Abstract
Purpose: We investigated the risk factors influencing MR changes associated with sacral injury from ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for uterine fibroids.
Methods: We retrospectively analyzed a total of 346 patients with symptomatic uterine fibroids who received USgHIFU ablation. All of the patients underwent contrast-enhanced magnetic resonance imaging (CE-MRI) before and after treatment. Injury to the sacrum was set as the dependent variable, while fibroid features and the treatment parameters were set as independent variables. These variables were used to assess respectively their correlation with sacral injury by using univariate and multivariate analyses.
Results: The results of univariate analysis revealed that the volume, distance from the fibroid to the skin, maximal diameter, distance from the fibroid to the sacrum, fibroid types, degree of enhancement, therapeutic dosimetry (TD), energy efficiency factor (EEF) and non-perfused volume (NPV) ratio manifested significant correlations with the sacral injury (p < .05). Multivariate analysis showed that the degree of enhancement, TD and EEF were independent risk factors for sacral injury (p < .05), while the distance from fibroid to sacrum and intramural or subserosal types were protective factors (p < .05). The incidence of sacral tail pain and leg pain showed a significant positive correlation with sacral injury (p < .05).
Conclusion: As important affecting factors, the degree of enhancement, distance from fibroid to sacrum and fibroid types all possess significant correlations with MR changes associated with sacral injury.
1. Introduction
Uterine fibroids are the most common benign tumors of female reproductive organs. These benign tumors are generally reported in 20–25% of women of reproductive age and 30–40% of women over 40 years of age [Citation1,Citation2]. Uterine fibroids have more recently been diagnosed in an increasing number of young female patients, and a series of fibroid-associated symptoms were observed in almost half of these patients, including menorrhagia, anemia, infertility, chronic pelvic pain, frequent urination and constipation. Traditional treatment methods included hysterectomy or myomectomy and uterine artery embolization; these methods were limited, however due to serious complications [Citation3].
The treatment of uterine fibroids has gradually progressed historically from surgical techniques to minimally invasive or non-invasive directions to avoid the risk of pelvic adhesions, longer hospitalizations or general anesthesia. As a non-invasive technique, USgHIFU has been widely used of late in the treatment of uterine fibroids, which can effectively relieve the fibroid-related symptoms [Citation4–6]. The ultrasound beam energy can be focused while it is passed through the soft tissues of the body, inducing coagulation necrosis of the target fibroid. However, thermal injury to the adjacent structures may occur during USgHIFU ablation including skin burning, fat edema, back or buttocks pain and/or vaginal discharge owing to the effects of ultrasound beams on living tissues [Citation7].
In clinical practice, we have often observed sacral injury on postoperative CE-MRI examination when the targeted fibroid was completely ablated. The sacrum was likely to sustain injury when USgHIFU was applied to fibroids with certain features that are known to be resistant to HIFU heating. The energy of the focus always extends along the axis of propagation of the ultrasound wave, with the sacrum more likely to be injured when it is perpendicular or nearly perpendicular to the USgHIFU beam axis. In addition, the sacrum is primarily supplied by terminal blood vessels and as its blood flow is relatively slow, the beam energy is not easily dissipated through the circulation, which results in significant thermal injury to the sacrum. However, there has been no specific investigation of sacral injury during USgHIFU ablation of uterine fibroids. Therefore, the aim of our study was to further analyze the factors that influence sacral injury and to evaluate the relationships between sacral injury and the features of fibroids.
2. Materials and methods
2.1. Patients
We retrospectively collected data on 346 patients who underwent USgHIFU at the First Affiliated Hospital of Kunming Medical University from August 2012 to December 2017 and the study was approved by the Ethics Committee of the Hospital. Importantly, we only collected data on patients who were treated for a single fibroid. All of the patient information was retrospectively collected based upon preoperative and postoperative MRI images, hospitalization records, treatment parameters and outpatient records. Patient baseline data included age, volume of the fibroid, distance from fibroid’s ventral side to the skin, maximal diameter, the distance from the fibroid’s dorsal side to the sacrum, uterine position (anteverted, mid position or retroverted), location of the fibroid [Citation8] (anterior, fundus, lateral wall or posterior), types of fibroid [Citation9] (intramural, subserosal, submucosal or transmural), T2WI [Citation10] (hypointense, isointense, heterogeneous or markedly homogeneous hyperintense) and degree of enhancement [Citation11] (slight, intermediate or progressive). Treatment parameters included TD, EEF, NPV, NPV ratio and treatment efficiency. All of the MRI images of the preoperative and postoperative region were evaluated and the three dimensions of the fibroids and non-perfused areas were measured by two experienced radiologists: longitudinal (a), anteroposterior (b) and transverse (c). The volume of fibroids and non-perfused regions were calculated according to the following equation: V = 0.5233 × a × b × c, as determined by Wang et al. [Citation12].
