Abstract
Objectives: Passive rise in core body temperature achieved by head-out hot water immersion (HHWI) results in acute increases in serum interleukin (IL)-6 but no change in plasma adrenaline in patients with cervical spinal cord injury (CSCI). The purpose of the present study was to determine the mechanism of heat stress-induced increase in serum IL-6.
Setting: A cross-sectional study.
Methods: The study subjects were nine with CSCI, ten with thoracic and lumbar spinal cord injury (TLSCI) and eight able-bodied (AB) subjects. Time since injury was 16.1 ± 3.4 years in TLSCI and 16.4 ± 4.1 years in CSCI. Subjects were subjected to lower-body heat stress (LBH) by wearing a hot water-perfused suit until 1 °C increase in core temperature. The levels of serum IL-6, plasma adrenaline, tumour necrosis factor (TNF)-α, C-reactive protein (CRP), and counts of blood cells were measured at normothermia and after LBH.
Results: Serum IL-6 concentrations increased significantly immediately after LBH in all the three groups. ΔIL-6% was lower in CSCI subjects compared with AB subjects. Plasma adrenaline concentrations significantly increased after LBH in AB and TLSCI subjects, but did not change throughout the study in CSCI subjects. Cardiac output and heart rate increased at the end of LBH in all three groups.
Conclusions: Under a similar increase in core temperature, ΔIL-6% was lower in the CSCI group compared with the AB group. These findings suggest that the observed rise in IL-6 during hyperthermia is mediated, at least in part, by plasma adrenaline.
Introduction
Individuals with cervical spinal cord injury (CSCI) and spinal cord injury (SCI) are often in a state of chronic low-grade inflammation. The depressed immunity is thought to be due to dysfunctional sympathetic nervous system and associated reduction of adrenergic response [Citation1–3].
Interleukin (IL)-6 is known as an inflammatory cytokine but it also plays important roles in anti-inflammation, immune responses, and metabolic functions [Citation4]. IL-6 reduces the production of tumor necrosis factor (TNF)-α and IL-1β and has local and systemic anti-inflammatory effects [Citation5,Citation6]. Exercise induces the release of IL-6 from skeletal muscle fibers, resulting in increases in plasma concentrations of IL-6 to about 100 times that at rest [Citation7]. Apart from IL-6, various other myokines, which are cytokines secreted from contracting muscles, seem to play important roles in protection against diseases, such as chronic systemic low-grade inflammation [Citation8–11]. The myokine response is governed by various muscle contraction-dependent and sympathetic nervous system-mediated signalling pathways [Citation12]. This may explain the blunted myokine response to exercise in CSCI, given their reduced muscle mass and sympathetic dysfunction [Citation13,Citation14].
Several studies have reported that factors other than exercise, increase IL-6 production from skeletal muscles. For example, hyperthermia [Citation15,Citation16], catecholamine [Citation17,Citation18], hyperglycemia [Citation19], excess lipopolysaccharide [Citation20,Citation21], reactive oxygen species [Citation22], and inflammatory cytokines [Citation23] increase IL-6 release from skeletal muscles. Several recent studies have examined the molecular mechanism of hyperthermia- and catecholamine-induced IL-6 production [Citation24–26]. These studies showed that heat stress increases IL-6 levels in skeletal muscles through the TRPV1, PKC, and CREB signal transduction pathways [Citation24] and that adrenaline may stimulate IL-6 gene transcription of protein kinase Avia β-adrenergic stimulation [Citation25]. Furthermore, in vitro studies demonstrated that heat potentiates epinephrine induced IL-6 gene expression [Citation26]. It is possible that the above mechanisms could increase serum IL-6 levels in SCI patients.
A recent study reported that a passive rise in core temperature caused by head-out hot water immersion (HHWI) is associated with an acute increase in serum IL-6 level in able-bodied (AB) individuals and subjects with CSCI [Citation27]. However, HHWI induces whole body heat stress, i.e., it does not only increase core body temperature but also muscle temperature, which dampened the response of plasma adrenaline concentration and increased cardiac output (CO) in both groups of subjects [Citation27]. Thus, it is not clear which change induced by whole body heat stress is the main factor for the observed increase in serum IL-6 level.
