Abstract
Purpose: To assess the feasibility of fusion imaging between intraprocedural ultrasound (US) and contrast-enhanced cone-beam CT (CBCT) for small (< 2 cm) hepatocellular carcinoma (HCC).
Materials and methods: Six patients (five males, one female, age range 58–80, mean 69 years), with small (mean diameter 16.8 mm) HCC poorly visible at US underwent percutaneous microwave ablation under US/CBCT fusion guidance. During general anesthesia with apnea control, a contrast- enhanced CBCT was acquired with an active tracker. Subsequently, real time US images were fused with CBCT images, and treatment performed under fusion imaging guidance. Feasibility of fusion imaging and percutaneous ablation were assessed, correct targeting (distance from center of tumor and center of ablation area <5 mm) and one-month primary technical efficacy were evaluated. Major and minor complications as well as overall procedural time were recorded.
Results: US/CBCT fusion was feasible in all cases, allowing for completion of the treatment as previously planned (technical success 100%). Correct targeting was achieved in 4/6 cases (66%), while in two cases, center of tumor and center of ablated area were respectively 7 and 8 mm distant. At 1 month CT scan, all tumors were completely ablated (primary technical efficacy 100%). No major or minor complications occurred. Mean overall procedure time was 127 min.
Conclusions: US/CBCT fusion is a feasible technique for liver ablation, and might represent a useful tool to increase the correct targeting of poorly US-visible HCC nodules in the angio suite.
Introduction
Image-guided ablations are currently proposed as first treatment choice for small HCC [Citation1]. Ultrasound (US) is the most commonly-used image guidance option for percutaneous ablations in consideration of its broad distribution, real time capability and good general visualization of liver tumors and normal structures. However, the technical feasibility of US-guided ablations is often limited by poor lesion visualization, due to lack of echogenicity difference or difficult localization in the liver [Citation2]. In these cases, other imaging modalities such as computed tomography (CT), magnetic resonance (MR), or cone beam CT (CBCT) can be used to perform liver ablations [Citation3]. Some techniques to improve conspicuity of target liver lesion for percutaneous ultrasound-guided ablations have been described, including contrast-enhanced US, and fusion imaging of US with previously acquired CT/MR [Citation4]. To our knowledge, US/CBCT fusion imaging has never previously been reported for the guidance of percutaneous ablation of small HCC poorly visible at US. CBCT is a new advanced tool integrated in the angiography suite: a c-arm with a flat panel detector rotates around the patients in a time lapse that is sufficient to ensure raw data collection; it makes 3D images available on a selected volume usually with a relatively small field of view (FOV). CBCT, being performed in the angio room during the treatment, with the patient already in the correct position, and acquired with an active tracker placed on the patient, has the potential to provide easier fusion with real time US imaging, and higher precision than that obtained by pre-acquired CT or MR images. Furthermore, application of US/CBCT fusion would allow to perform ablations in the angio room without the use of the CT machine.
The aim of the present study is to describe the technique for US/CBCT fusion imaging, and to report the preliminary data on its technical feasibility and primary technical efficacy as guidance in the percutaneous treatment of small HCC poorly visible at US.
Materials and methods
Patients
Institutional Review Board approval was obtained and the requirement for patients’ informed consent was waived due to the retrospective nature of the study. Data of patients treated with US/CBCT fusion from August 2017 were retrospectively reviewed. In the considered period, six patients (five males, one female age range 58–80, mean 69 years) underwent percutaneous thermal ablation for a single HCC <2 cm under US/CBCT fusion. All patients had a history of HCV related cirrhosis and were selected for ablation during a multidisciplinary board discussion in accordance to BCLC guidelines. All six patients were previously treated for HCC: two patients underwent laparoscopic ablations and two percutaneous radiofrequency ablations elsewhere with loco regional relapse, while the final two patients had a single new node in the remnant liver after surgery. Four HCCs were barely visible on US because of location in the liver dome while two were hardly detectable at US because of a low echogenicity difference with normal parenchyma. All HCCs were conversely well visible at CT in the arterial () or portal phase. At the time of treatment mean node dimension was 16.8 mm (range 9–20).
