or adjacent structures [Citation49]. These promising results require confirmation in animal and clinical studies.
The use of minimally-invasive thermo-protective techniques and/or osseous consolidation may render MRgHIFU possible in several cases that would originally have been considered unsuitable for treatment. In our series, 32.7% of OOs and 37.4% of metastases were targetable using adjunctive thermal protection or bone consolidation. These cases remain significantly less invasive than percutaneous ablation techniques, since HIFU avoids the need for large-bore access cannulae/thermo-probes, and only small-diameter needles (18–22-gauge) are required for hydro-dissection. In terms of bone consolidation, cementoplasty or percutaneous screw fixation could be performed either as a deferred two-step procedure, or more logically during the same procedure following ablation. Although HIFU does not appear to have a mechanical impact on bone [Citation50], metastatic lesions may be associated with spontaneous fractures. The decision to undertake consolidation is therefore based on the volume, location and density of the metastasis (osteolytic), rather than any possible influence of MRgHIFU on bone stiffness [Citation51].
Fluid injection next to vulnerable collateral structures (hydro-dissection) is a commonly used thermo-protective technique during percutaneous ablation. Spinal needles (18–22-gauge) are placed next to the sensitive structures and sterile water is injected to insulate structures from thermal damage. A thermosensor can be placed to monitor local temperature [Citation52,Citation53]. Particular care should be taken when inserting needles adjacent to target-lesions, due to possible interference with the ultrasound beam which may change the distribution of acoustic energy [Citation41]. Moreover, fluid injection may be accompanied by air bubbles, which may also alter the ultrasound beam. Although less invasive than percutaneous ablative therapies, MRgHIFU could not be reduced to a fully non-invasive technique in about one-third of our cases, since spinal needle placement was required for hydro-dissection to protect surrounding structures.
The optimal strategy for ultrasound beam focusing is not well established. The nature and integrity of cortical bone in metastatic lesions and OOs influence the distribution of acoustic energy, and altered cortical integrity may decrease the efficacy of heating for metastases [Citation15]. Optimal focus location either at the periosteum/soft-tissue interface or deep to the bone cortex is also an open question and appears case-dependent [Citation54]. However, the nature (osteolytic/sclerotic) and thickness of bone overlying the lesion are considered a mechanical factor requiring adjustment of ultrasonic power, rather than an impenetrable barrier to HIFU [Citation55]. Bone metastases may also demonstrate significant extra-osseous extension, resulting in pain due to muscular or neural tumor infiltration; these components must also be targeted to optimize therapy. Partial US beam obstruction was present in 14 lesions, 11 of which were considered targetable with <20% obstruction. Relatively mild obstruction (<20%) can safely be compensated for by increasing input power, since bone requires relatively low power anyway due to its high acoustic absorption [Citation27,Citation56–58], and efficiency/safety issues such as power loss and skin heating should not be critical. Finally, pre-treatment planning could be further improved by adding relevant information on predicted thermal lesions using acoustic and thermal simulations [Citation59–61].
This study is not intended to provide guidelines for a specific transducer or HIFU system, but to assess the general suitability of treatment using typical transducer geometries and properties. The study could certainly be refined by proposing optimal transducers well-suited for specific situations, but this lies beyond its scope. There are several further study limitations. Firstly, cases were analyzed retrospectively from department of interventional radiology electronic records. For bone metastases, the analysis excludes patients treated solely by EBRT (i.e. not referred for percutaneous ablation), resulting in significant selection bias. However, the population considered in this study nevertheless allows for clear and direct comparison between minimally-invasive thermal ablation and HIFU. Secondly, geometrical accessibility was not considered a limitation, since it was assumed that the transducer could be positioned freely on the patient regardless of its location. This assumption is valid for ‘transducer on patient’ configurations [Citation18], but may result in additional limitations and exclusion criteria for ‘in-table’ transducer systems. For example, for all cases requiring hydro-dissection or consolidation, the skin must remain accessible to allow insertion of needles next to the target, which is not possible with the in-table system. Thirdly, respiratory motion compensation for rib lesions was not taken into account. Lesions were considered targetable, despite possible difficulties related to breathing-motion and positioning of the HIFU transducer on the convex thoracic cage. High-frequency jet ventilation [Citation62] may be an interesting solution to overcome respiratory motion, but this technology is not yet available under high-field MR. Fourthly, no specific acoustic optimization was performed based on typical geometries for the treatment of bone lesions. The total number of cases potentially treatable by HIFU could certainly be improved by designing specific HIFU transducers with adequate geometry and acoustic properties. Finally, despite the possible use of non-invasive MR thermometry, which is particularly challenging in bone, placement of a thermal probe may potentially combine direct temperature measurement with the possibility of injecting cooling fluid next to surrounding structures, thereby facilitating a controlled ablation-volume without interrupting the procedure. Different thermal monitoring methods were not specifically considered in this study.
