Abstract
Background: Recurrence after wide excision or residual tumor after an unplanned excision of a malignant soft tissue sarcoma (STS) is a complex problem, due to a higher recurrence rate and poorer survival rate compared with primary resection. Regional hyperthermia was used, with the expectation that it will enhance the anti-tumor effects of chemotherapy and radiotherapy. This study aimed to assess the efficacy of neoadjuvant concomitant radiotherapy, hyperthermia, and chemotherapy (RHC) for salvage of recurrent or residual malignant STS.
Methods: We identified 64 patients with recurrent or residual STS treated between 1994 and 2013. After excluding those with low-grade malignancy, with recurrent bone tumor in the soft tissues, with truncal STS, and who declined to participate, 23 patients (7 with recurrence and 16 with residual tumor) underwent RHC. The histologic diagnoses were undifferentiated pleomorphic sarcoma (n = 11), synovial sarcoma (n = 3), leiomyosarcoma and myxoid liposarcoma (n = 2 each), and other histologic types. As primary outcomes, the 5-year overall survival (OS), distant metastasis-free survival (D-MFS), and local control (LC) rates were evaluated by Kaplan-Meier analysis.
Results: The median follow-up period was 112.3 months. The 5-year OS, D-MFS, and LC were 86.4%, 77.4%, and 86.7%, respectively. In the univariate analysis, tumor depth was considered as a negative prognostic factor for OS and D-MFS, and a positive margin was also a negative prognostic factor for OS, D-MFS LC with retained on Cox proportional hazards model in OS, and D-MFS.
Conclusion: RHC is an effective option for salvage treatment of recurrent and residual STS.
Introduction
Patients with localized soft tissue sarcoma (STS) can experience local recurrence, with or without concurrent distant metastases, after definitive surgery [Citation1]. Local recurrence of STS is considered to increase susceptibility to further local recurrence, with a prevalence rate of 20%–45% [Citation1–5]. The 5-year survival rate of 52%–83% for these patients was considered unsatisfactory [Citation5–7]. The management of locally recurrent sarcoma is significantly challenging for surgical oncologists as many patients with locally recurrent disease have previously received adjuvant radiation and/or chemotherapy or underwent skin grafting. Thus, these patients often present with a range of contractures, fibrosis, or other defects of the skin, subcutaneous tissues, tendons, or muscles [Citation8]. Amputation is inevitable in 5%–25% of patients treated for local recurrence [Citation9,Citation10]; in some cases, amputation may be the most effective treatment for STS after local recurrence with multiple local recurrence, multicompartment disease, or major invasion to the neurovascular structure [Citation6].
Moreover, unplanned resection of STS was associated with poor outcomes [Citation11,Citation12]. As malignant soft tissue tumors are rare, comprising only 1% of all malignancies [Citation13], general surgeons, plastic surgeons, orthopedic surgeons, or dermatologists may end up performing inappropriate and unplanned excisions, under local anesthesia. Unplanned resection may result in surgical site contamination, due to transverse excision [Citation14], postoperative hematoma [Citation14,Citation15], and a lack of careful preoperative planning with imaging studies. These unplanned resections may further disseminate the tumor or residue of the tumor. The prevalence of residual tumor at the operative site ranges from 18% to 60% [Citation12,Citation16–18], and local recurrence may be observed in 7%–39% of cases [Citation18–21].
Radio-hyperthermo-chemotherapy (RHC), a trimodal therapy that combines radiotherapy, hyperthermia, and chemotherapy was developed for the treatment of STS before performing definitive surgery; its use is expected to markedly improve local control rates and to eradicate possible invasion or metastasis of tumor cells from the primary lesion [Citation22–24]. In addition to its use before primary resection, RHC has been used in the treatment of recurrent STS or STS identified after unplanned resection with or without apparent residual tumor (termed second-look surgery, as originally described by the gynecologists Wangensteen et al. [Citation25], in which they aimed to determine – via direct laparoscopic observation – if any cancer cells remained after resection of ovarian cancer). The second-look surgery used for ovarian cancer was modified for the treatment of malignant STS, with RHC administered to the contaminated lesion after unplanned resection without apparent residual tumor to determine if any tumor cells remained around the cicatrized lesion, avoiding unnecessary wide resection [Citation26].