Individuals with SCI have impaired thermoregulatory control due to loss of pseudomotor and vasomotor effectors below the level of spinal cord lesion [Citation28]. Under heat stress, subjects with CSCI have impaired thermoregulation and peripheral blood flow response to prevent rise in core body temperature [Citation28]. Based on the findings of these studies, lower body heat stress may cause a much lower rise in upper peripheral temperature (i.e., muscle) in CSCI subjects, compared with AB, at the same core temperature.
HHWI induced hyperthermia as described previously [Citation27]. Other studies showed that head-out immersion (HOI) causes a cephalad blood shift in normal subjects and increases CO [Citation29,Citation30]. Our group has also reported recently an increase in CO during HOI in CSCI subjects [Citation31], similar to that reported in normal men [Citation31]. While these physiological changes are observed during HOI, it is not clear whether hyperthermia per se is involved in IL-6 elevation.
In this regard, the physiological features of individuals with CSCI are quite different from those with thoracic and lumbar spinal cord injury (TLSCI), because the dysfunctional sympathetic nervous system in CSCI reduces adrenergic responses during lower-body heat stress (LBH). The purpose of the present study was to determine the mechanism of heat stress-induced rise in serum IL-6 during. For this purpose, we compared the effects of LBH induced by perfusion of 50 °C water, on the IL-6 response in AB, TLSCI and CSCI.
Materials and methods
Subjects
The study subjects were all males and included eight AB, ten with TLSCI, and nine with CSCI (). Apart from the spinal cord injury, the participating TLSCI and CSCI subjects were free of other neurological/medical comorbid conditions, as confirmed by thorough clinical examination and motor and sensory system examinations at the time of the study. Furthermore, none of the participating subjects was taking medications that are known to affect the immune or endocrine system. The subjects were informed of the purpose and risks of the study before providing written informed consent. Both the study protocol and consent forms were approved by the human ethics committee of Wakayama Medical University. Furthermore, the protocol was performed in accordance with the Declaration of Helsinki. All subjects refrained from alcohol, caffeine, and exercise for 24 h before the start of the study.
Table 1. Characteristics of participating subjects.
Instrumentation and measurements
Upon arrival to the laboratory, a physician assessed the patient for the location of spinal cord injury level through a review of the medical records and physical examination. The border between the area of sensation and lack of sensation was mapped on the skin using tactile and pressure stimulation as well as temperature stimulation (placement of warm syringe containing 50 °C water on the skin at different locations). A thermocouple was inserted via the nasal passage into the esophagus to a depth equivalent to one-fourth the subject's height, for measurement of core body temperature. The skin temperature was also measured at six sites (chest, upper back, abdomen, lower back, thigh, and lower leg), and the mean skin temperature was estimated using six of these sites, as described in detail by Taylor et al. [Citation32]. The heart rate (HR) was monitored continuously from the electrocardiogram (Nihon Kohden; BSM-2401, Tokyo, Japan), recorded in supine position with limb silver-silver chloride electrodes. Blood pressure was recorded continuously using finger plethysmography (Penaz method, Portapres, Finapres Medical Systems, the Netherlands), which was confirmed occasionally by the cuff method on the arm. Each subject wore a water-perfused tube-lined suit (Med-Eng, Ottawa, Canada) that covered the entire body except the head, face, hands, one forearm, and feet. This suit had separate water inlets for the upper and lower parts of the body and each part was perfused with water of preset temperature. In a series of preliminary studies, we confirmed that the skin and internal temperatures could be easily changed by adjusting the temperature of the water perfusing the suit.