In conclusion, 50% of OOs and 35.7% of bone metastases treated in our department with minimally-invasive ablation could have been treated using MRgHIFU entirely non-invasively. This result should be considered in terms of targetability alone, without any intention to predict procedural efficacy. A further major conclusion is that about one-third of additional cases could be eligible for HIFU if adjunctive minimally-invasive thermo-protection and/or consolidation was performed.
Acknowledgements
We would like to thank Dr. Nitin Ramamurthy for the redaction in the English language.
Disclosure statement
There is no conflict of interest for any of the authors and there is no necessity for any financial disclosure.
References
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18.
- Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365–378.
- Mavrogenis AF, Angelini A, Vottis C, et al. Modern palliative treatments for metastatic bone disease: awareness of advantages, disadvantages, and guidance. Clin J Pain. 2016;32:337–350.
- Graham GN, Browne H. Primary bony tumors of the pediatric spine. Yale J Biol Med. 2001;74:1–8.
- Sherman MS, McFarland G. Jr. Mechanism of pain in osteoid osteomas. South Med J. 1965;58:163–166.
- Barlow E, Davies AM, Cool WP, et al. Osteoid osteoma and osteoblastoma: novel histological and immunohistochemical observations as evidence for a single entity. J Clin Pathol. 2013;66:768–774.
- Byers PD. Solitary benign osteoblastic lesions of bone. Osteoid osteoma and benign osteoblastoma. Cancer. 1968;22:43–57.
- Rosenthal D, Callstrom MR. Critical review and state of the art in interventional oncology: benign and metastatic disease involving bone. Radiology. 2012;262:765–780.
- Rosenthal DI, Alexander A, Rosenberg AE, et al. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183:29–33.
- Gangi A, Dietemann JL, Gasser B, et al. Percutaneous laser photocoagulation of osteoid osteomas. Semin Musculoskelet Radiol. 1997;1:273–280.
- Gangi A, Alizadeh H, Wong L, et al. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242:293–301.
- Tsoumakidou G, Thenint MA, Garnon J, et al. Percutaneous image-guided laser photocoagulation of spinal osteoid osteoma: a single-institution series. Radiology. 2016;278:936–943.
- Malietzis G, Monzon L, Hand J, et al. High-intensity focused ultrasound: advances in technology and experimental trials support enhanced utility of focused ultrasound surgery in oncology. Br J Radiol. 2013;86:20130044.
- Chan M, Dennis K, Huang Y, et al. Magnetic resonance-guided high-intensity-focused ultrasound for palliation of painful skeletal metastases: a pilot study. Technol Cancer Res Treat. 2017;16:570–576.
- Huisman M, Lam MK, Bartels LW, et al. Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Ther Ultrasound. 2014;2:16.
- Hurwitz MD, Ghanouni P, Kanaev SV, et al. Resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106.
- Gianfelice D, Gupta C, Kucharczyk W, et al. Palliative treatment of painful bone metastases with MR imaging-guided focused ultrasound. Radiology. 2008;249:355–363.
- Joo B, Park MS, Lee SH, et al. Pain palliation in patients with bone metastases using magnetic resonance-guided focused ultrasound with conformal bone system: a preliminary report. Yonsei Med J. 2015;56:503–509.
- Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16:140–146.
- Napoli A, Anzidei M, Marincola BC, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics. 2013;33:1555–1568.
- Lee H-L, Kuo C-C, Tsai J-T, et al. Magnetic resonance-guided focused ultrasound versus conventional radiation therapy for painful bone metastasis: a matched-pair study. J Bone Joint Surg Am. 2017;99:1572–1578.
- Harding D, Giles SL, Brown MRD, et al. Evaluation of quality of life outcomes following palliative treatment of bone metastases with magnetic resonance-guided high intensity focused ultrasound: an international multicentre study. Clin Oncol (R Coll Radiol). 2018;30:233–242.
- Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases – preliminary clinical experience. Ann Oncol. 2006;18:163–167.
- Geiger D, Napoli A, Conchiglia A, et al. MR-guided focused ultrasound (MRgFUS) ablation for the treatment of nonspinal osteoid osteoma. A prospective multicenter evaluation. J Bone Joint Surg Am. 2014;96:743–751.
- Masciocchi C, Zugaro L, Arrigoni F, et al. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: a propensity score matching study. Eur Radiol. 2016;26:2472–2481.
- Rovella MS, Martins GL, Cavalcanti CF, et al. Magnetic resonance-guided high-intensity focused ultrasound ablation of osteoid osteoma: a case series report. Ultrasound Med Biol. 2016;42:919–923.
- Temple MJ, Waspe AC, Amaral JG, et al. Establishing a clinical service for the treatment of osteoid osteoma using magnetic resonance-guided focused ultrasound: overview and guidelines. J Ther Ultrasound. 2016;4:16.
- Napoli A, Bazzocchi A, Scipione R, et al. Noninvasive therapy for osteoid osteoma: a prospective developmental study with MR imaging-guided high-intensity focused ultrasound. Radiology. 2017;285:186–196.
- Napoli A, Mastantuono M, Cavallo Marincola B, et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology. 2013;267:514–521.
- Sharma KV, Yarmolenko PS, Celik H, et al. Comparison of noninvasive high-intensity focused ultrasound with radiofrequency ablation of osteoid osteoma. J Pediatr. 2017;190:222–228.e1.
- Yu W, Tang L, Lin F, et al. High-intensity focused ultrasound: noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg Oncol. 2015;24:9–15.
- Feldmann A, Wili P, Maquer G, et al. The thermal conductivity of cortical and cancellous bone. Eur Cell Mater. 2018;35:25–33.
- Greenberg A, Berenstein Weyel T, Sosna J, et al. The distribution of heat in bone during radiofrequency ablation of an ex vivo bovine model of osteoid osteoma. Bone Joint J. 2014;96-B:677–683.
- Walker KE, Baldini T, Lindeque BG. Thermal conductivity of human bone in cryoprobe freezing as related to density. Orthopedics. 2017;40:90–94.
- Kim YS, Trillaud H, Rhim H, et al. MR thermometry analysis of sonication accuracy and safety margin of volumetric MR imaging-guided high-intensity focused ultrasound ablation of symptomatic uterine fibroids. Radiology. 2012;265:627–637.
- Dababou S, Marrocchio C, Scipione R, et al. High-intensity focused ultrasound for pain management in patients with cancer. Radiographics. 2018;38:603–623.
- Viallon M, Petrusca L, Auboiroux V, et al. Experimental methods for improved spatial control of thermal lesions in magnetic resonance-guided focused ultrasound ablation. Ultrasound Med Biol. 2013;39:1580–1595.
- Kobus T, McDannold N. Update on clinical magnetic resonance-guided focused ultrasound applications. Magn Reson Imaging Clin N Am. 2015;23:657–667.
- Hsiao YH, Kuo SJ, Tsai HD, et al. Clinical application of high-intensity focused ultrasound in cancer therapy. J Cancer. 2016;7:225–231.
- ten Eikelder HM, Bosnacki D, Elevelt A, et al. Modelling the temperature evolution of bone under high intensity focused ultrasound. Phys Med Biol. 2016;61:1810–1828.
