Abstract
Objective: To compare the treatment efficacy and safety of high intensity focused ultrasound (HIFU) on its own, HIFU combined with levonorgestrel-releasing intrauterine system (LNG-IUS) and HIFU combined with gonadotropin-releasing hormone agonist (GnRHa).
Method: Seventy-eight patients with adenomyosis who underwent HIFU treatment were retrospectively analyzed. Among them, 45 patients were treated only with HIFU, 15 patients were treated with HIFU combined with LNG-IUS and 18 patients were treated with HIFU combined with GnRHa. Dysmenorrhea scores, menstrual blood volumes, uterine volumes and adenomyotic lesion volumes were evaluated 1, 6 and 12 months after HIFU.
Result: After treatment, dysmenorrhea score, menstrual blood volume, uterine volume and adenomyotic lesion volume significantly decreased in all three groups (p < 0.05). No significant difference was observed among the HIFU group, HIFU with LNG-IUS group and HIFU with GnRHa group 1 month after HIFU. However, 6 and 12 months after HIFU, dysmenorrhea score, menstrual blood volume, uterine volume and adenomyotic lesion volume decreased significantly more in the HIFU with LNG-IUS group and HIFU with GnRH-a group than in the group treated with HIFU on its own (p < 0.05).
Conclusion: HIFU can be effectively used in the management of adenomyosis. Based on the results of this study with a limited number of patients, our study suggested that combining HIFU with LNG-IUS or GnRHa may provide a superior clinical effect compared to HIFU treatment on its own.
Introduction
Adenomyosis is a benign disorder characterized by the presence of ectopic glandular tissue found in the muscular wall of the uterus [Citation1,Citation2]. The main clinical manifestations include progressive dysmenorrhea and hypermenorrhea. It also has a great impact on the fertility of women. The traditional treatments for adenomyosis include medicinal and surgical methods. Hysterectomy is the only definitive treatment for adenomyosis, however, it is unviable for patients who wish to remain fertile. Because the boundary of the adenomyotic lesion is not clear, adenomyomectomy has proven effective in only 50% of the patients. Uterine artery embolisation (UAE) and medicinal treatments, including levonorgestrel-releasing intrauterine systems (LNG-IUS), gonadotropin-releasing hormone agonists (GnRH-a) and oral contraceptives, have been used in the management of adenomyosis, but the side effects of the treatments and the high recurrence of adenomyosis after cessation of medicinal treatment have limited their effects [Citation3,Citation4]. Recently, high intensity focused ultrasound (HIFU), as a novel non-invasive technique, has received attention in the treatment of adenomyosis. Although HIFU has proven to be safe and effective in the treatment of adenomyosis, symptom relief is only presented in around 80% of the patients [Citation5,Citation6]. Is HIFU combined with medicinal treatment able to achieve a higher symptom relief rate and reduce the recurrence rate? The aim of this study was to compare the treatment effects of the patients treated with HIFU on its own, HIFU combined with LNG-IUS and HIFU combined with GnRH-a.
Materials and methods
This study was approved by the ethics committee at the First Hospital of Shijiazhuang. Informed consent was obtained from every patient before HIFU treatment.
Patients
From January 2013 to January 2015, 78 patients with adenomyosis were enrolled in this study. The average age of these patients was 41.62 ± 4.98 (range: 28–51) years. Dysmenorrhea pain scores ranged from 0 to 10 points; scores for menstrual blood volume ranged from 3 to 5. Among them, 45 patients were treated only with HIFU, 15 patients were treated with HIFU combined with LNG-IUS and 18 patients were treated with HIFU combined with GnRH-a. No patients had high blood pressure, diabetes mellitus, hyperthyroidism, liver diseases or kidney diseases in this study. No significant difference was observed among the three groups in baseline characteristics () (p > 0.05).
Table 1. Baseline characteristics of patients with adenomyosis treated with HIFU, HIFU combined with LNG-IUS or HIFU combined with GnRHa.