To date, we have reported the favorable effects of RHC on the primary resection of malignant STS, with results suggesting that RHC strongly inhibits local recurrence, making preservation of the extremities possible [Citation22–24]. In this study, we investigated the efficacy of RHC as salvage therapy for patients with recurrent or residual malignant STS. This study aimed to analyze the oncologic outcomes (overall survival [OS], distant relapse-free survival [D-RFS], and local control rate [LC]) at 5 years.
Materials and methods
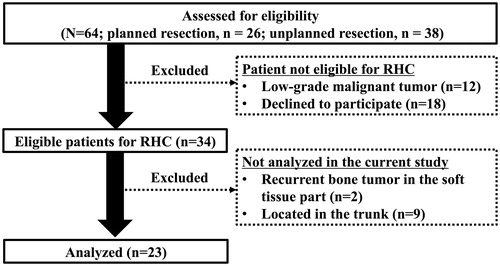
We included patients who presented with recurrent or residual malignant STS between 1994 and 2013. Patients were divided into a planned resection group (patients with local recurrence after a planned excision under general or conduction anesthesia at which time a histologic diagnosis was confirmed) and an unplanned resection group (patients with residual tumor after unplanned resection, that is, excision performed without considering the need to remove a margin of normal tissue surrounding the tumor and where the histologic diagnosis of the initial tumor was not established [Citation11–12]). A retrospective review identified a total of 64 patients (planned resection, n = 26; unplanned resection, n = 38) from the database of the Department of Orthopaedic Surgery of Nagoya City University Hospital. Of these, 30 patients were considered to be not eligible for RHC due to the following reasons: 12 had low-grade malignant tumors (atypical lipomatous tumors, n = 8 and dermatofibrosarcoma protuberans, n = 4) and 18 declined to undergo RHC. In addition, 11 patients were excluded from this analysis, of which 2 had recurrent bone tumors in the soft tissue, and 9 had STS involving the trunk. A total of 23 patients (7 in the planned resection group and 16 in the unplanned resection group) underwent RHC before subsequent surgery ().
RHC protocol
Patients who met the following inclusion criteria were enrolled for RHC treatment: patients aged 15–70 years with non-metastatic tumor, involving the extremities, and classified as Fédération Nationale des Centre de Lutte Contre le Cancer grade 2 or 3 STS (low-grade sarcomas, such as dermatofibrosarcoma protuberans or atypical lipomatous tumors, that were not suitable for RHC). All patients were informed about the RHC therapy and its potential adverse events, and provided written informed consent prior to their participation.
To minimize the rate of adverse events, patients were required to fulfil the following criteria before every cycle of RHC: white blood cell count >3.0 × 109/L, platelet count >75 × 109/L, hemoglobin level >70 g/L, creatinine clearance >60 ml/min, normal hepatic function, and left ventricular ejection fraction >60%.
The RHC protocol is described in , and a more detailed protocol is described in Supplemental Figures 1 and 2. Prior to RHC therapy, angiography was performed to assess tumor blood flow, and a catheter was simultaneously inserted with a reservoir placed into the artery feeding the tumor (intra-artery [IA] chemotherapy). Anticancer drugs were intra-arterially injected through this reservoir. Radiotherapy was initiated on day 1, and 2 Gy per fraction was delivered 20 times (on days 1–5, 8–12, 15–19, and 22–26), with a total of 40 Gy, by radiologists. Following radiotherapy, thermotherapy was simultaneously initiated with the IA injection of anticancer drugs by orthopedic surgeons. Cisplatin (100 mg/m2 on days 1, 15, and 29) and pirarubicin (a derivative of doxorubicin, 30 mg/m2 on days 8 and 22) were alternatively administered. The treatment interval was extended depending on the patient’s status, and, if necessary, colony-stimulating factor was administered. Two weeks after RHC therapy was completed (day 43), chemotherapy (ifosfamide, 2 g/m2 [5 days]; etoposide, 100 mg/m2 [3 days]; and pirarubicin, 30 mg/m2 [2 days]; modified Rosen T-16 regimen [Citation27]) was administered intravenously.
