Abstract
Objective: Isolated limb perfusion (ILP) and isolated limb infusion (ILI) are treatment options for patients with locally advanced melanomas and sarcomas of the extremities. ILP potentially have higher response rates, but requires open surgery for vascular access, whereas ILI is minimally invasive and easier to perform. We now present the technical details and outcome of a new approach to ILP by a minimally invasive vascular access (MI-ILP).
Methods: Six patients, five with melanoma in-transit metastases and one with squamous cell carcinoma, were included in a phase I feasibility trial. Percutaneous vascular access of the extremity vessels was performed and the inserted catheters were then connected to a perfusion system.
Results: All six treated patients underwent the procedure without the need for conversion to open surgery. The median operating time was 164 min and the median leakage rate was 0.1%. The complete response rate was 67%. Four patients (67%) had a Wieberdink grade II reaction and two patients (33%) had a grade III reaction.
Conclusions: MI-ILP is feasible and gives the same treatment characteristics as open ILP, but with the advantage of a minimally invasive vascular access.
Introduction
About 5–10% of patients with recurrent malignant melanoma develop in-transit metastases. Surgical resection is an option if the number of tumors is limited, but if this is not feasible or the tumors are rapidly recurring, isolated limb perfusion (ILP) or isolated limb infusion (ILI) are established treatment options. These treatment modalities can also be used for patients with soft-tissue sarcomas [Citation1,Citation2] and for other locally advanced tumors of the extremities such as squamous cell carcinoma (SCC) [Citation3], lymphoma [Citation4–6], desmoid tumors [Citation7] and Merkel cell carcinoma (MCC) [Citation8,Citation9].
The technique of ILP was pioneered in the 1950s by Creech and Krementz [Citation10] and offers the benefit of a regional delivery of high doses of a chemotherapeutic agent that can reach 20–100 times higher concentrations compared to systemic intravenous delivery [Citation11]. The method requires vascular dissection of the inflow and outflow vessels of the extremity and intraluminal placement of large bore vascular catheters [Citation10]. A tourniquet is applied proximally to the vascular access to further isolate the extremity by compression of collateral vessels. The catheters are connected to an extracorporeal by-pass heart-lung machine and the extremity is then perfused with heated chemotherapeutics for 60–90 min. The extremity is finally rinsed with 2–3 L of crystalloid solution, the catheters are removed and the artery and vein are repaired, followed by fascia and skin closure.
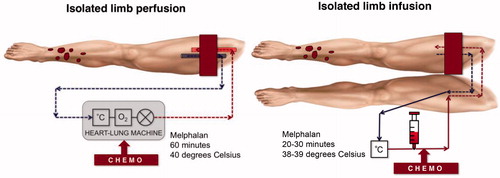
The minimally invasive counterpart of ILP is ILI. The method was developed in the late 1990s and proved to be safe with good response rates and a low rate of local toxicity [Citation12]. The vascular access is achieved by a percutaneous approach through the contralateral side and chemotherapy is infused under tourniquet isolation in an ischaemic environment for 20–30 min, no extracorporeal oxygenator is used [Citation13,Citation14]. A schematic representation of the two methods is shown in .
There are several retrospective studies reporting outcome after both ILP and ILI and in a meta-analysis including 2018 ILP procedures, the median overall response rate (ORR) was 90% including a complete response (CR) rate of 58% [Citation15]. This can be compared with a meta-analysis including 576 ILI procedures reporting an ORR of 73% including a CR rate of 33% [Citation13]. One recent publication comparing two high-volume centers using either ILP or ILI, showed both a significantly higher ORR for ILP (80% vs. 53%) and a significantly higher CR rate (60% vs. 29%), without any difference in local or systemic toxicity. This difference was also significant in a multivariate analysis including known predictive factors for response [Citation14]. However, no randomized data exists that can prove superiority for any of the two methods. An advantage of ILI is that it can be repeated more easily and that it is associated with fewer adverse events related to the open surgical technique, such as wound complications [Citation14]. Even though ILP potentially might have better response rates, several large centers in the world prefer ILI due to its percutaneous approach, but also in order to avoid the more complex perfusion technique which necessitates the use of an extracorporeal oxygenator. Potential benefits of the ILP procedure are the higher flow rate achieved, and the possibility to perform the perfusion during longer time due to the oxygenation. A typical catheter size used in ILI is a 6 Fr arterial and a 6–8 Fr venous catheter whereas in ILP a 10-14 Fr arterial and a 14–18 Fr venous catheter is used.