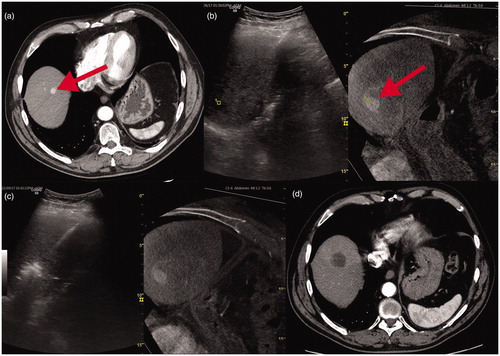
Figure 1. (a) Small HCC node in the liver dome at baseline MDCT evident as focal contrast enhancement (red arrow). (b) US fusion with contrast enhanced CBCT: HCC node is barely detectable with US alone but this visualisation improves due to fused CBCT node visualisation (red arrow). (c) the subsequent needle-antenna deployment and ablation. d-1 month MDCT control.
Procedure
All the six procedures were performed in general anesthesia in a sterile setting with supine decubitus and both arms gently placed above the head with dedicated soft supports. All procedures were performed by the same interventional radiologist with more than 15 years of experience.
A flat panel angiography system (Artis Zee, Siemens Healthcare Erlangen, Germany) was used to obtain contrast enhanced (maximum dose of 120 ml, ultravist 370 mg\ml and flow rate of 3.5 ml/sec) CBCT images with a 7 s c-arm rotation time in both arterial and portal phase. A prearranged delay of 35 s from injection was established for the arterial phase and a delay of 70 s for the portal phase. A breath-hold apnea at the same lung expansion was performed by the anesthesiologist during both acquisitions. A dedicated active patient tracer (omniTRAX, CIVCO, Coralville, Iowa, US) was placed on the upper abdomen and included in the field of view. The CBCT acquisition parameters were: rotation angle 200° and angulation step 0.5°. The slice dimension was 521 × 512 pixels with about 400 images per volume. The isotropic voxels had an approximate size of 0.5 mm3. The syngo X-workplace for Dyna CT was used (Siemens Healthcare) to process images after volume acquisition and for overlapping volume images: each CBCT volume could be fused with that previously acquired as a further control of needle deployment and ablation area position (Syngo InSpace 3d\3d Fusion software, Erlangen, Germany). Mean waiting time from acquisition to image visualization was about 20 s.
Fusion imaging was performed by co-registration of the 3 D contrast enhanced CBCT data set with real-time ultrasound images. Ultrasound imaging was performed using a dedicated ultrasound scanner configured with Volume Navigation (LOGIQ S8 XD clear 2.0, GE Healthcare, Ill-USA) and a C1-6 VN ultrasound transducer with electromagnetic sensor inside. An electromagnetic transmitter is placed near the area of interest and electromagnetic sensors are attached to a bracket connected to the US probe and to the patient’s active tracer. Both the transmitter and the sensors are connected to a position sensing unit embedded in the ultrasound machine. The position sensing equipment allows the ultrasound system to track the transducer's position, and therefore the image position, within the electromagnetic field.
CBCT images of the arterial phase were selected for US fusion in four nodes because of their significant contrast enhancement () while the portal phase was chosen for the last two nodes that were more evident as washout areas.
Fused US was performed exactly in the same decubitus as per CBCT, looking for a precise image matching. The same volume apnea was required in order to completely interrupt liver movements and under US fusion guidance, a needle was deployed along the planned path to the target lesion. The needle was inserted under continuous US visualization, aiming to target the center of the lesion, as preoperatively identified by means of US and CBCT. All the six nodes were treated with a commonly available microwave system (Covidien Emprint Ablation System with Thermosphere TechnologyTM, Boulder Colorado USA). All cases were treated with 100 W power for 3 min (). At the end, track ablation was performed. A final contrast enhanced CBCT was carried out in order to confirm the correct tumor ablation () and to exclude any complications.
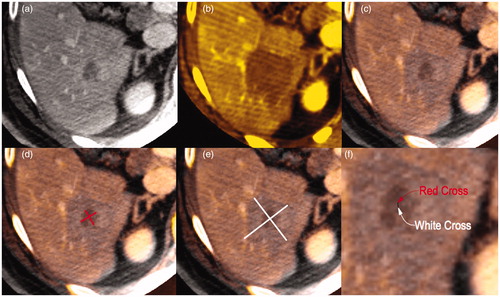
Figure 2. (a) pretreatment and (b) posttreatment CBCT for a 2 cm HCC with a well-defined wash-out in portal phase. (c) pre and post treatment overlapping of both previous CBCTs with Inspace 3d fusion software (see text). (d) centre of HCC (red cross) and (e) centre of necrosis area (white cross) evaluated on fused CBCT images with longitudinal and transverse diameters. (f) distance between the two centre points.