Figure 2. Radio-hyperthermo-chemotherapy protocol. Hyperthermia: Hyperthermia is performed using an 8-MHz radiofrequency capacity heating system (Thermotron RF-8; Yamamoto Vinita, Osaka, Japan) and repeated every week for a total of five courses. The objective of the hyperthermia was to achieve a temperature of 42.5 °C or more for 60 min. Chemotherapy: Chemotherapy is administered by intra-arterial (IA) infusion. During hyperthermia, cisplatin (first, third, and fifth sessions) or pirarubicin (an Adriamycin derivative; second and fourth sessions) are simultaneously injected. After five courses of IA chemotherapy, systemic chemotherapy using ifosfamide, pirarubicin, and etoposide are initiated, followed by surgery. Radiation: All patients are treated with daily radiotherapy at a dose of 40.0 Gy, for a total of 20 sessions. Irradiation is performed just before hyperthermia and chemotherapy on the day of hyperthermia and chemotherapy. The details of each course of RHC are described in Supplemental Figures 1 and 2.
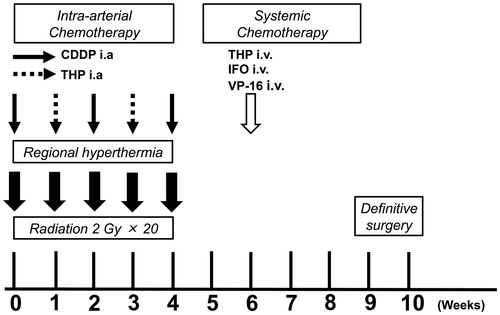
For thermotherapy, an 8-MHz radiofrequency capacitive heating system (Thermotron RF-8: Yamamoto VINITA, Osaka, Japan) was used. Tumor temperature was monitored by inserting a hyperthermia needle with a thermocouple thermometer (0.64 mm, Thermotron CE-150, Yamamoto VINITA, Osaka, Japan) into the tumor space. The treatment objective was to achieve a temperature of ≥42.5 °C for 60 min (T42.5 × 60). Three categories were established based on the number of cycles during which T42.5 × 60 was achieved: poor hyperthermia, T42.5 × 60 was not achieved; mild hyperthermia, T42.5 × 60 was achieved in 1–3 cycles; and complete hyperthermia, T42.5 × 60 was achieved in 4–5 cycles [Citation22].
Surgical procedure
After completing the RHC protocol, the area of the surgical excision was preoperatively evaluated based on the T1-weighted magnetic resonance imaging (MRI) findings (+gadolinium enhancement). Tumors were removed together with previous surgical scars and normal surrounding tissues. However, if a tumor was located in the vicinity of a critical neurovascular area and the response to RHC therapy was considered sufficient (more than partial response, based on the modified response evaluation criteria in solid tumors), marginal resection was permitted. The surgical margin was categorized as wide, marginal, and intralesional [Citation28]. Postoperatively, assessment of the resected tissue margins for tumor cells was performed by the Division of Pathology of the Nagoya City University Hospital.
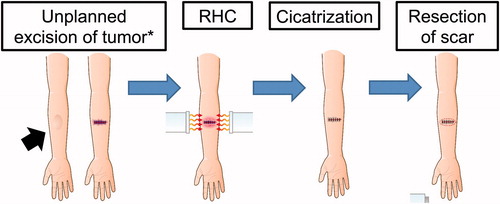
Second-Look surgery
Second-look surgery was performed after the unplanned excision of malignant STS with no apparent residual tumors (). After several cycles of RHC treatment around the resected site, the surgical scar was typically cicatrized and had regressed. Thereafter, the scar was resected with a small surrounding margin of skin and subcutaneous tissue. If necessary, skin flaps or grafts were used to cover the defect(s). Intraoperatively, the scar was pathologically assessed to detect whether the tumor cells were alive or not. If there were no apparent viable cells in the resected specimen, we closed the small surgical site; if viable cells were present, extension of the surgical site was considered.