Considering the advantages and disadvantages of the two methods, and in an effort to combine these two techniques, we developed a minimally-invasive ILP technique (MI-ILP). Six patients were included in a prospective feasibility trial, and the aim is to report on the technical details as well as the first outcome results.
Methods
Patients
Between June 2016 and March 2017, six patients with locally advanced extremity tumors were included in a phase I feasibility trial. The indications for ILP were: in-transit metastases of malignant melanoma (n = 5) and recurrent SCC (n = 1). None of the patients had general metastases and none had previously undergone lymph-node dissection (). The study was approved by the Regional Ethical Review Board at the University of Gothenburg (www.clinicaltrials.gov NCT03376126).
Table 1. Patient and treatment characteristics.
Minimal-invasive isolated limb perfusion (MI-ILP) technique
All procedures were performed in a hybrid operating theatre at the Sahlgrenska University Hospital. A hybrid theatre enables percutaneous ultrasound-guided vascular access with fluoroscopic control, and in case of failure or intraoperative complications, it offers the possibility for immediate conversion to open vascular surgery, as previously described [Citation16].
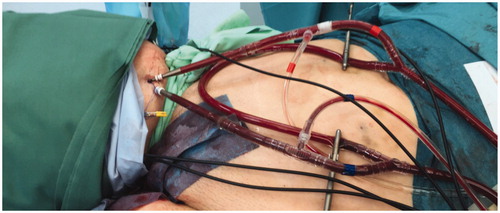
A small 5 mm incision is made with a scalpel in the skin, and for femoral access (n = 5) the superficial femoral artery was punctured first, using ultrasound guidance and a micropuncture technique with a Special Needle (20 G, 12 cm; Mediplast AB, Sweden) at approximately 15 cm below the inguinal ligament. Following placement of a micro-introducer (4 French, 10 cm MAK, Merit Medical, USA), the position was verified by fluoroscopy and a 0.035″ guidewire (Starter® Boston Scientific, MA, USA) was inserted. Using the pre-suture closing technique, two ProGlide® sutures (Abbott Vascular Devices, Santa Clara, CA, USA) were placed over the guidewire. A 7–9 French dilatator, according to need, was then advanced and the guidewire was replaced by a 0.018″ guidewire (V-18™; Boston Scientific, MA, USA). After percutaneous dilatation of the artery, a 10–12 Fr Bio-Medicus® NextGen Pediatric Cannulae (Medtronic, Minneapolis, MN, USA) was introduced into the artery and secured with a skin suture. Slightly proximal to the arterial level, the superficial femoral vein was then cannulated in a similar fashion, except that no ProGlide® sutures were placed, and a 12–14 F Bio-Medicus® NextGen Pediatric Cannulae (Medtronic, Minneapolis, MN, USA) was introduced and secured. For brachial access (n = 1) a similar procedure was used for both artery and vein, with a cannulation site that was approximately 10 cm below the humeral head using catheters of the same calibers as in the femoral procedure.
After cannulation, the limb was isolated with the placement of double Esmarch tourniquets proximal to the site of catheterization, this technique we use to achieve a ‘wider’ compressions zone (). In the case that there were no tumors in the foot or hand, we wrapped another Esmarch bandage around to minimize the toxicity in the hand and foot sole. The catheters were then connected to an oxygenated perfusion system and the limb was heated to 40 °C. The final position and connections for the femoral perfusion are illustrated in . Thermistors for temperature monitoring were placed intramuscularly and subcutaneously. Continuous leakage monitoring was carried out using a precordial scintillation probe (MedicView, Gothenburg, Sweden) to measure leakage of technetium-99 m labeled human serum albumin (Vasculosis, Cis-Bio International, Gif-sur-Yvette, France) injected into the perfusion circuit. Melphalan (Alkeran, Vitaflo, Gothenburg, Sweden), at a dose of 13 mg/L for upper limb and 10 mg/L for lower limb, was infused during 20 min with a total perfusion time of 60 min under hyperthermia. Limb volume was calculated by the summation of circumferential measurements every 5 cm calculating the volume of a cone as previously described [Citation17].
