Abstract
Background: High-intensity focused ultrasound (HIFU) is a noninvasive thermodestructive procedure targeting internal organs with concentrated sonification energy that may cause pain. We aimed to compare the effectiveness of epidural analgesia (EA) and monitored anesthesia care (MAC) in HIFU treatment of uterine adenomyosis.
Materials and Methods: Sixty-eight patients were included in this case-control study. Thirty-seven patients underwent MAC; 31 patients underwent fluoroscope-guided epidural analgesia. The primary outcome was a frequency of patients reporting severe or very severe intraoperative pain. Secondary outcomes were differences in dosages of analgesics, ablation ratio, and other clinical factors.
Results: The EA group reported a significantly lower frequency of severe or very severe intraoperative pain than did the MAC group (41.9% vs. 75.7%; p = .006). Consumption of remifentanil during treatment was significantly lower in the EA group (173 ± 189 µg vs. 426 ± 380 µg; p = .001), as was the use of fentanyl in the recovery room (52 ± 38 µg vs. 75 ± 44 µg; p = .030). Multivariable analysis revealed EA to be the largest contributing factor to increased nonperfused volume ratio (B = 0.41; 95% confidence interval = 0.29 to 0.53; p < .001). The frequency of thermal injury after HIFU was significantly lower in the EA group (22.6% vs. 54.1%; p = .008).
Conclusions: EA during HIFU treatment of uterine adenomyosis improved quality of pain control and ablation ratio over MAC without increasing risk of treatment-related complications. EA also reduced consumption of opioid analgesics during and after HIFU treatment.
Introduction
High-intensity focused ultrasound (HIFU) treatment is a noninvasive, radiation-free transcutaneous or transrectal thermodestructive procedure targeting internal tumors and lesions with concentrated ultrasound wave energy [Citation1]. HIFU treatment has several advantages for indicative cancer patients; it is less invasive than are surgical procedures, thereby permitting one-day intervention, and it avoids adverse reactions caused by chemotherapy or radiation therapy [Citation2]. Since the concept of HIFU was first described in the early 1940s, ultrasound-guided HIFU (USgHIFU) and magnetic resonance-guided HIFU (MRgHIFU) have been widely used to focus thermal energy on target structures over the last decades [Citation1]. MRgHIFU has several benefits, including precise identification of the target, temperature measurement using a magnetic resonance (MR) thermometer, and evaluation of the treatment process in real time [Citation3,Citation4]. MRgHIFU or MR-combined USgHIFU have been clinically approved for treatment of uterine fibroids [Citation5], prostate cancer [Citation6], bone metastases [Citation7], and essential tremor [Citation8], and they are under investigation for treating various type of cancers [Citation9,Citation10] and brain lesions [Citation11].
HIFU treatment is usually painful because of localized tissue heating, and it is time-consuming. Parenteral opioid analgesia is required during the procedure and may be continued into the postprocedural period as required. Therefore, an appropriate analgesic plan is imperative for patient tolerability and precise target ablation during the procedure, and for perioperative pain control [Citation12]. Use of HIFU for treatment of intraabdominal organs such as the liver, pancreas, and kidney requires general anesthesia or spinal anesthesia to control both visceral and somatic pain; ablation of uterine adenomyosis is less invasive, so monitored anesthesia care (MAC) or sedation have been utilized [Citation12,Citation13]. However, it is sometimes difficult to maintain a balance between pain control and spontaneous respiration during sedation. Moreover, patients under sedation may show disinhibited behavior or loss of cooperation, which could result in serious complications during HIFU treatment [Citation14]. Inability to self-report serious discomfort during deep sedation is another concern with HIFU.
Epidural analgesia (EA) is one of the best analgesic modalities for several types of surgery [Citation15,Citation16]. It facilitates early recovery by reducing use of general anesthetics, stabilizes perioperative vital signs, and reduces postoperative complications [Citation17]. For major abdominal surgery, thoracic EA provides better pain relief, mental status, and bowel activity than does intravenous analgesia [Citation18]. Although there may be individual variability in epidural spreading pattern of the injectates [Citation19], EA at the low thoracic spine provides consistent blockade of both somatic and visceral pain without risk of unintentional blockade of the sciatic nerve [Citation20]. To the best of our knowledge, however, the safety and effectiveness of EA in HIFU treatment have not been examined.
