Abstract
Introduction: Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) predispose to postoperative renal dysfunction. Dexmedetomidine is an α2 adrenoreceptor agonist, which has renoprotective effects after cardiac surgery.
Objective: To assess the effect of dexmedetomidine on renal function after CRS and HIPEC.
Materials: Thirty-eight patients undergoing CRS and HIPEC were randomized to receive dexmedetomidine (dexmedetomidine group, n = 19, loading 1 μg/kg over 20 min followed by infusion at 0.5 μg/kg/h) or 0.9% sodium chloride (control group, n = 19) during surgery. Creatinine clearance (CrCl) was assessed daily until postoperative day 7. Urine neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule (KIM)-1 were measured for 24 h after surgery.
Results: There was no difference in the lowest CrCl value during the first 7 days postoperatively, but the % change from baseline to the lowest value was lower in the dexmedetomidine group than in the control group (p = .037). Urine NGAL and KIM-1 levels were increased over time in both groups, but the increases were significantly less in the dexmedetomidine group (p = .018 and 0.038, respectively). In the dexmedetomidine group, the length of intensive care unit stay was shorter (p = .034).
Conclusions: Intraoperative dexmedetomidine infusion did not improve renal function in terms of serum Cr-related indices following CRS and HIPEC. However, as the decrease in CrCl was attenuated and early tubular-injury markers were lower in the dexmedetomidine group, dexmedetomidine may have protective effects against early tubular injury in CRS and HIPEC.
Clinical Trials Registry: http://clinicaltrials.gov (NCT02641938).
Introduction
Despite better patient selection and advanced perioperative management in recent years, the risk of postoperative morbidity after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) remains highly concerning [Citation1–3]. Particularly, the main features of CRS and HIPEC comprising multiple visceral resections, extensive peritonectomy and delivery of hyperthermic chemotherapy is inevitably accompanied by factors associated with renal injury [Citation4–6]. Hemodynamic instability and increased intraabdominal pressure by instilled perfusate contribute to renal hypoperfusion [Citation4,Citation5]. Thermal stress-induced renal sympathetic excitation further predisposes the kidney to ischemic insult [Citation7]. Moreover, systemic inflammation induced by extensive tissue injury is responsible for direct tubular damage [Citation8]. Indeed, postoperative renal dysfunction affects up to 30% of patients undergoing CRS and HIPEC [Citation9], which is undoubtedly linked to poor outcomes [Citation10–13]. However, few studies have identified or tested any potential renal protective strategies.
Dexmedetomidine is a highly selective α2 adrenoreceptor agonist that is widely used for sedation and analgesia [Citation14]. Recently, its organ-protective properties, beyond the original use, have emerged [Citation15,Citation16]. Especially, the renoprotective effect of dexmedetomidine has been supported by a large body of experimental evidence. In animal models of sepsis and ischemia-reperfusion (IR), dexmedetomidine reduced renal injury through its anti-inflammatory, anti-oxidative stress and anti-apoptotic actions [Citation17–19]. Dexmedetomidine showed an inhibitory effect on the excitement of the renal sympathetic nerves, leading to preserved renal perfusion and tubular function [Citation20–21]; it reportedly attenuates renin secretion and increases glomerular filtration by activation of adrenoreceptors in the renal vasculature and tubules [Citation21,Citation22]. In conjunction, several randomized clinical trials on cardiac surgery have reported that perioperative dexmedetomidine infusion reduced the incidence and severity of acute kidney injury (AKI) [Citation23–25]. In contrast, investigation on percutaneous nephrolithotomy revealed no benefit of dexmedetomidine except for reduced renin secretion [Citation26]. Moreover, its renal protective efficacy has never been explored in other clinical scenarios.
Thus, in this prospective, randomized, placebo-controlled study, we aimed to investigate the effect of intraoperative dexmedetomidine infusion on postoperative renal function after CRS and HIPEC. We assessed urinary biomarkers of early tubular injury, neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule (KIM)-1, in addition to the serum creatinine-derived measure of renal function.