Metabolic variables
Standard respiratory and metabolic data were obtained by using an automatic breath-by-breath respiratory gas analysis system (ARCO200-MET; Arcosystem, Chiba, Japan). CO was estimated by the carbon dioxide (CO2) rebreathing technique, and the CO2 content in the mixed venous blood was estimated using the CO2 rebreathing equilibrium technique [Citation33]. CO was determined using Fick's equation modified to accommodate the CO2 content in mixed venous blood, CO2 output, and arterial CO2 content. The CO2 content in mixed venous blood was estimated using the CO2 re-breathing equilibrium technique, in which a Hans Rudolf valve (model 8200, Hans Rudolf, Shawnee, KS, USA), a re-breathing attachment and an automatic breath-by-breath respiratory gas analysis system were used (ARCO2000-MET, Arcosystem).
Experimental protocol
Following instrumentation, the subject rested quietly in supine position for 30 min while normothermic water (33 °C) circulated through both the upper and lower parts of the suit. In the next step, LBH was induced by perfusing 50 °C water through the lower body part of the suit only, whereas a slightly warm water (36 °C) circulated through the upper body part of the suit. Once the target core temperature increased by 1 °C, the temperature of the water circulating through the suit was lowered to 47 °C in order to limit further increases in core temperature. The time marking the end of LBH was just after the body temperature reached the target of 1 °C increase relative to the baseline. All physiological data related to “increased temperature” were collected at that time point.
Blood samples were collected from the antecubital vein into heparinized tubes and ethylenediaminetetraacetic acid-2K and 2Na containing tubes for the measurements of various parameters: serum concentrations of IL-6 and TNF-α were measured using commercially available chemiluminescent ELISA kits (R&D Systems, Minneapolis, MN), which detect both soluble and receptor-bound IL-6 and TNF-α. All samples were run in duplicates and the average value was calculated. Serum C-reactive protein (CRP) levels were measured by the latex enhanced immunonephelometric assay (Siemens Healthineers, Tokyo). Plasma adrenaline was extracted using alumina and its concentration was measured by high-performance liquid chromatography (Wako Pure Chemical Industries, Osaka, Japan). Plasma cortisol levels were assayed using electro-chemiluminescence immunoassay (Roche, Tokyo). Total blood cell count was determined using a cell counter (MEK-6400; Nihon Kohden, Tokyo). Hematocrit (Hct) was measured by centrifugation.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Differences between groups of age, height, weight, BMI, and LBH time were tested by one-way analysis of variance (ANOVA). For the above parameters, subsequent post hoc tests were performed to determine significant differences among the three groups using the Tukey–Kramer test. Data of blood samples, thermal responses and hemodynamic variables were tested by Kruskal–Wallis test. For these variables, the Dunn's test was used as the subsequent post-hoc test to determine the significance of differences among the three groups. Differences between normothermic and LBH were examined by using the Wilcoxon signed-rank test. Differences were considered statistically significant at p < .05. All statistical analyses were conducted using the Graph Pad Prism 6 software (GraphPad Software Inc., CA).
Results
Anthropometric data
There were no differences among the three groups with respect to age and height. The body weight and body mass index (BMI) of the AB subjects were higher than those of the subjects with CSCI. The spinal lesion was between Th4-L1 in the participating TLSCI subjects and C5-C7 in the CSCI subjects. Time since injury was 16.1 ± 3.4 years in TLSCI and 16.4 ± 4.1 years in CSCI. The actual LBH time was 62.8 ± 4.4 min for AB, 65 ± 5.4 min for TLSCI and 41 ± 3.8 min for CSCI. LBH time was significantly shorter in CSCI than AB and TLSCI ().
Thermal response and hemodynamic variables during normothermia and LBH
There were no significant differences in core and mean skin temperatures among the three groups under normothermia. Furthermore, there were no differences in core temperature among the three groups after LBH. The mean skin temperature at the end of LBH was significantly lower in the CSCI than AB. The core and mean skin temperatures increased significantly immediately after LBH in all subjects of the three groups ().
Table 2. Thermal responses and hemodynamic variables during normothermia and LBH.