Tumor necrosis factor-alpha (TNF-alpha, Beromun®, Boehringer Ingelheim, Germany) was used in one patient, due to a bulky melanoma metastasis measuring 110 mm, and was injected as a bolus dose 30 min before melphalan infusion with a total perfusion time of 90 min. Our indications for adding TNF-alpha to the perfusion is when the largest tumor is more than 30 mm and for re-perfusion. After perfusion, the limb was rinsed with 3000 ml of Ringer’s solution (Ringer Acetate, Baxter Medical, Kista, Sweden).
After rinsing, the arterial catheter was removed first and the two ProGlide® sutures were tightened and secured. Thereafter the venous catheter was removed and pressure was applied to the groin for 5–10 min. Arterial and venous flow was verified by ultrasound. The patients remained in the postoperative unit for 4–6 h and were then clinically followed at the ward. All patients received thrombosis prophylaxis with low molecular weight heparin 5000 IE daily for 4 weeks (Fragmin, Pfizer Inc. New York, USA).
Follow-up
All patients had a follow-up visit 3 months after surgery for response evaluation, and then every 6 months, either at Sahlgrenska University Hospital or at the referring hospital, depending on the distance for the patient to travel.
Response evaluation
Clinical response was recorded prospectively as the response at 3 months (not the best response) using the WHO criteria definitions [Citation18]. Complete response (CR) was a complete disappearance of all lesions; Partial response (PR) was a reduction of at least 50%; Progressive disease (PD) was an increase of 25% in tumour size or the appearance of new lesions. Stable disease (SD) was an insufficient reduction or a sufficient growth of tumour burden to be classed as CR, PR or PD. Local toxicity was classified as the worst reaction at 3 months according to the Wieberdink classification [Citation17]. Complications were reported using the Clavien-Dindo classification system [Citation19].
Results
Minimal invasive approach
All patients (n = 6) were able to undergo ipsilateral percutaneous vascular catheter placement without the need for conversion to open surgery. The limb perfusion was successful in all cases and there were no immediate or late complications related to the percutaneous cannulation, for example, stenosis or thrombosis. The median operating time was 164 min (150–225 min) including a perfusion time of 60–90 min ().
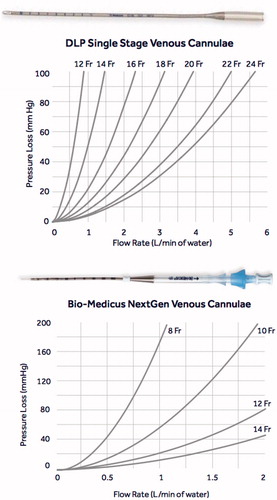
When comparing the DLP Single Stage venous cannula that we have traditionally used for open ILP with the Bio-Medicus NextGen venous cannula, we concluded that the venous cannula size can be reduced from 18 Fr to 14 Fr or from 16 Fr to 12 Fr while still having a comparable pressure loss ().
Perfusion characteristics
The median flowrate in the five femoral perfusions, was 610 ml/min (270–711 ml/min) while the flow rate in the brachial perfusion was significantly lower at 110 ml/min. The median temperature during the perfusion was 39.8 °C (range 37.5–40.0 °C). In one patient undergoing a femoral perfusion, both the median flow rate (270 ml/min) and the median temperature (37.5 °C) was lower than expected, but we could not identify any specific reason. The leakage rate during the perfusions was in median 0.1% (range 0.0%–3.2%).