Pre-HIFU preparation
Before HIFU treatment, the procedure of HIFU treatment was clearly explained to the patients. Every patient was required to undergo transvaginal ultrasonography and contrast-enhanced magnetic resonance imaging (MRI). The volume of the uterus and the adenomyotic lesion were measured on contrast-enhanced T1WI with a program to contour adenomyotic lesions, which were then calculated using the same program.
Every patient received careful bowel preparation before HIFU treatment. The patients were required to ingest a mixed fluid diet prior to the procedure, fast for 12 h before treatment and undergo a cleansing enema in the morning prior to procedure. In the morning of the procedure, the anterior abdominal wall from the umbilicus to the superior border of the symphysis pubis was shaved. Degreasing and degassing of the skin of the anterior abdominal wall were performed. A urinary catheter was inserted to control bladder volume during treatment. A degassed water balloon was used to push the bowel away from the acoustic pathway to preventing bowel injury.
HIFU treatment
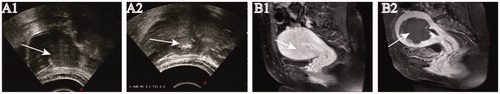
HIFU treatment was performed under conscious sedation using the JC200 focused ultrasound tumor therapeutic system (Chongqing Haifu Medical Technology Co, Ltd, Chongqing, China). The frequency of the transducer operated at 0.8 MHz, with a maximum sonication power of 400 W. Real-time ultrasound imaging was used to detect the adenomyotic lesions and monitor the response to HIFU treatment. Every patient was carefully positioned prone on a HIFU table. Point scan was used to sonicate the lesion. Sonication started from the inferior part to the superior part and from the posterior part to the anterior part of the lesion, with the focal point at least 1.5 cm away from the endometrium and 1 cm from the margin of the adenomyotic lesion. During HIFU treatment, the sonication power was adjusted based on both patient feedback and grayscale changes on ultrasonographic imaging. This process was repeated on a section-by-section basis until the entire target lesion was covered by hyperechoic changes. The comparison of pre-HIFU and post-HIFU ultrasound gray scale level is presented in .
Figure 1. Ultrasonography and MR imaging from a patient with adenomyosis.
A1. Pre-HIFU ultrasonography showed the hypoechoic lesion. A2. Real-time ultrasound imaging showed the significant grayscale change in the treated area. B1. Contrast-enhanced MRI obtained before HIFU showed the enhancement of the diffuse adenomyosis. B2. MRI obtained one day after HIFU showed the nonperfused area of the lesion without damage to the surrounding normal tissue.
Post-HIFU MRI
Contrast-enhanced MRI was performed 1–3 days after HIFU to evaluate the nonperfused volume of the lesions. The volume of the uterus, the adenomyotic lesion and the nonperfused volume were measured on the contrast-enhanced T1WI using a program to contour adenomyotic lesions, which was then calculated using the same program.
LNG-IUS insertion and GnRHa administration
In the patients from the group treated with HIFU following insertion of LNG-IUS, the LNG-IUS was inserted 1 month after HIFU. In the group of patients treated with HIFU combined GnRHa, GnRHa was administered three times. The first GnRHa was given on the first day of the first menstruation after HIFU. The interval between the two injections was 28 days.
Follow-up
The follow-ups were conducted 1, 6 and 12 months after HIFU. The size of the uterus, the lesion volume and symptom relief were evaluated. Ultrasound examination was used to evaluate the changes in blood flow, uterus volume and lesion volume before and after HIFU. The longest diameter (D1), left-right diameter (D2) and anteroposterior diameter (D3) of the uterus were measured. The following formula was used to calculate the uterus volume: V = 0.5233 × D1 × D2 × D3. The volume reduction rate is calculated as follows: Visual analogue scale (VAS) score was used to evaluate the scale of the dysmenorrhea. Pain intensity (0–10 score) was classified by the following standard: no pain (0 points); slight pain (1–3 points); pain that affects sleep (4–6 points) and severe pain that is hard to bear and affects appetite and sleep (7–10 points). Menstrual volume was measured using the number of completely soaked sanitary napkins: less than 5 pieces (1 point); 6–10 pieces (2 points); 10–15 pieces (3 points); 15–20 pieces (4 points) and more than 20 pieces (5 points).