There were no differences in mean upper body temperature among the three groups at normothermia. The mean upper body temperature at the end of LBH was significantly lower in the CSCI than AB. The mean lower body temperature at normothermia was significantly lower in the CSCI than AB. Furthermore, there were no differences in mean lower body temperature after LBH among the three groups. The mean upper and lower body temperatures increased significantly immediately after LBH in all subjects of the three groups ().
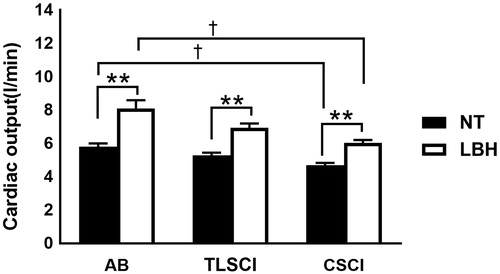
CO and HR increased at the end of LBH in the three groups, whereas no changes were observed in stroke volume. CO and HR at normothermia and end of LBH were significantly lower in CSCI than AB (, ). There were no differences in mean blood pressure under normothermia and after LBH among the three groups. The mean blood pressure remained constant throughout the study in the three groups ().
Changes in serum IL-6, TNF-α and CRP levels
There were no significant differences in serum IL-6 concentrations among the three groups under normothermia (AB, 1.7 ± 1.0 pg/ml; TLSCI, 1.5 ± 0.3 pg/ml; CSCI, 2.3 ± 0.8 pg/ml) and after LBH (AB, 2.8 ± 1.3 pg/ml; TLSCI, 2.0 ± 0.3 pg/ml; CSCI, 2.6 ± 0.8 pg/ml). In all three groups, serum IL-6 concentrations significantly increased immediately after LBH (. ΔIL-6% was lower in CSCI than AB subjects. There were no significant differences in ΔIL-6% between the AB and TLSCI (AB, 91.0 ± 21.4%; TLSCI, 38.1 ± 9.9%; CSCI, 22.1 ± 7.9%) (. In contrast, serum TNF-α levels remained constant throughout the study in the three groups. CRP was significantly after LBH in AB, compared with normothermia. On the other hand, CRP did not change throughout the study in TLSCI and CSCI. There were no significant differences in CRP among the three groups at normothermia and after LBH ().
Figure 1. Serum IL-6 level during LBH in AB TLSCI and CSCI. Data are mean ± SEM. *p < .05, **p < .01, relative to normothermia. AB: able-bodied; TLSCI: thoracic and lumbar spinal cord injury; CSCI: cervical spinal cord injury; NT: normothermia; LBH: lower body heat stress.
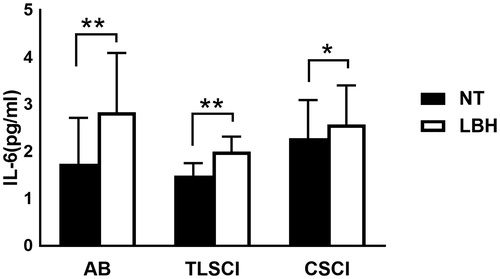
Figure 2. ΔIL-6 in AB TLSCI and CSCI. Data are mean ± SEM. †p < .05, compared with AB; by the post hoc test. AB: able-bodied; TLSCI: thoracic and lumbar spinal cord injury; CSCI: cervical spinal cord injury; NT: normothermia; LBH: lower body heat stress.
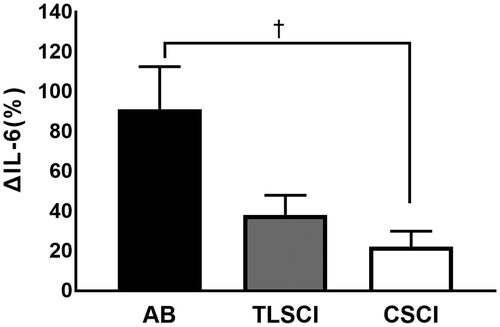
Table 3. Changes in blood cell count, haematocrit, CRP and TNF-α during lower body heat stress.