Statistical analysis
The primary goal of this study was to validate the efficacy of RHC by assessing oncologic outcomes. OS was defined as the time from surgery to death due to any cause, D-RFS as the time from surgery to distant metastasis or death due to any cause, and LC as the time from surgery to local recurrence; these variables were calculated using Kaplan-Meier analysis. Furthermore, the differences in the Kaplan-Meier analysis between the RHC group and reference group were compared using the log-rank test. Chi-square test was used for the statistical analysis of categorical data. For numerical data, two sample t tests or Mann-Whitney U tests were used, as appropriate, based on the result of the Shapiro-Wilk analysis of distribution. For the analysis of the risk factors for oncologic outcomes, hazard ratios were calculated using a Cox proportional hazards model. All statistical analyses were performed using IBM SPSS version 24 (IBM Corp., Armonk, NY). A p values of <.05 was considered significant.
Ethics approval
This retrospective study was approved by the local committees of Nagoya City University Hospital (clinical trial number, 60-17-0042) and was conducted in compliance with the guidelines of the 1975 Declaration of Helsinki.
Results
Among the 23 patients, the mean age was 55.2 (±14.2) years (). Tumors involved the upper and lower limb in 11 and 12 cases, respectively. All patients with local recurrence after planned resection (n = 7) underwent initial surgery under general anesthesia. Of the patients with residual tumors after unplanned resection, 12 underwent resection under local anesthesia and 4 underwent resection under conductive or general anesthesia. Among patients with local recurrence after planned resection, the median time from initial surgery to recurrence was 17.8 months (range: 3.0–106.6 months), and among patients with residual tumors after unplanned excision, the median time from the previous surgery to the first visit to our department was 3.0 months (0.7–23.4 months). The most common histologic diagnosis was undifferentiated pleomorphic sarcoma (n = 11), followed by synovial sarcoma (n = 3), leiomyosarcoma, and myxoid liposarcoma (n = 2 each). Physical examination and image analysis identified no apparent residual tumor in 8 of 17 patients after unplanned resection; second-look surgery was performed in these patients after RHC therapy. The representative case is shown in .
Figure 4. The representative case. A 64-year-old male patient was initially diagnosed with solitary fibrous tumor in the vastus femoris ([a] MRI T1WI, with gadolinium enhancement) and underwent wide resection 1 year ago. During the follow-up period, the recurrence of tumor was found around the femoral artery ([b], MRI T1WI, with gadolinium enhancement). Then, the RHC was performed for the recurrent tumor. The area of irradiation was determined by the radiologist with coverage of the prior surgical area. IA chemotherapy was administered from the catheter placed in the superficial femoral artery (c). The evaluation of thermal effect was mild (42.5 °C achieved in 3/5 RHC cycles). An example of thermal therapy is depicted in (d). Although the tumor size remained the same, the intake of tracer was attenuated (e). Then, a repeat wide resection was performed. Five years after surgery, the patient was still alive with no evidence of the disease.
![Figure 4. The representative case. A 64-year-old male patient was initially diagnosed with solitary fibrous tumor in the vastus femoris ([a] MRI T1WI, with gadolinium enhancement) and underwent wide resection 1 year ago. During the follow-up period, the recurrence of tumor was found around the femoral artery ([b], MRI T1WI, with gadolinium enhancement). Then, the RHC was performed for the recurrent tumor. The area of irradiation was determined by the radiologist with coverage of the prior surgical area. IA chemotherapy was administered from the catheter placed in the superficial femoral artery (c). The evaluation of thermal effect was mild (42.5 °C achieved in 3/5 RHC cycles). An example of thermal therapy is depicted in (d). Although the tumor size remained the same, the intake of tracer was attenuated (e). Then, a repeat wide resection was performed. Five years after surgery, the patient was still alive with no evidence of the disease.](/cms/asset/d8561c01-b5b0-449d-9065-e48c9540e0e6/ihyt_a_1518545_f0004_c.jpg)
Table 1. Clinicopathologic characteristics of patients with malignant soft tissue sarcomas undergoing radio-hyperthermo-chemotherapy (n = 23).