Statistical analysis
SPSS13.0 (SPSS Inc., Chicago, IL, USA) was applied to analyze the result. Normal distribution data was indicated using the format mean ± SD. Abnormal distribution data were reported as a median and interquartile range. One-way ANOVA analysis was applied when comparing variables between the two groups. The Wilcoxon test and chi-square test were applied for the analysis of the quantitative and enumerated data. A p value of less than 0.05 was defined as statistically significant.
Results
Peri-procedure evaluation
As shown in , in HIFU treatment settings, no significant difference was seen among the three groups (p > 0.05). Using similar sonication power and delivering similar sonication energy to the adenomyotic lesions, the NPV ratio (which was defined as the NPV divided by the volume of the target lesion) was 79.27 ± 0.08% in the group treated with HIFU on its own, 84.20 ± 0.12% in the group treated with HIFU combined with LNG-IUS and 78.4 ± 0.11% in the group treated with HIFU combined with GnRHa (p > 0.05).
Table 2. HIFU treatment parameters.
Symptom improvement
shows that the menstruational pain scores decreased significantly 1, 6 and 12 months after HIFU treatment in all the three groups (p < 0.05). We further compared the pain scores at each follow-up point among the three groups and found that there was no significant difference among the three groups 1 month after HIFU. The menstruational pain scores in HIFU combined with LNG-IU and HIFU combined with GnRHa were significantly lower than those of the patients treated with HIFU alone (p < 0.05), but no significant difference was observed between HIFU combined with LNG-IU and HIFU combined with GnRH-a (p < 0.05) 6 and 12 months after HIFU.
Table 3. Evaluation of treatment efficacy in patients with dysmenorrheal.
The menstrual volume scores were also significantly lower 1, 6 and 12 months after HIFU in all the three groups (p < 0.05) (). We further compared the scores of the menstrual volume at each follow-up point among the three groups and found that there was no significant difference among the three groups 1 month after HIFU. The menstrual volume scores in the groups treated with HIFU combined with LNG-IU and HIFU combined with GnRHa were significantly lower than those of patients treated with HIFU alone (p < 0.05), but no significant difference was observed in scores of the menstrual volume between HIFU combined with LNG-IU and HIFU combined with GnRH-a (p < 0.05) 6 and 12 months following HIFU.
Table 4. Evaluation of treatment efficacy in patients with menorrhgea after HIFU treatment.
Changes of the uterine volume after HIFU
showed that the volume of the uterus significantly decreased 1, 6 and 12 months after HIFU in every group. The uterine volume reduction rate 1 month after HIFU treatment was 13.01 ± 05.11% in the group treated with HIFU alone, 13.66 ± 4.05% in the group treated with HIFU combined with LNG-IUS and 14.23 ± 3.48% in the group treated with HIFU combined with GnRHa. The uterine volume decreased by 22.06 ± 9.99%, 27.81 ± 3.78% and 28.03 ± 4.01% 6 months after HIFU in the three groups, respectively. 12 months after HIFU, the reduction rate of uterine volume was 30.42 ± 15.3%, 38.47 ± 3.39% and 39.55 ± 5.53%. We further compared the volume changes of the uterus at each follow-up point among the three groups and found that there was no significant difference among the three groups 1 month following HIFU, but the volume of the uterus 6 and 12 months after HIFU were significantly lower in HIFU combined with LNG-IU and HIFU combined with GnRH-a than that in HIFU alone group (p < 0.05). No significant difference was observed in uterine volume change between HIFU combined with LNG-IU and HIFU combined with GnRH-a (p < 0.05) 6 and 12 months after HIFU.