In this study, EA was introduced in the middle of the study period following the radiologist’s request for improved perioperative pain management in ablation of uterine adenomyosis. We hypothesized that EA would provide effective pain control during and after HIFU treatment, possibly resulting in lower pain intensity during HIFU treatment than that observed with intravenous injection of analgesics. Therefore, the aim of our study was to investigate the effectiveness of fluoroscopy-guided, sciatic nerve-sparing EA, followed by patient-controlled EA (PCEA) for HIFU treatment of uterine adenomyosis.
Materials and methods
Patients
This retrospective case-control study was approved by the Institutional Review Board of Seoul National University Hospital (No. 1802–115–924). In this study, we reviewed medical charts of 70 patients who participated in a prospective clinical trial of a USgHIFU system (ALPIUS 900, Alpinion Medical Systems Co., Ltd. Seoul, South Korea) combined with magnetic resonance imaging (MRI) to treat adenomyosis. MAC was supplied by a nurse anesthetist under supervision of the attending radiologist using intravenous opioids and benzodiazepine for the first 39 participants between 14 January 2016, and 8 December 2016 (MAC group). Participants 40–70 received EA combined with MAC during the procedure, followed by PCEA between 29 December 2016, and 31 August 2017 (EA group). Inclusion and exclusion criteria for HIFU treatment of uterine adenomyosis in the original prospective study are described in . In this case-control study, we excluded subjects who did not complete the prospective HIFU study and participants who underwent the HIFU treatment with an aberrant analgesic protocol.
Table 1. Inclusion and exclusion criteria for HIFU treatment of adenomyosis.
Among 70 participants who underwent the HIFU study, two patients were excluded: one for withdrawing consent and one for a deviant epidural analgesic protocol. Therefore, 68 patients were included in this study. The EA group consisted of 31 patients (screening numbers 40–70), while the MAC group consisted of 37 patients (screening numbers 1–39).
Epidural catheterization in the EA group
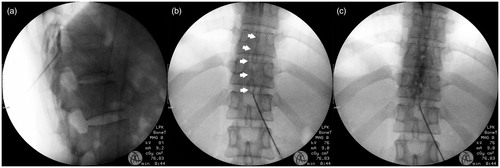
After obtaining written consent from each patient, a venous route was accessed, and Hartmann’s solution was administered at a rate of 100 ml/h, or as appropriate. During the procedure and postoperative observation in the recovery room, real-time 4-limb lead electrocardiogram, pulse oximetry, and automated noninvasive blood pressure monitoring were performed continuously. The patient was placed on a fluoroscopy table in a prone position. A cushion was placed beneath the patient’s abdomen to maintain neutral lumbar curvature. All procedures were performed by two anesthesiologists with over 6 years of experience. The skin was sterilized with betadine solution and then draped. Using a C-arm fluoroscope, the T11–12 interlaminar space was identified. After local infiltration with 1% lidocaine, a 17-gauge Tuohy needle (Perican®, B. Braun Melsungen AG, Germany) was advanced from the entry point on the L1 upper pedicle into the interlaminar space at a 45-degree angle against the skin. The epidural space was detected using a loss-of-resistance technique and confirmed with iopamidol contrast medum (Iopamiro 300; Bracco, Milan, Italy). Next, an 18-gauge closed-end epidural catheter with three lateral orifices (Portex; Smiths Medical, Hranice, Czech) combined with a custom metal guidewire was advanced through the epidural needle, and the tip was placed in the sagittal midline and the upper endplate of the T10 vertebral body. After the guidewire was withdrawn, 3 ml of contrast medium was administered to confirm the spread of injectate into the bilateral T9–12 epidural space and to exclude subdural spreading or vascular uptake (). Subcutaneous tunneling was then performed to prevent bacterial infection of the epidural catheter. Afterward, the patient was moved to the HIFU intervention room.
Figure 1. Serial images of epidural catheterisation. The tip of a 17G Tuohy needle was in the epidural space (a), epidural catheter with curved steel wire (white arrows) was advanced cephalad (b), and 3 ml of contrast media was injected (c). There is no evidence of vascular uptake or subdural spreading pattern.