Toxicity and complications
Four patients (67%) had a Wieberdink grade II reaction and two patients (33%) had a Wieberdink III reaction. No patient experienced any signs of systemic toxicity during the perioperative period. Patients stayed 4–6 h at a post-operative care unit and were then transferred to the surgical ward with a median hospital stay of 4.5 days (2–14). There were two postoperative complications. One patient developed erysipelas at the 2nd postoperative day with redness and swelling of the hand. He was treated with intravenous antibiotics but still had marked edema with reduced sensory and motoric nerve function which was attributed to his previously diagnosed carpal tunnel syndrome. After 6 months, the nerve function was normalized (Clavien-Dindo grade II). One patient with an 11 cm large melanoma tumor in the popliteal fossa developed a tumor necrosis associated with fever and a sinus from the tumour to the skin, and was treated with intravenous antibiotics. However, after the tumor responded, the sinus was healed and the fever disappeared (Clavien-Dindo grade II). No wound complications related to the percutaneous catheter insertion were registered ().
Response, loco-regional recurrences and survival
Three of the five patients (60%) with melanoma in-transit metastases had a complete response (CR). Two patients (40%) had stable disease (SD) and both these patients received immunotherapy (nivolumab) and later developed a CR in the treated limb. One patient was currently without any evidence of disease, whereas the other patient had progressed systemically. The patient with SCC had a CR at 3 months and was still without recurrence. After a median follow-up of 15 months, all the patients were still alive.
Discussion
In our institution, we have a long experience with ILP for the treatment of extremity malignancies, mostly melanomas. In an attempt to evolve our ILP technique, we have developed this new approach (MI-ILP) where large-bore arterial and venous catheters can be introduced percutaneously ipsilateral in the limb to be treated. The idea behind this was to apply a minimally invasive technique to an already successful open perfusion method associated with high response rates [Citation16]. This would combine the advantage of ILI, where thinner catheters are placed percutaneously, with the potential benefit of an oxygenated perfusion that allows for higher perfusion flows, longer perfusion time and higher tissue temperature in the treated limb ().
Table 2. Comparison of ILP, MI-ILP and ILI with approximate values of important parameters.
The biggest challenge to apply such a method was to choose the appropriate set of cannulas for percutaneous placement with sufficient diameter and length allowing for perfusion at high flow rates. The cannulas used in ILP and ILI are of critical importance since they constitute the narrowest part in the perfusion circuit, being the cause of sequential pressure loss. The evaluation of a cannula’s performance is traditionally done by measuring the pressure loss across the cannula using water. In the real setting, a higher-pressure drop is associated with hemolysis and denaturation of proteins [Citation20]. Using the Bio-Medicus NextGen catheter, having a thinner wall and also a shorter length, allowed for a significant decrease in catheter size compared to the traditional DLP Single Stage venous cannula. This was the main reason for choosing the latter for our minimally invasive method, however, there might very well be other, even more, suitable catheters available. These catheter sizes are also commonly used in vascular and cardiac procedures, and the risks for adverse events such as stenosis, thrombosis and pseudoaneurysms are usually regarded low [Citation21].
The flow rate achieved during the brachial ILP and one of the femoral ILPs were lower than we anticipated. Also, the temperature development was lower than anticipated in two of the femoral ILPs. Notably, we do not use external heating (which easily increase the temperature superficially in the skin), and we regard the temperature increase rather as a marker for a well-perfused limb. Potentially these effects could depend on the minimal invasive approach, but larger series are needed to draw any valid conclusions regarding this.
When comparing ILP with ILI, higher complete response rates have been reported for ILP [Citation22,Citation23], however no prospective trials comparing the two methods directly have been presented, and a limitation in most of the retrospective analyses is that they do not stratify for known predictors of response, such as disease burden [Citation24]. In a previous study comparing the outcomes between two large referral centres, Moffitt Cancer Center (Florida, USA) and Sahlgrenska University Hospital (Gothenburg, Sweden), where Moffitt only used ILI and Sahlgrenska only ILP, it was shown that the CR rate was significantly better for ILP, even in multivariate analysis adjusting for tumor burden (OR 3.86, p < .001). Both therapies were very well tolerated with a risk of less than 3% for major toxicity. Interestingly, there was no difference in survival between the two cohorts with a median survival of approximately 40 months for both groups [Citation14].