Table 5. Comparison of the uterine volume reduction rate after HIFU treatment.
The reduction rate of the adenomyotic lesion volume is shown in . The volume of the adenomyotic lesion significantly decreased 1, 6 and 12 months after HIFU in every group. The reduction rate 1 month after HIFU treatment was 25.27 ± 10.40%, 23.81 ± 3.33% and 23.99 ± 2.62% in the groups treated with HIFU alone, HIFU combined with LNG-IUS and HIFU combined with GnRHa, respectively. The uterine volume decreased by 31.72 ± 12.68%, 42.52 ± 19.15% and 37.23 ± 5.31% 6 months after HIFU in the three groups, respectively. At 12 months after HIFU, the reduction rate of uterine volume was 43.96 ± 17.29%, 54.48 ± 4.72% and 53.91 ± 3.66%, respectively, in the three groups. The reduction rate of the adenomyotic lesion volume 6 and 12 months after HIFU in the groups of HIFU combined with LNG-IU and HIFU combined with GnRH-a was significantly lower than that in HIFU group (p < 0.05), but no significant difference was observed between the group treated with HIFU combined with LNG-IU and the group treated with HIFU combined with GnRH-a 6 and 12 months after HIFU (p < 0.05).
Table 6. Comparison of the shrinkage rate of the adenomyotic lesion after HIFU treatment in the three groups.
Pregnancy post-HIFU
Three pregnancies were reported from the patients treated with HIFU alone at 6 months after HIFU. Two of them had an abortion, while the third patient delivered a healthy baby at term. One patient from that group treated with HIFU following with GnRH-a reported a pregnancy and had an abortion. No pregnancy was reported from the group of patients treated with HIFU combined LNG-IUS.
Recurrence
During the 12 month follow-up, recurrence occurred in three patients between 5 and 8 months after HIFU in the group treated with HIFU only. Two of them had hysterectomy, and one patient had a second session of HIFU treatment. No recurrence occurred in the other two groups of patients.
Adverse effects
No HIFU related severe adverse effects such as skin burn, leg pain and hematuresis occurred in this study. Vaginal bleeding occurred in one patient in the group treated with HIFU combined with LNG-IUS. This patient recovered 3 months later.
In the group of patients treated by HIFU combined with GnRH-a, all the patients had amenorrhea during treatment. Normal menstruation recovered within 2 months after drug withdrawal. Also in this group, three patients reported mood changes, hot flashes, night sweats, insomnia and loss of sexual desire during administration of GnRHa.
Discussion
As a non-invasive treatment technique, HIFU has been widely used to treat patients with adenomyosis in China. Over the last few years, many studies have demonstrated that HIFU is a safe and effective treatment for adenomyosis [Citation7,Citation8]. The previous studies also found that around 20% of the patients did not show symptom relief, and the recurrence rate was high [Citation7,Citation9]. Therefore, it is important to explore if HIFU combined with other treatment could achieve a higher symptom relief rate and reduce the recurrence rate.
LNG-IUS is a T type intrauterine device containing progesterone. It works by constantly and slowly releasing progesterone to reduce the menstrual blood volume and the menstruational pain [Citation10,Citation11]. Abou-Setta et al. reported that inserting LNG-IUS after a conservative operation could reduce the recurrence of adenomyosis [Citation12]. In this study, 15 patients used LNG-IUS after the first menstrual cycle post-HIFU.
GnRHa is a gonadotropin-releasing hormone agonist. Previous studies have shown that GnRHa had a remarkable analgesic effect on adenomyosis, and the recurrence rate of adenomyosis can be effectively reduced [Citation13–15]. In this study, a subcutaneous injection of GnRHa was given to the patients after HIFU, once every 28 days for 3 months.