Analgesic care during and after the HIFU treatment
Routine monitoring, including electrocardiography, noninvasive arterial pressure monitoring, pulse oximetry, and capnography, was performed in all patients. Mean arterial pressure, respiratory rate, and heart rate were measured every 3 min; peripheral oxygen saturation (SpO2) and end-tidal CO2 concentration (ETCO2) were monitored continuously. Oxygen was supplied at 5 L/min via a facemask. No premedication was administered to either group.
In the MAC group, intravenous remifentanil was administered with target-controlled infusion to a target site concentration of 3 ng/mL (Injectomat TIVA Agilia; Fresenius Kabi GmbH, Graz, Austria) in accordance with patient discomfort. In the EA group, 6 ml of 0.5% ropivacaine was administered epidurally prior to HIFU treatment to achieve spinal sensory block at the level of T9–12. Preservation of sensation at the lumbar and lumbosacral plexus was assessed with alcohol swab in both lower extremities and if muscle power in both hip flexors was assessed as ≥ grade 4 (movement against external resistance with less strength than usual) or 5 (normal strength) [Citation21]. An additional 4 ml of ropivacaine was administered every 1 h if the procedure took more than 2 h. When the patient complained of insufficient analgesia despite epidural sensory block, intravenous remifentanil was administered with target-controlled infusion to a target site concentration of 3 ng/mL. Intravenous midazolam was administered with a bolus dose of 1–2 mg when patients in either group asked to be sedated because of pain or anxiety. At the end of HIFU treatment, fentanyl 20 µg was injected in the operating room if the patient complained of moderate to severe pain.
After immediate postoperative MRI for approximately 45 min, all patients were observed in the recovery room for at least 60 min. If a patient still had pain of more than moderate severity, fentanyl was incrementally injected up to 100 µg in accordance with the patient’s discomfort. In the EA group, a PCEA device (Automed 3200; Ace Medical Co. Ltd., Seoul, South Korea) was connected using 0.125% ropivacaine 4 ml/h (bolus, 2 ml; lockout time, 30 min; and total volume, 250 ml) via the epidural catheter. After confirming stable vital signs and alert mental status without acute severe complications at 1 h in the recovery room, participants who exhibited a satisfactory modified Aldrete score [Citation22] ≥9 were discharged.
Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and other combination analgesics (except for strong opioids) were allowed as rescue analgesics in both groups. In the EA group, participants went home with their PCEA device and were instructed to use bolus delivery prior to taking oral analgesics when they required additional analgesia. Patients revisited the hospital to remove their epidural catheters at 3 d (i.e., if the HIFU treatment was performed on a Thursday, removal of the epidural catheter was performed on Saturday; if the HIFU treatment was conducted on Friday, the epidural catheter was removed the following Monday because the clinic was closed on Sunday).
Outcomes and safety
The primary outcome was the frequency of patients reporting severe or very severe intraoperative pain (4 or 5) as evaluated on a self-reported 6-point scale ranging from 0 (no pain) to 5 (very severe pain) at discharge on the day of the procedure.
Secondary outcomes included differences in dosages of intraoperative remifentanil and postprocedural fentanyl between the groups. The nonperfused volume (NPV) ratio was evaluated to assess the effectiveness of the HIFU procedure; NPV is a ratio of the volume of ablated adenomyosis to the initial volume on contrast-enhanced T1-weighted MR imaging [Citation23,Citation24]. The NPV is considered a predictor of long-term symptom relief, and ratio close to 1 suggests near complete ablation of the target lesion. Other clinical factors, including procedure time, abdominal wall thickness, volume of adenomyosis, scarring in the lower abdomen, and location of adenomyosis, were also investigated [Citation25]. The enhancement type of adenomyosis on T1-weighted imaging and the location of adenomyosis in the uterus were not considered because of their homogenous characteristics. Postprocedural pain intensity was assessed for 5 d over the phone, using a 4-point Likert scale ranging from 0 (no pain) to 3 (severe pain) as follows: 0 (none), no discomfort; 1 (mild), minimal discomfort without limitation of daily activity; 2 (moderate) discomfort with significant limitation of daily activity; and 3 (severe) significant discomfort caused impossibility of daily activity [Citation26]. In addition, the use of rescue analgesics was evaluated.