Variables
The primary endpoint of the study was to verify feasibility on US/CBCT fusion imaging. Secondary endpoints were precision of targeting under US/CBCT fusion imaging, technical success, primary technical efficacy and complications rate. Also, procedural time was evaluated.
The feasibility of fusion imaging was defined as the possibility of performing fusion and real time display of acquired CBCT images, with precise matching of the visible anatomical structures, on the judgement of the operator.
Correct targeting was defined as a distance from center of tumor and center of ablation area detected at post treatment CBCT <5 mm. The distance was assessed in all cases superimposing pre- and post-treatment CBCT (Syngo InSpace 3d\3d Fusion software, Erlangen, Germany) (). Safety-free margins were also evaluated as adequate if ≥5 mm [Citation5].
Technical success and primary technical efficacy were defined according to the standard definition [Citation5] and evaluated at immediate post-treatment CBCT and one month CT respectively.
Complications were defined as major and minor according to SIR criteria [Citation6]
Overall procedural time was defined as the time from anesthesia induction to the patient awakening.
Follow-up consisted in clinical examination and a contrast enhanced CT scan at 1 and 3 months
Results
US/CBCT fusion was feasible in all cases, and we were able, again in all cases, to complete the treatment as preoperatively planned (technical success 100%).
In four cases (66.6%), the correct targeting was confirmed at post treatment CBCT. In two cases, the center of tumor and center of ablated area were respectively 7 and 8 mm distant. In all cases, safety-free margins were adequate.
No major or minor complications occurred.
Mean overall time from induction of general anesthesia beginning to patient awakening was 127 min. Complete tumor ablation was achieved in all cases at one and three months (primary technical efficacy 100%).
Discussion
Our preliminary results demonstrate that US/CBCT fusion is a feasible technique for precisely guiding percutaneous thermal ablations.
Correct targeting still remains one of the most challenging problems for percutaneous image-guided ablations. Fusion imaging has been successfully used to improve liver and renal lesion targeting, particularly in cases of lesions which are poorly visible at real-time US [Citation7–9]. However, one of the main limits of fusion imaging is the use of CT or MRI images acquired days before the procedure, with the patient in a different position, and often with a different respiratory phase. Conversely, CBCT can be performed directly in the operating room, with the patient already in the desired position for the procedure, and with controlled respiratory apnea. Thus, the images obtained with CBCT performed immediately before the ablation represent the ideal data set for a precise matching with real-time US. CBCT has recently been described as a useful tool for identifying liver lesions during percutaneous thermal ablations, and it has been applied for targeting and ablating hepatic lesions in small cohorts of patients. In addition, CBCT has been proposed as a possible alternative or complementary technique for assessing ablation results [Citation10,Citation3]. However, several technical aspects limit the application of CBCT as the sole method for liver tumor ablation guidance. Motion artefacts for the slower rotation time, patient positioning in an off-center position and decubitus where the patient is forced to have both arms above the head are among the main limitations of this technique [Citation11]. In particular, the limited field of view compared to CT might limit CBCT application especially for lesions located in the periphery of the right liver or in severe obese patients [Citation11]. Our rationale for the present study was to evaluate feasibility of US/CBCT image fusion. With the patient already in the desired treatment position and under general anesthesia, it would be possible to avoid the CBCT limitations described above, such as motion artefacts due to breath or body unintentional movement. At the same time, the acquisition of CBCT exactly in the same position as that employed in the US-guided procedure should ensure a better image matching, mainly because of curarisation and a very precise apnea control. In fact the anesthesiologist was purposely requested to ensure that each apnea was of precisely the same volume and depth so as to ensure a standardization of diaphragmatic excursion. Furthermore, with this setting it would be possible to perform combination therapy of TACE and ablation at the same time, or to perform angiography during the ablation.
In our series, it was always possible to achieve a correct and precise registration of the CBCT images with real time US images. This was also possible due to the application of the patient active tracer that, being located on the patient at the moment of CBCT acquisition, enabled precise and automatic image coregistration. Moreover, it was also possible to correctly target the liver tumor in 4/6 (66%) of cases, while in two cases the center of the ablation area was slightly more distant than 5 mm from the center of the tumor. This might be due to some degree of needle bending and organ displacement during the procedure, which is not yet possible to take into account with present fusion systems. Furthermore, as this series represents our initial experience with the method, and due to the small number of cases included, our results should be regarded as preliminary, and further studies are necessary to thoroughly asses the clinical impact of this method.