Changes in plasma adrenaline and cortisol levels
Plasma adrenaline levels at normothermia and end of LBH were significantly lower in CSCI than in AB subjects. Furthermore, plasma adrenaline concentrations were significantly higher after LBH in AB and TLSCI subjects, but did not change throughout the study in subjects with CSCI (normothermia: AB, 42.4 ± 5.7 pg/ml; TLSCI, 20.3 ± 4.6 pg/ml; CSCI, 8.4 ± 1.3 pg/ml; LBH: AB, 66.8 ± 12.1 pg/ml; TLSCI, 31.0 ± 7.3 pg/ml; CSCI, 8.2 ± 1.3 pg/ml) (. Serum cortisol concentrations decreased significantly after LBH in CSCI subjects, compared to normothermia. Serum cortisol levels remained stable throughout the study in both AB and TLSCI subjects ().
Figure 3. Plasma adrenaline levels during LBH in AB TLSCI and CSCI. Data are mean ± SEM. †p < 0.05, compared with AB; *p < .05, compared with normothermia; by the post hoc test. AB: able-bodied; TLSCI: thoracic and lumbar spinal cord injury; CSCI: cervical spinal cord injury; NT: normothermia; LBH: lower body heat stress.
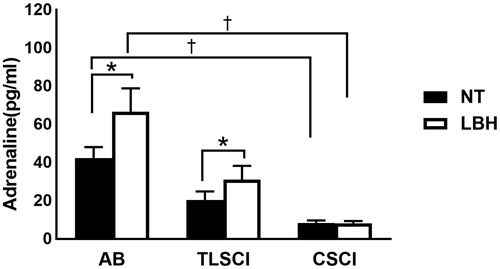
Figure 4. Cardiac output during LBH in AB SCI and CSCI. Data are mean ± SEM. †p < .05, compared with AB; **p < .01, compared with normothermia; by the post hoc test. AB: able-bodied; TLSCI: thoracic and lumbar spinal cord injury; CSCI: cervical spinal cord injury; NT: normothermia; LBH: lower body heat stress.
Changes in blood cell counts
At the end of LBH, Hct values were significantly lower in TLSCI and CSCI compared with AB subjects. Hct significantly increased after LBH in AB, compared with normothermia. On the other hand, Hct remained constant throughout the study in TLSCI and CSCI. In all three groups, leukocyte count increased significantly immediately after LBH. The monocyte count increased significantly after LBH in AB, compared with normothermia. On the other hand, the monocyte count did not change throughout the study in TLSCI and CSCI. There were no significant differences in monocyte count among the three groups at normothermia and after LBH ().
Discussion
This is the first study to compare the acute cytokine response induced by LBH, representing heat stress at paresis area, among AB, TLSCI and CSCI subjects. The major findings of the present study were: (1) serum IL-6 concentrations increased significantly immediately after LBH in both TLSCI and CSCI, (2) the increase in serum IL-6 levels after LBH in CSCI subjects was lower than that of the AB subjects, (3) the mean skin temperature and mean upper body temperature at the end of LBH were significantly lower in CSCI than AB subjects, and (4) plasma adrenaline concentrations increased significantly after LBH in both AB and TLSCI subjects, but not CSCI subjects.
Serum IL-6 concentrations immediately after LBH increased significantly in both TLSCI and CSCI. In previous studies, HHWI was used as the hyperthermic stimulus to examine the IL-6 response [Citation27]; however, the present study was designed to limit heat exposure to the lower body area only. Therefore, it seems that the LBH protocol used in the present study increased core and muscle temperatures over the critical threshold to mount an adequate response. However, despite the similar increase in core temperature in the three groups, ΔIL-6% was lower in CSCI subjects compared with AB subjects. This result suggests that core temperature is not the only factor involved in the observed increase in serum IL-6.