For the safety assessment, all adverse events during and immediately following the HIFU treatment were observed, including nausea/vomiting, tingling sensation in the lower extremities, voiding difficulty, itching sensation, dizziness, headache, shivering, and abnormal blood pressure. In addition, intraprocedural and postprocedural usages of medications to deal with those adverse events were monitored. Thermal injury to adjacent structures or any complications possibly related to the HIFU or EA procedures were evaluated using a 4-point Likert scale with values ranging from 0 to 3 as reported in telephone interviews over a period of 5 d. Routine follow-up of all participants was conducted at 1-month and 3-month visits.
Statistical analysis
Depending on the data distribution, independent t-tests or Wilcoxon rank-sum tests were performed to compare two independent groups. Pearson’s χ2 test or Fischer’s exact test was used to analyze categorical or ordinal data. For continuous variables, the normality distribution was assessed with the Kolmogorov-Smirnov test, and an independent t-test was used to compare the normally distributed variables. Mann–Whitney U test was used to compare continuous variables that deviated from a normal distribution. Multivariable linear regression analysis with stepwise selection was conducted to assess the impact of EA and other covariates on the NPV ratio to determine the effectiveness of the HIFU procedure. Clinical factors with p values < .2 in univariable analysis were considered in the regression model. Statistical analyses were performed using SPSS version 23 for Windows (IBM Corp., Armonk, NY, USA). Data are reported as mean ± standard deviation, median [first interquartile, third interquartile], or number (%) of patients. All p values presented are two-tailed, and p values < .05 were considered to indicate statistical significance.
Results
Patient characteristics
Demographic characteristics and procedure-related variables in both groups are presented in . There were no differences in demographics or in preexisting conditions associated with the NPV ratio, including abdominal wall thickness, location of adenomyosis, or volume of adenomyosis, with the exception of preexisting postoperative scarring in the lower abdomen (p = .014).
Table 2. Demographic and clinical data of the study population.
Effectiveness of EA
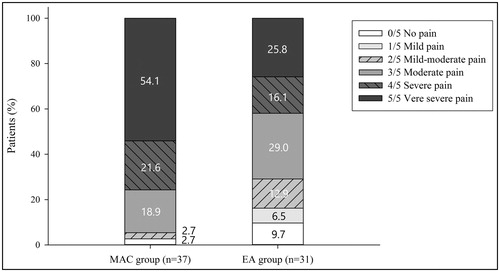
The frequency of patients reporting severe or very severe intraoperative pain—the primary outcome—was significantly lower in the EA group than in the MAC group [41.9% (n = 13) vs. 75.7% (n = 28); p = .006]. Patient’s reports of pain levels are detailed in . In addition, the percentage of patients suffering from moderate pain or more severe pain was also significantly lower in the EA group than in the MAC group [71.0% (n = 22) versus 94.6% (n = 35); p = .017].
Figure 2. The proportion of intraprocedural pain intensity during HIFU treatment in the MAC group (n = 37) and the EA group (n = 31). The pain intensity was reported using a 6-point Likert scale (0, no pain; 1, mild pain; 2, mild-moderate pain; 3, moderate pain; 4, severe pain; 5, very severe pain).
In terms of secondary outcomes, the total dosages of intraoperative remifentanil and postoperative fentanyl administered were significantly lower in than EA group than in the MAC group. Consumption of remifentanil was significantly lower in the EA group than in the MAC group during treatment (173 ± 189 µg versus 426 ± 380 µg; p = .001), and use of fentanyl in the recovery room was significantly lower in the EA group than in the MAC group (52 ± 38 µg versus 75 ± 44 µg; p = .030) (). There were no patients in the EA group who needed midazolam; conversely, six patients (16.2%) in the MAC group required midazolam injection (p = .028). Nonetheless, the NPV ratio was significantly higher in the EA group than in the MAC group (0.87 ± 0.18 versus 0.43 ± 0.32; p < .001). The incidence of any postprocedural pain or discomfort as reported at a 5-d follow-up phone call was much lower in the EA group than in the MAC group [38.7% (n = 12) versus 64.9% (n = 24); p = .031). The percentage of patients who took rescue analgesics such as acetaminophen, tramadol, NSAIDs, and/or codeine was lower in the EA group than in the MAC group but was not statistically significant (19.4% versus 35.1%; p = .149).