Before surgery, the average number of RHC cycles performed was 4.5 (3 cycles for 1 patient; 4 cycles for 7 patients, and 5 cycles for 15 patients). As for the quality of hyperthermia, T42.5 × 60 was achieved in 19 (82.6%) patients, and 14 (60.8%) patients were considered to have achieved complete hyperthermia. However, poor hyperthermia was observed in 4 (17.3%) patients. Surgery was performed with wide margins in 17 (73.9%) patients and marginal resection in 6 (26.1%) patients; of these, 4 patients were assessed as having positive margins on microscopy and required adjuvant chemotherapy for the eradication of possible micro-residual tumors. A total of 6 (26.1%) patients required skin flaps or grafts for coverage after wide tumor resection.
Second-look surgery
Eight patients underwent second-look surgery. In three patients, the tumor arose in the vicinity of a neurovascular structure (median nerve, radial nerve, or femoral artery/nerve); these structures were preserved at surgery. One patient developed synovial sarcoma in the ankle joint, and the presence of tumor cells in the scar was noted; this patient had amputation and subsequent pulmonary metastasectomy. All others (n = 7) recovered with good functionality (Enneking score [Citation29]: 100%).
Oncologic outcomes
The median follow-up period was 112.3 months (range: 13.1–201.5 months). A total of 4 (17.4%) patients died of the disease. The 5-year OS, D-RFS, and LC rates were 86.4% ± 7.3%, 77.4% ± 9.0%, and 86.7% ± 7.1%, respectively (). The oncologic outcome of each histological tumor type is summarized in .
Figure 5. Oncologic outcomes of patients with highly malignant soft tissue sarcomas treated by radio-hyperthermo-chemotherapy (RHC). (a) Overall survival, (b) distant relapse-free survival, and (c) local control. The straight lines indicate the patients’ group who underwent RHC for salvage after unplanned resection and the dotted lines indicate the patients’ group who underwent RHC for recurrent lesion after planned resection. RHC: radio-hyperthermo-chemotherapy; OS: overall survival; D-RFS: distant relapse-free survival; LC: local control.
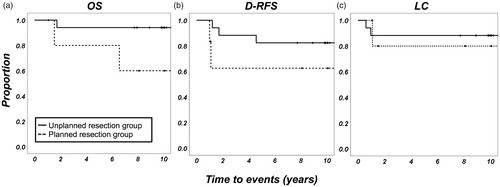
Table 2. Oncologic outcomes of the different histologic tumor types after undergoing radio-hyperthermo-chemotherapy (n = 23).
The 5-year OS, D-RFS, and LC rates for each characteristic are summarized in In the univariate analysis, tumor depth was considered a negative prognostic factor for OS and D-MFS, and a positive margin was also a negative prognostic factor for OS; D-MFS and LC, with retained on Cox proportional hazards model in OS (p = 0.042); and D-MFS (p = 0.024). The thermal effect, histologic tumor grade, and tumor size were not associated with oncologic outcomes, despite the fact that all the patients who underwent second-look surgery had no evidence of the disease at the final follow-up.
Table 3. Oncologic outcomes of patients undergoing radio-hyperthermo-chemotherapy (n = 23).
Limb sparing surgery was achieved in 100% and 81.3% of the patients in the planned resection group and unplanned resection group, respectively. Amputation was inevitable in three patients. The first patient had a malignant peripheral nerve sheath tumor of the shoulder. This patient had been diagnosed with neurofibromatosis Type 1 and had already undergone four unplanned excisions prior to referral. Surgery was performed with a negative margin, but multiple recurrences were observed after surgery. The second patient had a synovial sarcoma of the ankle and underwent second-look surgery; viable cells in the surgical lesion extended circumferentially around the ankle joint. The third patient had an alveolar soft part sarcoma of the hand that had infiltrated the bones and tendons. In this case, regardless of the favorable response to RHC therapy, appropriate margins had to be assured.
Complications
In the RHC group, delayed skin healing (>2 weeks post-surgery) due to skin burns and irradiation was observed in six patients, one of whom required additional procedures (debridement and skin grafting). One patient experienced acute renal failure during RHC and required transient hemodialysis. All comorbidities were well managed without permanent complications.