Our study also showed that ΔIL-6% was lower in CSCI than AB subjects. Furthermore, the mean skin and upper body temperatures at the end of LBH were significantly lower in CSCI than AB. When body temperature rises in humans, body heat balance is normally restored by increased blood flow to the skin and by sweating. Regulation of core temperature is achieved through behavioral and autonomic mechanisms that actively balance heat production and heat loss. These mechanisms are largely controlled by the hypothalamus and depend on inputs from afferent neurons from various sites within the body [Citation34]. Peripheral vasodilatation occurs under warmer conditions, which increases blood flow to the periphery and exacerbates heat loss. Neural reflex control of cutaneous blood flow is mediated by two populations of sympathetic nerves: the adrenergic vasoconstrictor system and a less well-understood sympathetic vasodilator system [Citation35]. However, both systems are disturbed in paresis lesion of CSCI subjects, therefore the mean upper skin temperature in CSCI was lower compared with AB in this LBH study and resulted in lower mean skin temperature. This finding suggests that muscle and adipose tissue temperatures in CSCI were lower than AB and helped attenuate IL-6 production. In addition, the mean skin temperature and mean upper temperature at the end of LBH were not different between TLSCI and AB. This suggests that muscle and adipose tissue temperatures during LBH were similar in TLSCI and AB and synergistically enhanced IL-6 production.
The present study showed that ΔIL-6% was lower in CSCI subjects than AB subjects. All patients with CSCI have sympathetic nervous system impairment and thereby reduced circulating adrenaline concentrations, compared with AB subjects [Citation36]. Adrenaline may stimulate IL-6 gene transcription of protein kinase Avia β-adrenergic stimulation [Citation25], and previous in vitro studies demonstrated that heat potentiates epinephrine induced IL-6 gene expression [Citation26]. Our results demonstrated no changes in plasma adrenaline levels throughout the study in the CSCI group. In contrast, significant increases in plasma adrenaline concentrations were noted after LBH in AB and TLSCI subjects. These findings suggest that adrenaline may play a role in the observed increase in serum IL-6 levels during hyperthermia.
The mean skin and mean upper body temperatures at the end of LBH were similar in TLSCI and AB subjects. This finding suggests that muscle and adipose tissue temperatures in TLSCI were similar to those of AB and probably enhanced IL-6 production during LBH.
Previous studies reported an increase in CO during HOI in CSCI subjects, similar to normal men [Citation31] and that HOI induced activation of prostaglandin E and the renin–angiotensin–aldosterone system [Citation37,Citation38]. Leicht et al. [Citation27] applied HHWI using hot water and reported an increase in serum IL-6 following 2 °C increase in core body temperature. They concluded that HOI probably induced a shift in the blood and activation of the endocrine system. The present study used LBH to increase core temperature and showed that LBH induced IL-6 production in CSCI, TLSCI and AB without hydrostatic pressure-induced blood shift to the intrathoracic space. However, hyperthermia per se affects the cardiovascular system. In normal subjects, Nakamitsu et al. [Citation39] reported that an increase in core temperature to only 0.5 °C above resting temperature increased CO by approximately 80%. In the present study, CO increased by less than 30% in spite of 1 °C increase in core temperature during LBH. Therefore, we conclude that LBH used in this study has a drastically reduced effect on CO than HOI in hot water. In line with in vitro studies [Citation16], it appears that the increase in IL-6 in subjects with TLSCI and CSCI is related, at least in part, to the rise in core temperature, which is associated by only a slight increase in CO.
In all three groups of subjects, the leukocyte count increased significantly immediately after LBH. In AB subjects, the monocyte count increased significantly after LBH compared with normothermia, whereas it did not change throughout the study in TLSCI and CSCI subjects. Previous studies have demonstrated that human HSP60 and inducible HSP72 induce the release of proinflammatory cytokines from human monocytes [Citation40–42]. TNF is a multifunctional cytokine produced by activated monocytes and macrophages as well other cell types [Citation43–45]. However, serum TNF-α levels remained constant throughout the present study in the AB group. The results of our study exclude any role for monocytes in the observed increase in serum IL-6 during LBH.