Materials and methods
This study was conducted at Yonsei University College of Medicine, Seoul, Republic of Korea between December 2015 and November 2017 after approval by the institutional review board (3-2015-0262) and registration at http://clinicaltrials.gov (NCT02641938). Patients aged ≥20 years scheduled for CRS and HIPEC for peritoneal carcinomatosis from non-ovarian malignancies were considered eligible. Patients were excluded if they had pre-existing chronic kidney disease (CKD) stage ≥4 (estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73m2) defined by Kidney Disease: Improving Global Outcomes (KDIGO) [Citation27], congestive heart failure, atrioventricular block >1°, bradycardia <45 beats/min, myocardial infarction within 3 months or pregnancy. Written informed consent was obtained from all study participants.
Intervention and study protocol
Enrolled patients were allocated randomly into either the control or dexmedetomidine group at a 1:1 ratio using a computer-generated randomization list. Group assignment was concealed using opaque and sequentially numbered envelopes. Envelopes were opened on the morning of surgery by the attending anesthesiologist who would prepare 200 µg of dexmedetomidine (Precedex® 100 µg/mL; Hospira, Lake Forest, IL) added to 0.9% sodium chloride 48 ml to achieve a concentration of 4 µg/mL or the same volume of 0.9% sodium chloride. After induction of anesthesia, the dexmedetomidine group received an intravenous dose of 1 µg/kg over 20 min followed by continuous infusion of 0.5 µg/kg/h until the end of surgery. The control group received the same volume of 0.9% sodium chloride. The participants, surgeon, medical team in the intensive care unit (ICU), and outcome assessor were blinded to group allocation until the end of the study.
All participants underwent a standardized surgical technique [Citation28]. In brief, a midline laparotomy was followed by resection of the metastatic organs and peritonectomy aiming at removal of all visible disease. HIPEC was subsequently performed by circulating 3 L of hypertonic solution (Dianeal, 1.5% Dextrose Peritoneal Dialysis Solution; Boxter Healthcare Corp., Deerfield, IL) maintained at 42 to 43 °C mixed with three divided doses of mitomycin-C (totally 35 mg/m2) at a rate of 800–1000 ml/min for 90 min using a HIPEC pump (Belmont Hyperthermia Pump; Belmont Instrument Corp., Billerica, MA). The anastomosis of the resected bowels was followed by complete abdominal closure. Diverting ostomy was performed at the surgeon’s discretion.
Hemodynamic monitoring included the cardiac index (CI) and stroke volume variation (SVV) derived from the VigileoTM System (Edwards Lifesciences, Irvine, CA) and central venous pressure (CVP) obtained from the internal jugular vein. Anesthesia was maintained with sevoflurane inhalation and remifentanil infusion. Mean arterial pressure (MAP) was maintained between 60 and 80 mmHg with adequate fluid resuscitation and the use of norepinephrine. Goal-directed fluid therapy was performed under the guidance of SVV with concomitant consideration of preoperative deficit, maintenance and surgical loss. Balanced crystalloid (Plasma Solution A Inj.; CJ Pharma, Seoul, Korea) was administered as a primary resuscitation fluid and balanced synthetic colloid (Volulyte; Fresenius Kabi, Bad Homburg, Germany) was administered up to 1000 ml to compensate for blood loss. Packed red blood cells (pRBC) were transfused when the hematocrit was <25%. A 20% human albumin solution (SK chemical, Seoul, Korea) was administered to maintain serum albumin concentration at >2.0 g/dL. Active cooling with a cooling mattress forced air and infusion of cold fluids was performed during HIPEC. After surgery, all participants were transferred to the ICU with or without extubation at the attending anesthesiologist’s discretion. Postoperative care was performed by the attending physician and intensivist.
To minimize perioperative drug-induced nephrotoxicity, nonsteroidal anti-inflammatory drugs were discontinued before surgery. A third-generation cephalosporin was administered as a prophylactic antibiotic agent. Patients on neoadjuvant chemotherapy received the last dose at least 6 weeks before surgery.