Discussion
The effect of hyperthermia on the anti-tumor activity of agents, such as bleomycin [Citation30], cisplatin [Citation31], and Adriamycin [Citation32–33] has been demonstrated; hyperthermia was associated with the inhibition of their excretion and/or augmentation of cancer cell sensitivity to these agents. A previous in vitro and in vivo study suggested that hyperthermia acts synergistically with radiotherapy, as the effects of hyperthermia do not attenuate at the S-phase (the radio-resistant phase in cell cycles); therefore, seamless anti-tumor actions are expected [Citation34]. Furthermore, hyperthermia encourages anaerobic metabolism and a low-pH environment may inhibit the repair of thermal damage and sensitizes the tumor cells, including hypoxic tumor cells that are thought to be impervious to radiotherapy [Citation35].
With the expectation that IA chemotherapy enhances the intra-tumoral concentration of anti-cancer agents and the fact that hyperthermia acts synergistically with chemotherapy, we performed IA, despite the fact that evidence for IA chemotherapy remained limited and controversial. In 1996, the Riozzoli Institute in Italy randomly treated osteosarcoma by IA or intra-venous (IV) cisplatin + adriamycin-based regimens. Despite the higher response rate in the IA group versus the IV group (78% vs. 46%), the IA regimen was discontinued because of its side effects, including soft tissue damage [Citation36]. On the contrary, in 2008, a report from the United States published 18 years of data regarding oncologic outcomes for osteosarcoma and MFH using IV Adriamycin and IA cisplatin and found a favourable response rate (77%, greater than 90% necrosis) and local control rate (92%). They insisted that the side effects were manageable. Of 53 patients, 2 had cardiomyopathy, 1 had osteonecrosis, and 3 had myocutaneous inflammation [Citation37]. During this same timeframe, IA chemotherapy was initiated; soft tissue damage occurred (6 cases of delayed skin healing after operation, and one of whom underwent additional skin flap in 23 cases), but these adverse effects were regarded as manageable. Hence, the IA regimens were continued.
In a clinical setting, Issels et al. [Citation38] performed a multicenter randomized Phase 3 trial for STS, comparing the oncologic outcomes of patients treated with etoposide, ifosfamide, and doxorubicin alone or doxorubicin plus regional hyperthermia. A significant improvement in local progression-free survival and disease-free survival was observed when hyperthermia was added to conventional chemotherapy [Citation38]. This therapeutic strategy may offer a new treatment option for patients with highly malignant STS.
In this study, we reported the application of hyperthermia as a trimodal neoadjuvant therapy utilizing radiotherapy, hyperthermia, and chemotherapy for recurrent lesions after planned excision or residual tumors after performance of an inappropriate procedure. The primary sites were well managed by RHC in 83.3% of patients in the planned resection group and in 88.2% of those in the unplanned resection group. However, 37.5% and 17.6% of patients in the planned and unplanned resection groups, respectively, experienced distant metastases within 5 years post-surgery. The Kaplan-Meier analysis revealed that patients with recurrence after planned resection tended to experience metastasis earlier. This is partially because of the differences in the interval from the first surgery and subsequent salvage surgery between the planned and unplanned resection groups; these pre-entry conditions may account for the worse D-RFS rate in the planned excision group. Moreover, in the univariate analysis, the tumor depth was related to the OS. Considering the fact that unplanned resection was often performed for the tumor located in the superficial tissue, the worse prognosis was most likely due to the location of tumor.
With regard to second-look surgery, histologic analyses revealed that only 13% of the patients (1/8) had residual tumors; this result is consistent with the range (8–60%) reported in other studies [Citation12,Citation16–18], leading to uncertainty about whether RHC fully eradicates the residual tumor. Furthermore, given the variability of pre-treatment status, evaluating the efficacy of second-look surgery in terms of oncologic outcomes remains difficult. However, at least seven patients with no residual tumors were able to undergo excision with cicatrized tissues and did not undergo additional wide resection and the sacrifice of a neurovascular bundle. These patients recovered from surgery without any functional loss.