Perspectives and study limitations
Recent studies described the effects of exercise on other anti-inflammatory cytokines, such as IL-1ra, IL-8, IL-10, and IL-15 [Citation46]. However, we could not measure the levels of these anti-inflammatory cytokines in the present study. One of the reasons was the importance of IL-6 as a muscle-derived cytokine. Another reason was that we were not allowed by the ethics committee to collect a large quantity of blood samples from the subjects, for fear that such intervention could be an infringement to participation [Citation7].
Previous studies indicated that exercise-induced increase in IL-6 is related to the duration of the exercise [Citation46]. The present results showed no differences in core temperature among the three groups after LBH. However, LBH time was significantly shorter in CSCI than AB and TLSCI. This may explain why the decrease in Δ IL-6 observed in CSCI.
Conclusions
The present study showed significant changes in serum IL-6 with 1 °C increase in core body temperature induced by heating of the lower body area. Although the increase in core temperature was similar in all three groups, ΔIL-6% was lower in CSCI subjects compared with AB subjects. The results of this study suggest that the rise in serum IL-6 during hyperthermia may involve peripheral temperature (i.e., muscle) and adrenaline.
Acknowledgements
We thank Mr. Takashi Moriki, Tokio Kinoshita, Toshihito Mitsui, and Masaki Ogawa for the excellent technical help, and Mr. Takumi Ooko, Daisuke Kozima and Yoshinori Yasuoka for conducting the assay. We also thank the clinical assistance of Drs. Kazuya Ishida and Tomoyuki Ito. We also thank Dr. Faiq G. Issa for the careful reading and editing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yamanaka M, Furusawa K, Sugiyama H, et al. Impaired immune response to voluntary arm-crank ergometer exercise in patients with cervical spinal cord injury. Spinal Cord. 2010;48:734–739.
- Wang TD, Wang YH, Huang TS, et al. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc. 2007;106:919–928.
- Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46:466–476.
- Pedersen BK. Muscle as a secretory organ. Compr Physiol. 2013;3:1337–1362.
- Schindler R, Mancilla J, Endres S, et al. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–47.
- Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320.
- Fischer CP. Interleukin-6 in acute exercise and training: What is the biological relevance?. Exerc Immunol Rev. 2006;12:6–33.
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212.
- Matthys P, Mitera T, Heremans H, et al. Anti-gamma interferon and antiinterleukin-6 antibodies affect staphylococcal enterotoxin B-induced weight loss, hypoglycemia, cytokine release in D-galactosamine-sensitized and unsensitized mice. Infect Immun. 1995;63:1158–1164.
- Mizuhara H, O’Neill E, Seki N, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin-6. J Exp Med. 1994;179:1529–1537.
- Starkie R, Ostrowski SR, Jauffred S, et al. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. Faseb J. 2003;17:884–886.
- Welc SS, Clanton TL. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol. 2013;98:359–371.
- Kouda K, Furusawa K, Sugiyama H, et al. Does 20-min arm crank ergometer exercise increase plasma interleukin-6 in individuals with cervical spinal cord injury? Eur J Appl Physiol. 2012;112:597–604.
- Paulson TAW, Goosey-Tolfrey VL, Lenton JP, et al. Spinal cord injury level and the circulating cytokine response to strenuous exercise. Med Sci Sports Exerc. 2013;45:1649–1655.
- Welc SS, Judge AR, Clanton TL. Skeletal muscle interleukin-6 regulation in hyperthermia. Am J Physiol Cell Physiol. 2013; 305:C406–C413.
- Welc SS, Phillips NA, Oca-Cossio J, et al. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Cell Physiol. 2012;303:C455–C466.