Table 3. Clinical outcomes of epidural analgesia and patient-controlled epidural analgesia for HIFU treatment.
Multivariable linear regression analysis was used to assess the correlation between clinical variables and the NPV ratio. EA was found to contribute to an improvement in NPV ratio [B = 0.41; 95% confidence interval (CI) = 0.29 to 0.53; p < .001] (). Conversely, the anteroposterior location of adenomyosis decreased the NPV ratio (B = −0.22; 95% CI = −0.38 to −0.07; p = .006) in comparison with the anterior location of adenomyosis. The NPV ratio increased by 0.08 with each 100 cm2 of adenomyosis volume increase (95% CI = 0.03 to 0.14; p = .002).
Table 4. Multivariable analysis between the NPV ratio and Clinical Variables.
Safety profile
Pre-, intra-, and postoperative adverse events are presented in . Tingling sensation in the lower extremities was reported by only one-third of the EA group (10/31) after the bolus injection of local anesthetics into the epidural space. All occurrences were limited to the anterior thighs, without any sensory blockade. No new intraoperative tingling sensation was reported in the EA group, whereas three patients (8.1%) in the MAC group reported it. Among those who complained of tingling sensations, two patients in each group were discharged with improved but lingering symptoms that completely resolved within a few days. The frequency of intraoperative nausea/vomiting during HIFU treatment was not different between groups (29.0% versus 37.8%; p = .445); however, administration of antiemetics such as dexamethasone during the HIFU treatment and 5-HT3 antagonists in the recovery room was more frequent in the MAC group than in the EA group (0.0% versus 37.8%; p < .001 and 25.8% versus 62.2%; p = .003, respectively). In the recovery room, the sensation of urinary retention occurred only in the EA group (22.6%); however, the condition spontaneously resolved in all patients before discharge. Disconnection or pump failure (n = 2) and local inflammation at the catheter insertion site (n = 1) were reported in the EA group after discharge. However, all adverse events were mild and transient and had disappeared at the 5-d follow-up phone call.
Table 5. Adverse Events (AEs), Adjuvant Drug Usage, and Complications.
Thermal injury associated with the HIFU treatment was observed in both groups, but it was more frequent in the MAC group (54.1%) than in the EA group (22.6%) (p = .008). One severe complication occurred in the MAC group: a case of proctitis with focal mucosal wall defect confirmed by sigmoidoscopy that required hospitalization and conservative management for 7 d. Three moderate complications in the MAC group were one case of intensive coccygeal pain and two cases with of second-degree skin burn on the rectus abdominis muscle. All these moderate adverse events were improved by conservative treatment within 8 weeks. In the EA group, one severe complication and one moderate complication were reported as thermal injury. The severe complication was left gluteal burn despite sensory preservation in the pericoccygeal area (S2-4 dermatome) during HIFU treatment; only pain and redness were present at discharge, but distress was clearly noted at the 5-d follow-up phone call and local flap surgery performed by a plastic surgeon was required a month following HIFU treatment. The moderate complication was unilateral lumbosacral plexus injury confirmed electromyography/nerve conduction velocity test; the condition was alleviated with gabapentin up to 1800 mg daily for 6 months after the procedure.
Discussion
In this study, the EA group reported a significantly lower frequency of severe or very severe pain intensity in association with the HIFU treatment of uterine adenomyosis than did the MAC group (41.9% in the EA group versus 75.7% in the MAC group; p = .006). The total dosages of intraoperative remifentanil and postoperative fentanyl administered were significantly lower in the EA group than in the MAC group (p = .001 and .030, respectively). Moreover, there was no use of midazolam in the EA group during HIFU treatment, as opposed to 16.2% (6/37) of patients in the MAC group requiring midazolam injection. When we analyzed variables using multivariable analysis, EA was the largest contributing factor to increased ablation ratio. Although adverse events related to EA occurred in some patients, they were mild and transient. The frequency of thermal injury after HIFU treatment was higher in the MAC group (54.1%) than in the EA group (22.6%).