2.2. Magnetic resonance imaging
To evaluate the feasibility of USgHIFU ablation and the efficacy of treatment, CE-MRI was performed on each patient before and after USgHIFU treatment. A Philips 3.0 T MR scanner (Best, Netherlands) or GE 1.5 T MR scanner (Waukesha, USA) was used. The images in sagittal, axial and coronal planes were acquired, respectively, with the slice thickness/spacing =5.0 mm/1.0 mm. With regard to T2-weighted imaging (T2WI), fibroids were classified as hypointense (with a signal intensity similar to that of skeletal muscle), isointense (signal intensity higher than skeletal muscle, but lower than myometrium), heterogeneous (the overall signal intensity composed of interspersed hypo-intense and iso-intense areas), or markedly homogeneous hyperintense (a signal intensity similar to or higher than that of myometrium), based on the relevant studies [Citation13]. The degrees of fibroid enhancement were classified as a slight enhancement (where the degree of fibroid enhancement was lower than that of myometrium), intermediate (where the degree of enhancement was similar to that of the myometrium) or progressive enhancement (where the degree of enhancement was higher than that of the uterine myometrium).
2.3. UsgHIFU treatment
Treatment was performed with a Model-JC 200 focused ultrasound tumor therapeutic system (Chongqing HIFU Technology Inc., Chongqing, China), and the system was connected to a B-model ultrasound scanner (MyLab 70, Esaote, Italy) for monitoring the target area and adjacent structures. Before treatment, each patient signed informed consent and completed preoperative preparation (lower abdominal skin preparation included degreasing and degassing, and a cleansing enema); and the patient’s bladder was filled with normal saline using a Foley catheter to control volume and prevent intestinal injury.
During the procedure, each patient was placed on the treatment table in a prone position so that the skin of the abdomen was in complete contact with the degassed water. In addition, the position and size of the degassed water balloon were adjusted by a real-time guided ultrasonographic device to move the intestines out of the acoustic pathway. Vital signs of patients, including blood pressure, heart rate, oxygen saturation and breathing rate, were observed during the operation. Ultrasonic contrast agents (SonoVue, Bracco, Italy) were used to evaluate the condition of the necrotic tissue when the fibroid was completely ablated. All of the treatment parameters were recorded immediately after treatment, including treatment time, sonication power and sonication time.
To evaluate the safety of USgHIFU ablation, we recorded the AEs, including lower abdominal pain, sacrum/buttock pain, vaginal discharge, leg numbness/pain, erythema on skin, skin burns, sciatic nerve injury and other signs after HIFU treatment. Following the Society of Interventional Radiology (SIR) classification system, the severity of AEs were evaluated as follows: (1) Class A: no therapy, no consequence; (2) Class B: nominal therapy or no consequence, including overnight admission for observation only; (3) Class C: require therapy, minor hospitalization (<48 h); (4) Class D: required major therapy, including unplanned increased in level of care, or prolonged hospitalization (>48 h); (5) Class E: permanent adverse sequelae; (6) Class F: death [Citation14].
The MRI examination was performed for each patient after HIFU ablation. The three dimensions of the non-perfused area were measured in the CE-MRI images and the observation indices were further calculated based upon the treatment parameters and the NPV, including total energy (J, the energy required to treat the entire fibroid); EEF (J/cm3, the energy required to ablate the fibroid per unit volume); treatment efficiency (s/mm3, the sonication time required to ablate the fibroid per unit volume); and NPV ratio (the ratio [%] of NPV-to-fibroid volume).
2.4. Statistical analysis
Continuous variables were expressed as means ± standard deviation (if the data were normally distributed) or median and interquartile range (if the data were not normally distributed). Univariate analysis was performed to compare the volume, distance from the fibroid ventral side to skin, maximal diameter and the distance from the fibroid dorsal side to the sacrum. Qualitative data (position of uterus, location of fibroids, fibroid types, T2WI and degree of enhancement) were analyzed with the χ2 test. The significant independent variables were evaluated as to their relationships with the sacrum by using an unconditional logistic regression model. The treatment parameters (TD, EEF, NPV, treatment efficiency and NPV ratio) were assessed by the independent sample t test or the rank-sum test; and the TD, EEF and NPV ratio were then evaluated by multivariable analysis. The statistical analysis was completed using SPSS 17.0 (IBM, Armonk, NY), and p < .05 was considered to be statistically significant. Our statistical analysis using the rank-sum test was two-sided and conducted at an alpha level of 0.05.
3. Results
3.1. Patients and uterine fibroids
Patient baseline data were divided into two sets: with sacral injury (39.0%) or without sacral injury (61.0%). The average age of the 346 patients was 38.3 ± 6.1 years (mean ± SD, range 23–53). The mean volume and maximal diameter of the fibroid, respectively, were 83.5 ± 69.0 cm3 and 5.6 ± 1.6 cm. The average distance from the fibroid to skin was 5.2 ± 2.1 cm. The independent sample t test or the χ2 test was used to analyze data and we found that the age, distance from fibroid to skin, uterine location, T2WI and degree of enhancement were not statistically significant between the two data sets, while the volume of fibroid, maximal diameter, distance from the fibroid dorsal side to sacrum and types of fibroid showed significant associations with sacral injury ().
Table 1. Univariate analysis to evaluate the relationship between the sacral injury and the features of fibroids.