Outcome measures
The primary endpoint was creatinine clearance (CrCl) derived by the Cockroft–Gault equation [(140–age) × weight (kg)/72 × serum creatinine (mg/dL)] × (0.85 only in female patients) [Citation29]. The secondary endpoints were the incidence of AKI according to the KDIGO guideline [Citation30] (stage 1 = absolute increase in serum Cr ≥0.3 mg/dL within 48 h or increase to 150–200% of the baseline value within 7 days or urine output <0.5 ml/kg/h for 6–12 h, stage 2 = serum Cr increase to 2.0–2.9 the baseline level or urine output <0.5 ml/kg/h for ≥12 h, stage 3 = serum Cr ≥3 the baseline level, ≥4 mg/dL renal replacement therapy or urine output <0.3 ml/kg/h for ≥24 h or anuria for ≥12 h), eGFR, and urinary biomarkers for AKI including NGAL and (KIM)-1. Serum Cr levels were assessed within 2 weeks prior to surgery (baseline), immediately after surgery, and thereafter at least once per day for the first 7 postoperative days. Urinary NGAL and KIM-1 were assessed immediately before surgery, immediately after surgery and 24 h after surgery. Thirty-day morbidity endpoints including cardiovascular complications (myocardial ischemia, arrhythmia, stroke), pulmonary complications (pleural effusion requiring intervention, pneumonia, mechanical ventilation >24 h), gastrointestinal complications (leakage, ischemia, obstruction) and requirement of renal replacement therapy were assessed. The length of ICU and hospital stays after surgery was also recorded.
Perioperative variables were demographic data, history of chronic illness, cancer pathology, serum hemoglobin and albumin concentration, prior surgical score, neoadjuvant chemotherapy, total surgery time, intraoperative peritoneal cancer index, completeness of cytoreductive score, combined surgery, fluid balance, pRBC transfusion, urine output, blood loss and amount of norepinephrine infusion. Intraoperative hemodynamic variables including MAP, heart rate (HR), CI, SVV and CVP were assessed after induction of anesthesia, immediately before HIPEC, during HIPEC at 15 min intervals, and immediately after surgery.
Statistical analysis
Sample size calculation was performed using PASS (version 13.0.11; NCSS Statistical Software, Kaysville, UT). The postoperative minimum CrCl in patients who underwent CRS and HIPEC in our institution for 1 year prior the present study was 76 ± 18 ml/min. Expecting an improvement of 20% in CrCl with dexmedetomidine administration, 17 patients were required in each group in order to obtain a power of 80%, considering a type I error of 0.05. Assuming a 10% dropout rate, we decided to enroll 38 patients.
Data were analyzed using SPSS 23.0 (IBM Corp., Armonk, NY) and R package version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) and expressed as mean ± SD, median [interquartile range], or a number of patients (%). After Shapiro–Wilk testing for normality of variable distribution, continuous variables were compared between the groups using the independent t-test or Mann–Whitney U test, as appropriate. Categorical variables were compared between the groups using the chi-square or Fisher’s exact test, as appropriate. For between-group comparisons of repeated measures including urinary biomarkers and hemodynamic data, the Brunner–Langer method or linear mixed model was used after normality testing. Group, time and group-by-time as fixed effects were considered, and times were clustered within patients. A p value <.05 was considered statistically significant.
Results
Fifty patients were assessed for eligibility and 12 patients were excluded before randomization. The data of all 38 enrolled patients were statistically analyzed (). Patient characteristics were comparable between the groups (). All patients with preexisting hypertension had been using calcium channel blockers. Three patients in each group were diagnosed as having CKD and all were classified as stage 2, defined as eGFR 60–89 ml/min/1.73 m2 [Citation27]. The most common origin of peritoneal carcinomatosis was colorectal cancer in both groups.
Figure 1. CONSORT diagram showing the flow of participants through each stage of the randomized trial.
Table 1. Patient characteristics.
As shown in , operative details operative details including perioperative fluid balance, transfusion and vasopressor use were similar between the groups were similar between the groups. All participants except one in the dexmedetomidine group were extubated in the operating room. The patient who was transferred to the ICU in an intubated state was extubated 6 h postoperatively.
Table 2. Perioperative data.