Our results showed that HIFU treatment is effective in treating patients with adenomyosis. In this study, we found that the average menstruation pain score and the menstruation volume were significantly lower 1, 6 and 12 months after HIFU treatment. The volume of the adenomyotic lesion and the volume of uterus were also significantly lower at the same follow-up intervals after HIFU treatment. We further compared the menstruation pain score and the menstruation volume score at different follow-up intervals among the three groups and found that the scores in the groups treated with HIFU combined with LNG-IUS and HIFU combined with GnRHa were significantly lower than those of patients in the group treated with HIFU alone. We also found that the uterine volume reduction rate and the adenomyotic lesion volume reduction rate in the group treated with HIFU combined with LNG-IUS and the patients treated with HIFU combined with GnRHa were significantly higher than those rates of the patients in the group treated with HIFU alone. Our results also showed that the recurrence was only observed in the group treated with HIFU alone. Therefore, LNG-IUS or GnRHa combined with HIFU could improve the long-term results of HIFU treatment in patients with adenomyosis.
In this study, no HIFU-related severe adverse effects occurred. One patient in the group treated with HIFU combined with LNG-IUS reported vaginal bleeding 3 months after inserting the LNG-IUS. This vaginal bleeding could be explained by the side effects of Mirena [Citation16]. The patient recovered 3 months later without any specific treatment. In the group of patients treated with HIFU combined with GnRHa, three of them reported night sweat and emotion change because GnRHa lowers the estrogen level through adjusting the systemic hormones. No other adverse effects were observed. Therefore, HIFU combined with LNG-IUS or GnRHa is safe in the management of adenomyosis.
This study is limited because it is a retrospective study and the non-randomization of patients may cause result bias. Another limitation of this study is that the number of subjects is small and the follow-up time was short. A prospective study with a large number of randomized patients is required.
Conclusions
In summary, our study indicated that the combined treatments are safe and the symptom relief rate and the reduction rate of uterus and adenomyotic lesions seem to be higher than those of the group treated with HIFU alone. The combined treatments can also reduce the recurrence rate more significantly than HIFU on its own can. Between the two groups treated with HIFU combined with LNG-IUS and GnRHa, no significant difference was observed in symptom relief and the uterus and lesion volume reduction. LNG-IUS releases hormone locally, so it has less systemic adverse effects. A prospective study with a large number of randomized patients is required.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus-revisited. Am J Obstet Gynecol. 1972;112:583–593.
- Ascher SM, Arnold LL, Patt RH, et al. Adenomyosis: prospective comparison of MR imaging and transvaginal sonography. Radiology. 1994;190:803–806.
- Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:603–616.
- Kim KA, Yoon SW, Lee C, et al. Short-term results of magnetic resonance imaging-guided focused ultrasound surgery for patients with adenomyosis: symptomatic relief and pain reduction. Fertil Steril. 2011;95:1152–1155.
- Liu X, Wang W, Wang Y, et al. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study. Medicine (Baltimore). 2016;95:e2443.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem. 2015;27:677–681.
- Dong X, Yang Z. High-intensity focused ultrasound ablation of uterine localized adenomyosis. Curr Opin Obstet Gynecol. 2010;22:326–330.
- Fan TY, Zhang L, Chen W, et al. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol. 2012;81:3624–3630.
- Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96:707–714. doi: 10.1111/aogs.13159. Review
- Fong YF, Singh K. Medical treatment of a grossly enlarged adenomyotic uterus with the levonorgestrel-releasing intrauterine system. Contraception. 1999;60:173–175.
- Lindh I, Milsom I. The influence of intrauterine contraception on the prevalence and severity of dysmenorrhea: a longitudinal population study. Hum Reprod. 2013;28:1953–1960.
- Abou-Setta AM, Al-Inany HG, Farquhar CM. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;4: CD005072.
- Silva PD, Perkins HE, Schauberger CW. Live birth after treatment of severe adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril. 1994;61:171–172.
- Huang FJ, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741–744.
- Olive DL. Medical therapy of endometriosis. Semin Reprod Med. 2003;21:209–222.
- Hidalgo M, Bahamondes L, Perrotti M, et al. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years[J]. Contraception. 2002;65:129–132.