In this study, we did not find a relationship between the elevation of temperature during hyperthermia and oncologic outcome. This was partially because thermal effect was not a strong dependent factor in prolonging the oncologic outcomes, and the response to hyperthermia was determined by the composition of tumor (e.g., myxoid component, fibrous tissue, fatty tissue, or vascularity). For example, patients with alveolar soft part sarcoma, where the tumor is considered to have high vascularity, ended up having poor hyperthermia and were categorized as alive with disease due to the chemosensitive characteristics of the tumor. Likewise, myxoid liposarcoma, where the tumor is composed of small signet-ring lipoblast in prominent myxoid stroma, reacts to hyperthermia well [Citation39] and results in a favourable oncologic outcome. Thus, the heterogeneity of tumor subtypes was a potential confounding factor for the oncologic outcomes.
Our study has some limitations. First, due to the rarity of highly malignant STS and the limited availability of hyperthermic devices, only a small number of patients was enrolled in the study. Additionally, due to the study’s retrospective and non-randomized design, various biases or confounding factors may have affected the results. While the treatment protocol for RHC did not change during the study period, surgical techniques, the role of chemotherapy or radiotherapy, and surgical skills in covering skin defects as part of the standard treatment protocol varied across surgical teams and institutions.
Nevertheless, the biggest merit of RHC is its strong impact on LC, resulting in prolonged OS. However, during RHC, some issues may arise, including skin burns and delayed skin healing (observed in 26% [6/23] of patients). Moreover, the potential adverse effects of chemotherapy, including cytopenia, renal failure, nausea, general malaise, and myocardiopathy, should be fully monitored and managed during RHC. The risks and benefits should be balanced and, if possible, candidates for whom the maximum effects of RHC are expected should be identified.
Supplemental Material
Download MS Power Point (61.1 KB)Acknowledgments
We thank the staff of the Division of Pathology of Nagoya City University Hospital for the evaluation of the histological specimens and the Department of Radiology of Nagoya City University Hospital for the performance of the radiotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author SY on reasonable request.
References
- Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:739–747.
- Karakousis CP, Proimakis C, Rao U, et al. Local recurrence and survival in soft-tissue sarcomas. Ann Surg Oncol. 1996;3:255–260.
- LeVay J, O'Sullivan B, Catton C, et al. Outcome and prognostic factors in soft tissue sarcoma in the adult. Int J Radiat Oncol Biol Phys. 1993;27:1091–1099.
- Moureau-Zabotto L, Thomas L, Bui BN, et al. Management of soft tissue sarcomas (STS) in first isolated local recurrence: a retrospective study of 83 cases. Radiother Oncol. 2004;73:313–319.
- Sugiura H, Nishida Y, Nakashima H, et al. Surgical procedures and prognostic factors for local recurrence of soft tissue sarcomas. J Orthop Sci. 2014;19:141–149.
- Stojadinovic A, Jaques DP, Leung DH, et al. Amputation for recurrent soft tissue sarcoma of the extremity: indications and outcome. Ann Surg Oncol. 2001;8:509–518.
- Trovik CS, Gustafson P, Bauer HC, et al. Consequences of local recurrence of soft tissue sarcoma: 205 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand. 2000;71:488–495.
- Torres MA, Ballo MT, Butler CE, et al. Management of locally recurrent soft-tissue sarcoma after prior surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:1124–1129.
- Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226.
- Abatzoglou S, Turcotte RE, Adoubali A, et al. Local recurrence after initial multidisciplinary management of soft tissue sarcoma: is there a way out? Clin Orthop Relat Res. 2010;468:3012–3018.
- Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. JCO. 1985;3:1344–1348.
- Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78:650–655.
- Hajdu SI. Soft tissue sarcomas: classification and natural history. CA Cancer J Clin. 1981;31:271–280.
- Hoshi M, Ieguchi M, Takami M, et al. Clinical problems after initial unplanned resection of sarcoma. Jpn J Clin Oncol. 2008;38:701–709.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121–1127.
- Pretell-Mazzini J, Barton MD Jr, Conway SA, et al. Unplanned excision of soft-tissue sarcomas: current concepts for management and prognosis. J Bone Joint Surg Am. 2015;97:597–603.