Although HIFU treatment of uterine adenomyosis may be less invasive than treatment of other intraabdominal organs, it still requires appropriate control of both visceral and somatic pain in addition to the administration of sedatives. The MAC procedure that utilized remifentanil, fentanyl, midazolam, and a combination of antiemetics may be more popular than EA in the HIFU treatment of uterine adenomyosis [Citation27]. However, intravenous infusion of remifentanil could not satisfactorily eliminate nociceptive pain such as somatic pain in the abdominal wall or visceral pain originating from the parietal peritoneum during HIFU treatment [Citation28]. Furthermore, remifentanil suspension may cause immediate rebound hyperalgesia after the treatment [Citation29]. Bilateral transversus abdominis plane block has been suggested to manage postoperative pain caused by abdominal surgery [Citation30,Citation31], but it is not sufficient to relieve intraoperative pain. In this study, EA provided consistent intra- and postoperative analgesia for HIFU treatment of uterine adenomyosis. Analgesia to nociception from the abdominal wall and sympathetic stimulus from visceral organs was effectively achieved through epidural catheterization and intermittent additional administration of local anesthetics through the catheter. Use of the EA procedure led to decreased consumption of opioids and sedatives during both surgery and recovery. Although further study is warranted, EA is expected to achieve similar pain control in more invasive HIFU procedures on intraabdominal organs treating the liver, pancreas, and kidney, or uterine fibroids.
Another remarkable finding in this study was a strong correlation between the EA and the NPV ratio. Previous studies have suggested that an increase in NPV ratio results in improvement of long-term symptom relief in the HIFU treatment of adenomyosis [Citation23,Citation24]. It was assumed that reduction of pain under EA may lead to a higher NPV ratio in HIFU treatment, possibly resulting in a successful outcome. When considering other factors that could not be externally adjusted in the multivariable analysis, such as age, abdominal wall thickness, scarring in lower abdomen, and volume or location of adenomyosis, EA was the only factor shown to improve the outcome of HIFU treatment. Therefore, EA may be considered an appropriate analgesic procedure to achieve excellent procedural outcomes when performed safely by an experienced pain physician.
In accordance with a recent study, abdominal wall thickness, enhancement type on T1-weighted sequence, and the number of hyperintense points were negatively correlated with the NPV ratio. In addition, the smallest NPV ratio was seen in diffuse adenomyosis (both anterior wall and posterior wall of the uterus) rather than at the anterior wall, posterior wall, or fundus. A positive correlation between the volume of adenomyosis and the NPV ratio was reported. In our multivariable linear regression, volume of adenomyosis had a positive correlation, and anteroposterior location had a negative correlation, which concurred with the previous report [Citation25]. However, abdominal wall thickness was not related to the NPV ratio in this study. We considered that a limited distribution of abdominal wall thicknesses among our participants may have minimized the impact of abdominal wall thickness on the NPV ratio.
Thermal injury can be one of the most serious complications associated with HIFU treatment. There was one severe case of thermal injury in each group in this study; the case in the EA group required a surgical flap procedure to treat her gluteal lesion. Ironically, there had been no self-reported discomfort during the procedure for either group; all thermal injuries were detected after discharge. According to Dewhirst et al., local temperature >49 °C may cause skin necrosis in 10–20 min sonification time [Citation32]. However, the thermal threshold is known to be variable in normal tissue, and gradual temperature increases may not be detected until substantial skin defect occurs [Citation33]. Furthermore, the compressed region—in particular, the gluteal area—may be relatively vulnerable to heat accumulation during HIFU treatment, [Citation34]. Accordingly, rigorous temperature monitoring and sufficient cooling time may be critical to avoiding severe thermal injury during HIFU treatment.
The major risks of epidural catheterization are epidural hematoma and abscess, but the risks are extremely low when there is no comorbidity [Citation35,Citation36]. In this study, the most common adverse event reported by patients in the EA group was tingling sensation in the lower extremities, followed by intraprocedural nausea and postprocedural urinary retention. However, these complications were mild and transient, and no participants required early catheter removal due to any serious adverse events. A particular concern with EA during the HIFU treatment may be the possibility of delayed awareness of thermal injury to adjacent structures. However, the frequency of thermal injury was higher in the MAC group than in the EA group. Furthermore, there were only two moderate-to-severe thermal injuries in the EA group, both of which occurred in unanesthetized areas. Therefore, we consider that, when an appropriate protocol is employed, EA may not significantly increase complications during HIFU treatment.