- Hassanuddin A, Choi JH, Seo DW, et al. Factors affecting tumor ablation during high intensity focused ultrasound treatment. Gut Liver. 2014;8:433–437.
- Lam MK, Huisman M, Nijenhuis RJ, et al. Quality of MR thermometry during palliative MR-guided high-intensity focused ultrasound (MR-HIFU) treatment of bone metastases. J Ther Ultrasound. 2015;3:5.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119–1125.
- Issels RD, Lindner LH, Wessalowski R, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for soft-tissue sarcoma − authors’ reply. Lancet Oncol. 2017;18:e630.
- Partanen A, Yarmolenko PS, Viitala A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia. 2012;28:320–336.
- Tillander M, Hokland S, Koskela J, et al. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med Phys. 2016;43:1539–1549.
- Bedard G, Chow E. The failures and challenges of bone metastases research in radiation oncology. J Bone Oncol. 2013;2:84–88.
- Shim J, Staruch RM, Koral K, et al. Pediatric sarcomas are targetable by MR-guided high intensity focused ultrasound (MR-HIFU): anatomical distribution and radiological characteristics. Pediatr Blood Cancer. 2016;63:1753–1760.
- Karakitsios I, Mihcin S, Saliev T, et al. Feasibility study of pre-clinical Thiel embalmed human cadaver for MR-guided focused ultrasound of the spine. Minim Invasive Ther Allied Technol. 2016;25:154–161.
- Yeo SY, Arias Moreno AJ, van Rietbergen B, et al. Effects of magnetic resonance-guided high-intensity focused ultrasound ablation on bone mechanical properties and modeling. J Ther Ultrasound. 2015;3:13.
- Herman A, Avivi E, Brosh T, et al. Biomechanical properties of bone treated by magnetic resonance-guided focused ultrasound − an in vivo porcine model study. Bone. 2013;57:92–97.
- Filippiadis DK, Tutton S, Mazioti A, et al. Percutaneous image-guided ablation of bone and soft tissue tumours: a review of available techniques and protective measures. Insights Imaging. 2014;5:339–346.
- Tsoumakidou G, Buy X, Garnon J, et al. Percutaneous thermal ablation: how to protect the surrounding organs. Tech Vasc Interv Radiol. 2011;14:170–176.
- Kopelman D, Inbar Y, Hanannel A, et al. Magnetic resonance guided focused ultrasound surgery. Ablation of soft tissue at bone-muscle interface in a porcine model. Eur J Clin Invest. 2008;38:268–275.
- Hudson TJ, Looi T, Pichardo S, et al. Simulating thermal effects of MR-guided focused ultrasound in cortical bone and its surrounding tissue. Med Phys. 2018;45:506–519.
- Bucknor MD, Ozhinsky E, Shah R, et al. Effect of sonication duration and power on ablation depth during MR-guided focused ultrasound of bone. J Magn Reson Imaging. 2017;46:1418–1422.
- Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417–430.
- Lehmann JF, DeLateur BJ, Warren CG, et al. Heating produced by ultrasound in bone and soft tissue. Arch Phys Med Rehabil. 1967;48:397–401.
- de Greef M, Kok HP, Correia D, et al. Optimization in hyperthermia treatment planning: the impact of tissue perfusion uncertainty. Med Phys. 2010;37:4540–4550.
- de Greef M, Kok HP, Correia D, et al. Uncertainty in hyperthermia treatment planning: the need for robust system design. Phys Med Biol. 2011;56:3233–3250.
- Kok HP, Korshuize-van Straten L, Bakker A, et al. Feasibility of on-line temperature-based hyperthermia treatment planning to improve tumour temperatures during locoregional hyperthermia. Int J Hyperthermia. 2017;16:1–10.
- Muller A, Petrusca L, Auboiroux V, et al. Management of respiratory motion in extracorporeal high-intensity focused ultrasound treatment in upper abdominal organs: current status and perspectives. Cardiovasc Intervent Radiol. 2013;36:1464–1476.