- Fiore M, Casali PG, Miceli R, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13:110–117.
- Chandrasekar CR, Wafa H, Grimer RJ, et al. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. Bone Joint Surg Br. 2008;90-B:203–208.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66:81–87.
- Lewis JJ, Leung D, Espat J, et al. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231:655–663.
- Zornig C, Peiper M, Schröder S. Re-excision of soft tissue sarcoma after inadequate initial operation. Br J Surg. 1995;82:278–279.
- Otsuka T, Yonezawa M, Kamiyama F, et al. Results of surgery and radio-hyperthermo-chemotherapy for patients with soft-tissue sarcoma. Int J Clin Oncol. 2001;6:253–258.
- Aiba H, Kimura H, Otsuka T. Hyperthermic Oncology from Bench to Bedside, 2016 Jan 227-43. Chapter: Combination by Hyperthermia and Radiation and Chemotherapy: Soft Tissue Sarcoma, Springer, Singapore; 1st ed; 2016.
- Aiba H, Yamada S, Mizutani J, et al. Clinical outcomes of radio-hyperthermo-chemotherapy for soft tissue sarcoma compared to a soft tissue sarcoma registry in Japan: a retrospective matched-pair cohort study. Cancer Med. 2018;7:1560–1571.
- Gilbertsen VA, Wangensteen OH. A summary of thirteen years' experience with the second look program. Surg Gynecol Obstet. 1962;114:438–442.
- Hayashi K, Yamada S, Inatani H, et al. Salvage method for unplanned excision of soft tissue sarcoma: long-term results of second-look surgery following radio-hyperthermo-chemotherapy. Anticancer Res. 2015;35:493–498.
- Obata H, Ueda T, Kawai A, , et al. Clinical outcome of patients with Ewing sarcoma family of tumors of bone in Japan: the Japanese Musculoskeletal Oncology Group cooperative study. Cancer. 2007;109:767–775.
- Kawaguchi N, Matumoto S, Manabe J. New method of evaluating the surgical margin and safety margin for musculoskeletal sarcoma, analysed on the basis of 457 surgical cases. J Cancer Res Clin Oncol. 1995;121:555–563.
- Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246.
- Hahn GM, Braun J, Har-Kedar I. Thermochemotherapy: synergism between hyperthermia (42-43 degrees) and adriamycin (of bleomycin) in mammalian cell inactivation. Proc Natl Acad Sci USA. 1975;72:937–940.
- Urano M, Kahn J, Kenton LA. The effect of cis-diamminedichloroplatinum(II) treatment at elevated temperatures on murine fibrosarcoma, FSa-II. Int J Hyperthermia. 1990;6:563–570.
- Herman TS. Temperature dependence of adriamycin, cis-diamminedichloroplatinum, bleomycin, and 1,3-bis(2-chloroethyl)-1-nitrosourea cytotoxicity in vitro. Cancer Res. 1983;43:517–520.
- Nagaoka S, Kawasaki S, Sasaki K, et al. Intracellular uptake, retention and cytotoxic effect of adriamycin combined with hyperthermia in vitro. Jpn J Cancer Res. 1986;77:205–211.
- Westra A, Dewey WC. Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19:467–477.
- Rhee JG, Kim TH, Levitt SH, et al. Changes in acidity of mouse tumor by hyperthermia. Int J Radiat Oncol Biol Phys. 1984;10:393–399.
- Bacci G, Ferrari S, Forni C, et al. The effect of intra-arterial versus intravenous cisplatinum in the neoadjuvant treatment of osteosarcoma of the limbs: the experience at the Rizzoli Institute. Chir Organi Mov. 1996; 81:369–382.
- Hugate RR, Wilkins RM, Kelly CM, et al. Intraarterial chemotherapy for extremity osteosarcoma and MFH in adults. Clin Orthop Relat Res. 2008;466:1292–1301.
- Issels RD, Lindner LH, Verweij J, , et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–570.
- Aiba H, Yamada S, Mizutani J, et al. Preoperative evaluation of the efficacy of radio-hyperthermo-chemotherapy for soft tissue sarcoma in a case series. PLoS One. 2018;13:e0195289.