- Batistaki C, Kostopanagiotou G, Myrianthefs P, et al. Effect of exogenous catecholamines on tumor necrosis factor α, interleukin-6, interleukin-10 and β-endorphin levels following severe trauma. Vascul Pharmacol. 2008;48:85–91.
- Parrado AC, Canellada A, Gentile T, et al. Dopamine agonists upregulate IL-6 and IL-8 production in human keratinocytes. Neuroimmunomodulation. 2012;19:359–366.
- Bernal-Lopez MR, Llorente-Cortes V, Calleja F, et al. Effect of different degrees of impaired glucose metabolism on the expression of inflammatory markers in monocytes of patients with atherosclerosis. Acta Diabetol. 2013;50:553–562.
- Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R698–R709.
- Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: Role of the Jun NH2-terminal kinase. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1153–R1164.
- Kosmidou I, Vassilakopoulos T, Xagorari A, et al. Production of interleukin-6 by skeletal myotubes: Role of reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26:587–593.
- Luo G, Hershko DD, Robb BW, et al. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-k B. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1249–R1254.
- Obi S, Nakajima T, Hasegawa T, et al. Heat induces interleukin-6 in skeletal muscle cells via TRPV1/PKC/CREB Pathways. J Appl Physiol. 2017;122:683–694.
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ?. Exerc Sport Sci Rev. 2005;33:114–119.
- Welc SS, Morse DA, Mattingly AJ, et al. The impact of hyperthermia on receptor-mediated interleukin-6 regulation in mouse skeletal muscle. PLoS One. 2016;11:1–22.
- Leicht CA, Kouda K, Umemoto Y, et al. Hot water immersion induces an acute cytokine response in cervical spinal cord injury. Eur J Appl Physiol. 2015;115:2243–2252.
- Guttmann BY, Silver J, Wyndham CH. Thermoregulation in spinal man. J Physiol (Lond).). 1958;142:406–419.
- Epstein M. Renal effects of head-out water immersion in man: implications for an understanding of volume homeostasis. Physiol Rev. 1978;58:529–581.
- Tajima F, Sagawa S, Iwamoto K, et al. Renal and endocrine responses in the elderly during head-out water immersion. Am J Physiol. 1988;254:R977–R983.
- Tajima F, Sagawa S, Iwamoto K, et al. Cardiovascular, renal, and endocrine responses in male quadriplegics during head-out water immersion. Am J Physiol. 1990;258:R1424–R1430.
- Taylor WF, Johnson JM, Kosiba WA, et al. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592.
- Jones KR. A respiration monitor for use with CT body scanning and other imaging techniques. Br J Radiol. 1982;55:530–533.
- Insler SR, Sessler DI. Perioperative Thermoregulation and Temperature Monitoring. Anesthesiology Clin. 2006;24:823–837.
- Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–612.
- Kjaer M, Pollack SF, Mohr T, et al. Regulation of glucose turnover and hormonal responses during electrical cycling in tetraplegic humans. Am J Physiol. 1996;271:R191–R199.
- Epstein M, Loutzenhiser R, Friedland E, et al. Relationship between renal prostaglandin E and renal sodium handling during immersion induced central hypervolemia in normal humans. J Clin Invest. 1987;79:738–745.
- Epstein M, Preston S, Weitzman R. Suppression of plasma rennin and plasma aldosterone during water immersion in normal man. J Clin Endocrinol Metab. 1975;41:618–625.
- Nakamitsu S, Sagawa S, Miki K. Effect of water temperature on diuresis-natriuresis: AVP, ANP, and urodilatin during immersion in men. J Appl Physiol. 1994;77:1919–1925.
- Chen W, Syldath U, Bellmann K, et al. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219.
- Kol A, Lichtman AH, Finberg RW, et al. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17.
- Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442.
- Old LJ. Tumor necrosis factor (TNF). Science. 1985;230:630–632.
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452.
- Vandenabeele P, Declercq W, Beyaert R, et al. Two tumour necrosis factor receptors: Structure and function. Trends Cell Biol. 1995;5:392–539.
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406.