Even though the ILP technique might provide a better response rate, it is the use of ILI that is increasing worldwide [Citation25], and this is primarily due to the minimally invasive approach. When comparing ILP with ILI, it is not just the vascular access that differs. ILP also requires a heart-lung machine and a leakage monitoring system, especially when using TNF-alpha. In order to further expand the use of ILP, there will also be a significant need for future development of the perfusion system and the leakage monitoring system, reducing the complexity of the procedure and using a more standardized technique. It is known that hypoxia enhances the cytotoxic effect of melphalan in cell lines, an effect even further enhanced by the combination of hypoxia and acidosis [Citation26]. In ILI it has been shown that increasing tourniquet time leads to increased hypoxia and acidosis, a known predictive factor for response [Citation27,Citation28]. In order to, even more, minimize the complexity of the current ILP procedure, the need for oxygenation can be questioned, and the heart-lung machine might even be replaced by a much simpler roller pump. These and several other questions remain to be answered, however, a first step might be to establish the MI-ILP technique as a valid treatment option.
The introduction of BRAF/MEK inhibitors [Citation29–31], as well as immunotherapy with anti–CTLA-4 [Citation32] and anti-PD-1 antibodies [Citation33,Citation34], has radically changed melanoma treatment in the last years. In many melanoma centers, the first-line treatment for in-transit metastasis is nowadays anti-PD-1 therapies, without any specific response data being reported so far. However, data for inoperable stage III disease (mainly including extensive lymph node metastases) has been reported and the response rates are still far from the results achieved by ILP or ILI. Other rapidly expanding local treatment options include talimogene laherparepvec (TVEC) [Citation35], Rose Bengal [Citation36] or electrochemotherapy (ECT).
Our initial plan was to include ten patients in a feasibility trial, however, the possibility of using our new hybrid operating theatre became very limited, and we, therefore, chose to present these first six patients together with a thorough description of the technique. Larger prospective series are obviously needed to validate the technique, and we have now instead of using a hybrid operating decided to move into a regular angiographic suite, simplifying the procedure even more.
Taken together, this series of six patients show the feasibility of the MI-ILP technique. Perfusion flows, tissue temperature, leakage rates and clinical outcomes are similar to historical data for ILP. The significant advantage of MI-ILP when compared to ILI is the oxygenated perfusion under desirable temperature, which potentially offers better response rates while employing a similar minimally invasive vascular access. The advantage of the method compared to open ILP is the percutaneous access, which minimizes wound complications and also potentially allows for easier repetition of the treatment if necessary. Any conclusions regarding response rates, toxicities and complications are not possible in this limited series of patients and the use of MI-ILP should be restricted to clinical trials. We are now planning to move forward with a prospective phase II trial with response, toxicity and complications as end-points.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Grunhagen DJ, de Wilt JH, van Geel AN, et al. Isolated limb perfusion with TNF-alpha and melphalan in locally advanced soft tissue sarcomas of the extremities. Recent Results Cancer Res. 2009;179:257–270.
- Olofsson R, Bergh P, Berlin O, et al. Long-term outcome of isolated limb perfusion in advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2012;19:1800–1807.
- Olieman AF, Lienard D, Eggermont AM, et al. Hyperthermic isolated limb perfusion with tumor necrosis factor alpha, interferon gamma, and melphalan for locally advanced nonmelanoma skin tumors of the extremities: a multicenter study. Arch Surg. 1999;134:303–307.
- Paramo JC, Benavides C, Tang LW, et al. Complete remission of previously intractable peripheral cutaneous T-cell lymphoma of the lower extremity using isolated hyperthermic limb perfusion with melphalan (1-phenylalanine mustard). Cancer Invest. 2004;22:545–549.