Another point to be addressed in continuous EA with catheter insertion and MRI scan is the iatrogenic risk associated with MRI compatibility of the catheters [Citation37]. In contrast to classical catheters made of nylon, which were used in this study, or polyether block amide, a number of new catheters are magnetic with coil-reinforcement, which can lead to significant catheter displacement under the magnetic field of an MRI scanner. Furthermore, even if a few coil-reinforced catheters are not associated with magnetic displacement, hazardous temperature increases at the entry point may occur under a 3-T magnetic field [Citation37]. This would cause a cutaneous burn at the catheter entry site and/or along the subcutaneous tunneling track. Therefore, physicians must rigorously assure the MRI compatibility of catheters before performing EA prior to MRI scanning.
Limitations of this study include its nonrandomized, retrospective case-control design, which may have been affected by characteristic confounders that include bias and variability in the quality of available information. Otherwise, there are several issues that should be more discussed as drawbacks. First, the two cohorts in the study were sequential and time-based, which may be the major confounding factor (patients in the MAC group underwent the procedure between January 14, 2016, and December 8, 2016, while patients in the EA group underwent the procedure between December 29, 2016, and August 31, 2017). Although there were no differences in demographics or initial clinical variables, with the exception of preexisting postoperative scarring in the lower abdomen, progression on the learning curve by the radiologist over the study period would be another limitation. However, there was no correlation between the passage of time and the NPV ratio between the two groups as determined by scatterplot (Supplement 1). Second, higher prevalence of preexisting postoperative scarring in the lower abdomen may have been associated with increased analgesic needs in the MAC group. It has been reported that preexisting abdominal scarring may contribute to skin burns, followed by procedural pain during HIFU treatment [Citation38]. Although no significant correlation was found between preexisting scarring and pain (Spearman’s ρ = .028, p = .822) in this study, preexisting scarring might have contributed to the higher frequency of procedural pain in the MAC group. Finally, unless it is performed by a pain specialist, EA may present technical or economic burdens to physicians and patients. In addition, discharge with PCEA requires additional attention, and there are concerns related to device failure or disconnection of the catheter, and a return visit is needed for removal. Since only a limited number of patients in the EA group (6/31, 19.4%) needed rescue analgesics after discharge, it may be that the epidural catheter could be removed upon procedure termination, and oral analgesics could be used to control pain. This would obviate the burden of maintaining an epidural catheter at home and the need for a return visit to accomplish removal.
In conclusion, this study showed that EA reduced pain and reduced analgesic requirements during HIFU treatment of adenomyosis from levels observed with MAC. Furthermore, PCEA effectively reduced postprocedural pain. EA also increased NPV and reduced the frequency of thermal injury after HIFU. EA caused minor adverse events, but they were mild and transient. Based on our investigation, EA may be considered an effective analgesic option in HIFU treatment of adenomyosis.
Supplemental Material
Download TIFF Image (2.3 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. 2007;23:89–104.
- Orsi F, Arnone P, Chen W, et al. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther. 2010;6:414–420.
- Cline HE, Hynynen K, Watkins RD, et al. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194:731–737.
- Kim Y. Advances in MR image-guided high-intensity focused ultrasound therapy. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. 2015;31:225–232.
- Thiburce AC, Frulio N, Hocquelet A, et al. Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: Mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperthermia. 2015;31:764–770.
- Lindner U, Ghai S, Spensieri P, et al. Focal magnetic resonance guided focused ultrasound for prostate cancer: initial North American experience. CUAJ. 2012;6:283–E286.
- Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Ann Surg Oncol. 2009;16:140–146.
- Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375:730–739.
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–895.
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40:1080–1086.
- Coluccia D, Fandino J, Schwyzer L, et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound. 2014;2:17.
- Yao C-L, Trinh T, Wong GTC, et al. Anaesthesia for high intensity focused ultrasound (HIFU) therapy. Anaesthesia. 2008;63:865–872.