Intraoperative hemodynamic variables are shown in . The change in HR over time was significantly different between the groups (p < .001). On post-hoc analysis, increase in HR from baseline was significantly lower in the dexmedetomidine group than in the control group immediately before HIPEC (p < .001), during HIPEC (p < .001) and immediately after surgery (p = .033). There were no significant differences in MAP, CI, SVV and CVP changes over time between the groups. Within the groups, HR and SVV were significantly elevated compared with the baseline value throughout the surgery in the control group. The dexmedetomidine group showed significantly decreased HR immediately before HIPEC and elevated SVV immediately after surgery compared with the baseline values. CI was significantly elevated immediately before and during HIPEC compared with the baseline value in both groups. MAP and CVP were significantly decreased immediately before HIPEC in the dexmedetomidine group and restored during HIPEC.
As shown in , the highest serum Cr and the lowest eGFR during the first 7 postoperative days were similar between the groups. Although there was no statistically significant difference in the lowest CrCl, the value was 20% higher in the dexmedetomidine group than in the control group (95.57 ± 30.56 and 82.49 ± 31.20 ml/min, respectively, p = .082). The change in CrCl over time was not different between the groups, whereas the % change of CrCl from the baseline to the lowest CrCl during first 7 postoperative days was significantly lower in the dexmedetomidine group (p = .037). There was no difference in the incidence of AKI according to the KDIGO guideline between the groups, and all patients who developed AKI were classified as stage 1 [Citation30].
Table 3. Renal outcomes and 30-day morbidity endpoints.
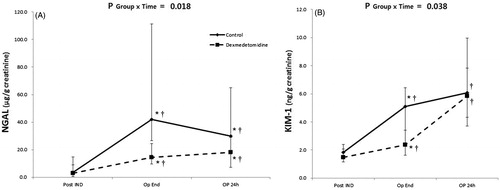
As shown in , there was a significant difference in the change in urine NGAL and KIM-1 over time between the groups (p = .018 and 0.038, respectively). On post-hoc analysis, increase in urine NGAL from the baseline value was significantly lower in the dexmedetomidine group immediately after surgery (p = .004) and 24 h after surgery (p = .037). Post-hoc analysis of the change in urine KIM-1 from baseline revealed a significantly lesser change in the dexmedetomidine group at 24 h after surgery (p = .034).
Figure 2. Serial changes in hemodynamic parameters. (A) MAP (mean arterial pressure), (B) HR (heart rate), (C) CI (cardiac index), (D) SVV (stroke volume index), (E) CVP (central venous pressure). Pre-HIPEC: immediately before hyperthermic intraperitoneal chemotherapy (HIPEC); HIPEC: during HIPEC (average of the values assessed at 15 min interval), OP end: immediately after surgery. P Group × Time = p values of the group and time interaction obtained by the linear mixed model. *p < .05 versus control group; †p < .05 versus before surgery. The values are expressed as mean with SD.
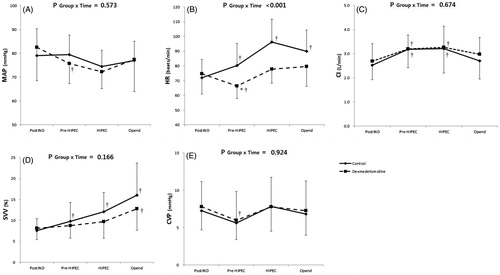
Figure 3. Perioperative urinary biomarkers. Serial changes in urine neutrophil gelatinase-associated lipocaline (NGAL)/creatinine ratio (A) and kidney injury molecule (KIM)-1/creatinine ratio (B). PostIND: after induction of anesthesia; OpEnd: immediately after surgery; OP 24 h: 24 h after surgery. P Group × Time = p values of the group and time interaction obtained by the Brunner–Langer model. *p < .05 versus control group; †p < .05 versus before surgery. The values are expressed as median with IQR.
The incidence of postoperative 30-day morbidity was comparable between the groups (). The length of ICU stay was significantly shorter in the dexmedetomidine group than in the control group (p = .034).