- Kobold S, Killic N, Lutkens T, et al. Isolated limb perfusion with melphalan for the treatment of intractable primary cutaneous diffuse large B-cell lymphoma leg type. Acta Haematol. 2010;123:179–181.
- Jansen RF, van Geel BN, van der Zee J, et al. Intractible cutaneous non-Hodgkin lymphoma of the lower limb. Complete remission after sequential regional isolated hyperthermic perfusion and perfusion with 1-phenylalanine-mustard (melphalan, L-Pam). Cancer. 1989;64:392–395.
- Grunhagen DJ, de Wilt JH, Verhoef C, et al. TNF-based isolated limb perfusion in unresectable extremity desmoid tumours. Eur J Surg Oncol. 2005;31:912–916.
- Zeitouni NC, Giordano CN, Kane JM, 3rd, et al. In-transit Merkel cell carcinoma treated with isolated limb perfusion or isolated limb infusion: a case series of 12 patients. Dermatol Surg. 2011;37:357–364.
- Belgrano V, Ben-Shabat I, Bergh P, et al. Isolated limb perfusion as a treatment option for rare types of tumours. Int J Hyperthermia. 2016;32:595–599.
- Creech O, Jr., Krementz ET, Ryan RF, et al. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–632.
- Minor DR, Allen RE, Alberts D, et al. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer. 1985;55:2638–2644.
- Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–715.
- Kroon HM, Huismans AM, Kam PC, et al. Isolated limb infusion with melphalan and actinomycin D for melanoma: a systematic review. J Surg Oncol. 2014;109:348–351.
- Dossett LA, Ben-Shabat I, Olofsson Bagge R, et al. Clinical response and regional toxicity following isolated limb infusion compared with isolated limb perfusion for in-transit melanoma. Ann Surg Oncol. 2016;23:2330–2335.
- Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, et al. Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist. 2010;15:416–427.
- Olofsson R, Mattsson J, Lindner P. Long-term follow-up of 163 consecutive patients treated with isolated limb perfusion for in-transit metastases of malignant melanoma. Int J Hyperthermia. 2013;29:551–557.
- Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910.
- World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization; 1979.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213.
- Lequier L, Horton SB, McMullan DM, et al. Extracorporeal membrane oxygenation circuitry. Pediatr Crit Care Med. 2013;14:S7–S12.
- Vierhout BP, Pol RA, El Moumni M, et al. Editor’s choice - arteriotomy closure devices in EVAR, TEVAR, and TAVR: a systematic review and meta-analysis of randomised clinical trials and cohort studies. Eur J Vasc Endovasc Surg. 2017;54:104–115.
- Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–2205.
- Raymond AK, Beasley GM, Broadwater G, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg Aug. 2011;213:306–316.
- Muilenburg DJ, Beasley GM, Thompson ZJ, et al. Burden of disease predicts response to isolated limb infusion with melphalan and actinomycin D in melanoma. Ann Surg Oncol. 2015;22:482–488.
- Testori A, Verhoef C, Kroon HM, et al. Treatment of melanoma metastases in a limb by isolated limb perfusion and isolated limb infusion. J Surg Oncol. 2011;104:397–404.
- Siemann DW, Chapman M, Beikirch A. Effects of oxygenation and pH on tumor cell response to alkylating chemotherapy. Int J Radiat Oncol Biol Phys. 1991;20:287–289.
- Kroon HM, Coventry BJ, Giles MH, et al. Australian multicenter study of isolated limb infusion for melanoma. Ann Surg Oncol. 2016;23:1096–1103.
- Lindner P, Doubrovsky A, Kam PC, et al. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–136.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39.
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:1290–1723.
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–1862.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. JCO. 2014;32:1020–1030.
- Luu C, Khushalani NI, Zager JS. Intralesional and systemic immunotherapy for metastatic melanoma. Expert Opin Biol Ther. 2016;16:1491–1499.
- Thompson JF, Agarwala SS, Smithers BM, et al. Phase 2 study of intralesional PV-10 in refractory metastatic melanoma. Ann Surg Oncol. 2015;22:2135–2142.