- Baco E, Gelet A, Crouzet S, et al. Hemi salvage high-intensity focused ultrasound (HIFU) in unilateral radiorecurrent prostate cancer: a prospective two-centre study. BJU Int. 2014;114:532–540.
- Robin C, Trieger N. Paradoxical reactions to benzodiazepines in intravenous sedation: a report of 2 cases and review of the literature. Anesth Prog. 2002;49:128–132.
- Marlowe S, Engstrom R, White PF. Epidural patient-controlled analgesia (PCA): an alternative to continuous epidural infusions. Pain. 1989;37:97–101.
- Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995;82:1474–1506.
- Grass JA. The role of epidural anesthesia and analgesia in postoperative outcome. Anesthesiol Clin North America. 2000;18:407–428.
- Hwang BY, Kwon JY, Jeon SE, et al. Comparison of patient-controlled epidural analgesia with patient-controlled intravenous analgesia for laparoscopic radical prostatectomy. Korean J Pain. 2018;31:191.
- Kaneko T, Iwama H. The association between injected volume of local anesthetic and spread of epidural anesthesia: a hypothesis. Reg Anesth. Pain Med. 1999;24:153–157.
- Yokoyama M, Hanazaki M, Fujii H, et al. Correlation between the distribution of contrast medium and the extent of blockade during epidural anesthesia. Anesthesiology. 2004;100:1504–1510.
- Tammy P, Ouellette H. Musculoskeletal system - Examination. Orthop. Made Ridiculously Simple. Miami, FL: Medmaster, Inc.; 2009. p. 59.
- Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. J Perianesth Nurs. 1998;13:148–155.
- Wang W, Wang Y, Tang J. Safety and efficacy of high intensity focused ultrasound ablation therapy for adenomyosis. Acad Radiol. 2009;16:1416–1423.
- Zhou M, Chen J-Y, Tang L-D, et al. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril. 2011;95:900–905.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32:496–503.
- Common Terminology Criteria for Adverse Events Version 4.03 (CTCAE). US Dep. Health Hum. Serv. 2010;1. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- Vaessen HHB, Knuttel FM, van Breugel JMM, et al. Moderate-to-deep sedation technique, using propofol and ketamine, allowing synchronised breathing for magnetic resonance high-intensity focused ultrasound (MR-HIFU) treatment for uterine fibroids: a pilot study. J Ther Ultrasound. 2017;5:8.
- Douma MR, Middeldorp JM, Verwey RA, et al. A randomised comparison of intravenous remifentanil patient-controlled analgesia with epidural ropivacaine/sufentanil during labour. Int J Obstet Anesth. 2011;20:118–123.
- Hansen EG, Duedahl TH, Rømsing J, et al. Intra-operative remifentanil might influence pain levels in the immediate post-operative period after major abdominal surgery. Acta Anaesthesiol Scand. 2005; 0:050920011853007–050920014641470.
- Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg. 2003;238:663–673.
- Charlton S, Cyna AM, Middleton P, et al. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Libr. [Internet]. John Wiley & Sons, Ltd; 2010 [cited 2017 Oct 15]. Available from: http://libproxy.snu.ac.kr/2cc385d/_Lib_Proxy_Url/onlinelibrary.wiley.com/doi/10.1002/14651858.CD007705.pub2/full.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19:267–294.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia. 2011;27:320–343.
- Suzuki T, Hirayama T, Aihara K, et al. Experimental studies of moderate temperature burns. Burns. 1991;17:443–451.
- Miller RD. Chapter 51. Spinal, Epidural, and Caudal Anesthesia. Mill. Anesth. Ed. Ronald Mill. 6th ed. New York: Elsevier/Churchill Livingstone; 2005. p. 1611–1638.
- Alley EA, Mulory MF. Neuraxial anesthesia for outpatients. Anesthesiol Clin. 2014;32:357–369.
- Owens S, Erturk MA, Ouanes J-PP, et al. Evaluation of epidural and peripheral nerve catheter heating during magnetic resonance imaging. Reg Anesth Pain Med. 2014;39:534–539.
- Xiong Y, Yue Y, Shui L, et al. Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: A retrospective study. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group. 2015;31:777–783.