Discussion
In the present study, we observed a possible renoprotective effect of intraoperative dexmedetomidine infusion in patients undergoing CRS and HIPEC. Although there were no significant differences in serum Cr, CrCL and AKI incidence, urine levels of NGAL and KIM-1, which are sensitive markers for early tubular damage were significantly less elevated in the dexmedetomidine group. The length of ICU stay was also significantly reduced in the dexmedetomidine group.
Previous randomized controlled trials on cardiac surgery with cardiopulmonary bypass have reported that perioperative infusion of dexmedetomidine reduced the incidence and severity of AKI [Citation23–25]. Conversely, we could not find a meaningful impact of dexmedetomidine on CrCl and AKI incidences based on the increase of serum Cr in CRS and HIPEC. However, the urinary levels of NGAL and KIM-1 were significantly less increased by dexmedetomidine. Such renal biomarkers were also shown to be affected or not affected by dexmedetomidine depending on the study [Citation24–26]. This inconsistency could be attributed to differences in the degree of insults and responses arising from the nature of the surgery and patient-related risks. The absence of clinical benefit in percutaneous nephrolithotomy shown by a recent clinical study might be explained in the same context, considering that there were lesser inflammatory responses and preexisting post-renal damage [Citation26]. In this regard, patients undergoing CRS and HIPEC for ovarian cancer who are at much higher risk for renal failure associated with cisplatin [Citation31] may receive a greater benefit from dexmedetomidine [Citation32], but this requires further analysis.
Despite the fact that renal compromise is the major complication of CRS and HIPEC, few studies have suggested protective strategies. Amifostine, an organic thiophosphate prodrug, showed positive results in a phase I study nearly 2 decades ago, but there were no follow-up next-phase trials [Citation33]. Sodium thiosulfate, a well-known cytoprotective adjuvant in chemotherapy or high-volume hydration combined with magnesium supplementation [Citation34] has also been used empirically but never been tested in clinical trials on this surgery. Moreover, the protective mechanisms of these agents are mainly associated with cisplatin-induced nephrotoxicity. In contrast, dexmedetomidine has an experimental background and clinical experience upon the IR injury, inflammation and sympathetic excitation [Citation17–25].
Of note, intraoperative infusion of dexmedetomidine provided hemodynamic stability during HIPEC in the present study. Tachycardia is the most prominent hemodynamic change observed during HIEPC [Citation4]. It occurs as a consequence of heating-induced sympathetic stress and as a compensatory mechanism to preserve cardiac output in response to markedly decreased systemic vascular resistance. However, impaired ventricular filling caused by shortened diastolic time may additionally contribute to reduced preload causing hypovolemic states during HIPEC [Citation35]. Moreover, tachycardia may severely disturb ventricular-arterial mismatch, leading to reduced stroke volume and cardiovascular efficiency despite the similar blood pressure and cardiac output [Citation36]. Hence, attenuation of tachycardia by dexmedetomidine may exert augmenting effects on systemic perfusion, which might also provide protection against hypoxic damage of the kidney. The SVV during HIPEC was greater in the control group indicating more hypovolemic states in patients who did not receive dexmedetomidine, although not reaching statistical significance (p = .081). Higher HR and SVV often require aggressive fluid resuscitation that may harm the patients in various ways [Citation37]. Indeed, we observed a trend toward the greater volume of fluid administration in the control group, but its association with the postoperative outcomes cannot be determined from the current results.
Along with renoprotective effects, perioperative 24 h infusion of dexmedetomidine in valvular heart surgery was associated with a shorter duration of ICU stay and lower incidence of stroke and mechanical ventilation >48 h [Citation23]. Possible mechanisms described by authors were anti-inflammation and anti-apoptosis-mediated protective effects on multiple organs and sedative action without respiratory depression. Intraoperative infusion of dexmedetomidine in our study was also related to shorter ICU stay, although the occurrence of major morbidities was not influenced. Considering that most of our study participants were extubated in the operating room unlike cardiac surgical patients, the lower requirement of intensive care may be mainly attributable to intraoperative hemodynamic stability and organ protective effects provided by dexmedetomidine.