Additionally, the possibility to repeat the same CBCT acquisition with the same conditions after treatment might be helpful to precisely assess the completeness of treatment with adequate safety margins during the same ablative session, and, if needed, to immediately administer a second treatment [Citation12]. In our series it was always possible to perform a final CBCT to immediately assess the result, with a 100% technical success in all cases. Again, a further contrast enhanced CBCT evaluation at the end of the procedure is of paramount importance to exclude early complications. Furthermore, if bleeding should occur, treatment with embolization can be directly performed in the same room, with the patient still under general anesthesia. In fact, we completely agree with Cazzato’s attitude [Citation3] in performing general anesthesia not only for the abovementioned technical considerations but moreover for the possibility of completely concentrating on the ongoing procedure. In our series, no major of minor complications occurred, even though the lesions were poorly visible at real time US and treatments were mainly performed relying on the image fusion guidance. In addition, the 1 month CT always confirmed an accurate targeting of the lesion, and complete tumor ablation, with a 100% primary technical efficacy.
Furthermore, as the role played by CBCT in interventional oncology is increasing, in line with the current recommendations in the CIRSE/SIR protocol guidelines for selective TACE [Citation11], its availability in angiographic suites will probably increase in the near future.
Some limitations of the present study must be disclosed: firstly, this is a retrospective evaluation on a very limited cohort of patients. Thus, our results should be regarded as preliminary, and need confirmation in larger trials. Secondly, precision of matching was performed on the subjective judgement of the operator. However, the long and extensive experience of the operator in image guided ablations, and the confirmed correct targeting at postprocedural CBCT lend support to the correct judgement of the operator on matching precision. Thirdly, results were assessed only at short-term follow up, while for future studies, a longer observation period would be desirable.
In conclusion, US/CBCT image fusion is technically feasible, and appears to be an effective image guidance modality for achieving correct targeting and complete ablation of small HCC which is not clearly visible at US. This method, providing reference images acquired with the patient in the desired position for ablation, bear the potential to overcome the majority of the limitations of US/CT or US/MRI fusion imaging, which are generally performed with reference images acquired days before the procedure. Further studies on larger series will clarify the role of this technique in the clinical practice.
Disclosure statement
Giovanni Mauri is a consultant for Elesta Srl. All other Authors declare no conflicts of interest.
References
- Lucchina N, Tsetis D, Ierardi AM, et al. Current role of microwave ablation in the treatment of small hepatocellular carcinomas. Ann Gastroenterol. 2016;29: 460–465.
- Lee MW, Kim YJ, Park HS, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010;194: W396–W400.
- Cazzato RL, Buy X, Alberti N, et al. Flat-panel cone-beam CT-guided radiofrequency ablation of very small (≤ 1.5 cm) liver tumors: technical note on a preliminary experience. Cardiovasc Intervent Radiol. 2015; Feb38: 206–212.
- Hakime A, Yevich S, Tselikas L, et al. Percutaneous thermal ablation with ultrasound guidance. Fusion imaging guidance to improve conspicuity of liver metastasis. Cardiovasc Intervent Radiol. 2017; May40: 721–727.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014; 273:241–260.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology Clinical Practice Guidelines. J Vasc Interv Radiol. 2003; 14:S199–S202.
- Mauri G, Cova L, De Beni S, et al. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143–151.
- Mauri G. Expanding role of virtual navigation and fusion imaging in percutaneous biopsies and ablation. Abdom Imaging. 2015; Oct40:3238–3239.
- Mauri G, Nicosia L, Varano GM, et al. Tips and tricks for a safe and effective image-guided percutaneous renal tumour ablation. Insights Imaging. 2017;8:357–363.
- Abdel-Rehim M, Ronot M, Sibert A, et al. Assessment of liver ablation using cone beam computed tomography. World J Gastroenterol. 2015;21:517–524.
- Bapst B, Lagadec M, Breguet R, et al. Cone Beam Computed Tomography (CBCT) in the field of interventional oncology of the liver. Cardiovasc Intervent Radiol. 2016;39:8–20.
- Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014; 5:209–216.