This study is subject to certain limitations. First, we did not include patients with ovarian cancer. As the mechanism of renal damage and severity and incidence of AKI are different [Citation31], our results cannot be extrapolated to patients undergoing CRS and HIPIC due to ovarian cancer. Considering the theoretical background of the protective effect of dexmedetomidine against cisplatin-induced nephrotoxicity [Citation32], further study in this cohort might be promising. Second, we used an indirect measure of CrCl as the primary endpoint to determine renal function, considering the great likelihood of loss and error in urine collection during 24 h [Citation38]. Although CrCl calculated by the Cockroft–Gault equation is often considered inaccurate, the trend of changes is highly correlated to the 24 h urine-derived value and able to reflect the alterations in renal function appropriately [Citation38]. Third, the small sample size may account for our inconclusive evidence regarding the effects of dexmedetomidine on the clinical indices of renal function. Although CrCl was 20% higher in the dexmedetomidine group as we hypothesized, the difference was not significant because both the mean value and standard deviation were higher than we expected. Nonetheless, a significant decrease in NGAL and KIM-1 can support our argument for the use of dexmedetomidine in this patient cohort during surgery. Indeed, biomarker analysis has recently been recognized as an alternative to serum Cr-related variables, as those are debated over diagnostic relevance on account of time delay and confounders [Citation39]. Moreover, NGAL and KIM-1 are widely accepted indicators of early tubular injury, which is the major mechanism of renal compromise associated with CRS and HIPEC including hypoperfusion and severe inflammation [Citation8,Citation40].
In conclusion, intraoperative infusion of dexmedetomidine in patients undergoing CRS and HIPEC did not significantly improve renal function in terms of serum Cr-related indices. However, it showed a protective effect against early tubular injury, which was reflected by urinary NGAL and KIM-1 levels. Dexmedetomidine also provided hemodynamic stability during surgery and was associated with shorter length of ICU stay. Further large-scale investigations with mechanistic insights are necessary to clarify our findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rubino MS, Abdel-Misih RZ, Bennett JJ, et al. Peritoneal surface malignancies and regional treatment: a review of the literature. Surg Oncol. 2012;21:87–94.
- Chua TC, Yan TD, Saxena A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900–907.
- Malfroy S, Wallet F, Maucort-Boulch D, et al. Complications after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis: risk factors for ICU admission and morbidity prognostic score. Surg Oncol. 2016;25:6–15.
- Coccolini F, Corbella D, Finazzi P, et al. Time course of cytokines, hemodynamic and metabolic parameters during hyperthermic intraperitoneal chemotherapy. Minerva Anestesiol. 2016;82:310–319.
- Ceresoli M, Coccolini F, Ansaloni L. HIPEC and nephrotoxicity: a cisplatin induced effect?. Eur J Surg Oncol. 2016;42:909–910.
- Meersch M, Schmidt C, Zarbock A. Perioperative acute kidney injury: an under-recognized problem. Anesth Analg. 2017;125:1223–1232.
- Lambert E, Schlaich M. The role of renal sympathetic nerves in ischemia reperfusion injury. Auton Neurosci. 2017;204:105–111.
- Prowle JR, Bellomo R. Sepsis-associated acute kidney injury: macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin Nephrol. 2015;35:64–74.
- Arjona-Sanchez A, Cadenas-Febres A, Cabrera-Bermon J, et al. Assessment of RIFLE and AKIN criteria to define acute renal dysfunction for HIPEC procedures for ovarian and non ovarian peritoneal malignances. Eur J Surg Oncol. 2016;42:869–876.
- Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448.
- Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370.
- Thakar CV, Yared JP, Worley S, et al. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003;64:239–246.
- Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858.
- Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs. 2015;75:1119–1130.
- Jiang L, Hu M, Lu Y, et al. The protective effects of dexmedetomidine on ischemic brain injury: a meta-analysis. J Clin Anesth. 2017;40:25–32.
- Cai Y, Xu H, Yan J, et al. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury. Mol Med Rep. 2014;9:1542–1550.
- Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153.
- Kocoglu H, Ozturk H, Ozturk H, et al. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70–74.
- Hsing CH, Lin CF, So E, et al. α 2 -Adrenoceptor agonist dexmedetomidine protects septic acute kidney injury through increasing BMP-7 and inhibiting HDAC2 and HDAC5. Am J Physiol Renal Physiol. 2012;303:F1443–F1453.
- Xu H, Aibiki M, Seki K, et al. Effects of dexmedetomidine, an alpha2-adrenoceptor agonist, on renal sympathetic nerve activity, blood pressure, heart rate and central venous pressure in urethane-anesthetized rabbits. J Auton Nerv Syst. 1998;71:48–54.
- Gellai M, Ruffolo RR. Renal effects of selective alpha-1 and alpha-2 adrenoceptor agonists in conscious, normotensive rats. J Pharmacol Exp Ther. 1987;240:723–728.
- Taoda M, Adachi YU, Uchihashi Y, et al. Effect of dexmedetomidine on the release of [3H]-noradrenaline from rat kidney cortex slices: characterization of alpha2-adrenoceptor. Neurochem Int. 2001;38:317–322.
- Cho JS, Shim JK, Soh S, et al. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. 2016;89:693–700.
- Zhai M, Kang F, Han M, et al. The effect of dexmedetomidine on renal function in patients undergoing cardiac valve replacement under cardiopulmonary bypass: a double-blind randomized controlled trial. J Clin Anesth. 2017;40:33–38.
- Jo YY, Kim JY, Lee JY, et al. The effect of intraoperative dexmedetomidine on acute kidney injury after pediatric congenital heart surgery: a prospective randomized trial. Medicine. 2017;96:e7480.
- Bayram A, Esmaoglu A, Akin A, et al. The effects of intraoperative infusion of dexmedetomidine on early renal function after percutaneous nephrolithotomy. Acta Anaesthesiol Scand. 2011;55:539–544.
- Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735.
- Park EJ, Baik SH, Hur H, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colorectal cancer with peritoneal carcinomatosis: clinical outcomes at 2 tertiary referral centers in Korea. Medicine. 2017;96:e6632.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Kellum JA, Lameire N. KDIGO AKI Guideline Work Group, Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204.
- Tan GHC, Shannon NB, Chia CS, et al. Platinum agents and mitomycin C-specific complications in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Int J Hyperthermia. 2018;34:595–600.
- Liang H, Liu HZ, Wang HB, et al. Dexmedetomidine protects against cisplatin-induced acute kidney injury in mice through regulating apoptosis and inflammation. Inflamm Res. 2017;66:399–411.
- Vaira M, Barone R, Aghemo B, et al. Renal protection with amifostine during intraoperative peritoneal chemohyperthermia (IPCH) with cisplatin (CDDP) for peritoneal carcinosis. Phase 1 study. Minerva Med. 2001;92:207–211.
- Yamamoto Y, Watanabe K, Tsukiyama I, et al. Hydration with 15 mEq magnesium is effective at reducing the risk for cisplatin-induced nephrotoxicity in patients receiving cisplatin (≥ 50 mg/m2) combination chemotherapy. Anticancer Res. 2016;36:1873–1877.
- Zimpfer M, Khosropour R, Lackner F. Effect of dobutamine on cardiac function in man: reciprocal roles of heart rate and ventricular stroke volume. Crit Care Med. 1982;10:367–370.
- Morelli A, Singer M, Ranieri VM, et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med. 2016;42:1528–1534.
- Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–632.
- Herrera-Gutierrez ME, Seller-Perez G, Banderas-Bravo E, et al. Replacement of 24-h creatinine clearance by 2-h creatinine clearance in intensive care unit patients: a single-center study. Intensive Care Med. 2007;33:1900–1906.
- van Meer L, Moerland M, Cohen AF, et al. Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br J Clin Pharmacol. 2014;77:947–957.
- Vlaeminck-Guillem V, Bienvenu J, Isaac S, et al. Intraperitoneal cytokine level in patients with peritoneal surface malignancies. A study of the RENAPE (French Network for Rare Peritoneal Malignancies). Ann Surg Oncol. 2013;20:2655–2662.