3.2. Correlation between fibroid features and sacral injury
In , the sacral injury was set as a dependent variable and the transmural type was as the control group set as the categorical variable. An unconditional logistic multivariate analysis was performed to assess the statistically significant factors of univariate analysis, and after adjusting for the logistic regression analysis, the volume of the fibroids and maximal diameter were eliminated. Finally, the fibroid types, distance from the fibroid dorsal side to the sacrum and degree of enhancement were used in the final step of the logistic regression analysis. The results showed that the degree of enhancement was an independent risk factor for sacral injury during the USgHIFU procedure (p < .05), while the distance from the fibroid’s dorsal side to the sacrum was a protective factor (p < .001). Regarding fibroid types, intramural and subserosal types were protective factors with respect to sacral injury.
Table 2. Multivariable binary logistic regression analysis to evaluate the correlation of sacral injury with the significant factors of univariate analysis.
3.3. Relationships between sacral injury and treatment parameters
shows each parameter of USgHIFU treatment that we evaluated by univariate analysis. Sacral injury was set as the dependent variable, and TD, EEF, NPV, treatment efficiency and NPV ratio were set as the dependent variables. The independent sample t test or the rank-sum test was used to assess the relationship between sacral injury and treatment parameters. The results of the t test were as follows: TD (t=–4.493), EEF (t=–2.225), NPV (t=–1.021), and treatment efficiency (t=–1.934); and showed that TD and EEF both correlated well with sacral injury (p < .05), but NPV and treatment efficiency did not. According to the results of the rank-sum test, the NPV ratio (Z=–2.151, p = .031) showed a significant positive correlation with sacral injury (p < .05). Finally, the variables of TD, EEF and NPV ratio were entered into the logistic regression model.
Table 3. Evaluation of the relationship between sacral injury and treatment parameters according to univariate analysis.
3.4. Correlations between sacral injury and significant treatment parameters
Significant treatment parameters were further evaluated by multivariate analysis as shown in . The results showed that EEF and TD were risk factors for sacral injury (p < .05), indicating that the higher the EEF or TD, the more easily the sacrum was injured. The NPV ratio had no significant relationship with sacral injury in the multivariate analysis.
Table 4. corralation of the sacral injury with the significant treatment parameters according to multivariate analysis.
3.5. Adverse events
Following the SIR classification as shown in , a total of 229 (61.9%) adverse events (AE) were classified as Class A, 71 (19.1% of) events were classified as Class B, 3 (0.9% of) events were classified as Class C, and 2 (0.6% of) events were classified as Class D. No Class E or F adverse effects occurred in this study. Among the Class A AEs, the main AE was lower abdominal pain, reported by 44.8% of patients, and included 46.7% (63/135) of cases in the injury group, and 43.6% (92/211) of cases in the group without injury. The incidence of sacrum/buttock pain and erythema on skin was 14.1% and 5.2% in the injury group, respectively, and 9.5% and 3.8% in the group without injury, respectively. A total of 6 patients suffered from vaginal discharge and 13 patients reported leg numbness, pain or both after treatment. All of these AEs recovered spontaneously within three days of HIFU ablation without any treatment. Among the Class B AEs, the lower abdominal pain was reported by 15.90% of patients and included 16.3% (63/135) of cases in the injury group, and 15.6% (33/211) of cases in the group without injury. The incidence of sacrum/buttock pain and leg numbness, pain or both was 3.7% and 2.2% in the injury group, respectively, and 0.9% and 0.5% in the group without injury, respectively. A total of 5 patients reported erythema on the skin. Among the Class C AEs, 1 patient in the group without injury reported skin burns and 2 patients in the injury group had leg pain. Among the Class D AEs, 2 patients had sciatic nerve injury and had symptom relief after 3 months with NSAIDs. No significant differences were observed between the two groups in terms of postprocedural AEs (p > .05).
Table 5. Summary of postprocedural adverse effects.
4. Discussion
In the present study, sacral injury was evaluated by postoperative CE-MRI, which is an objective, accurate and imaging-based endpoint. Although the safety of USgHIFU ablation has been demonstrated by various research groups [Citation15–17], there are no published reports from researchers who specifically investigated sacral injury during HIFU ablation. Therefore, it is still important for patients who are suitable for USgHIFU ablation to take potential sacral injury into consideration. As we demonstrated in the present study, many factors were highly correlated with sacral injury; while some were shown to have originated from the treatment parameters, others were related to the fibroid features. It is worth noting that treatment parameters were affected by the characteristics of fibroids and we therefore not only analyzed the relationship between fibroid features and sacral injury in this study, but also investigated the correlation between treatment parameters and sacral injury. We propose to further evaluate the risk factors that influence sacral injury as related to treatment parameters.
In one recent study [Citation18], investigators reported that the degree of enhancement was strongly correlated with the temperature rise due to HIFU treatment, and that perfusion of hyper-enhancing fibroids was improved with greater blood vessel distribution. It is therefore difficult to undergo HIFU ablation since the therapeutic responses of target fibroids depend upon energy deposition. According to the multivariable binary logistic regression, we found that the degree of enhancement was an independent risk factor for sacral injury during treatment, and showed an increasingly commensurate and significant correlation with therapeutic responses. The greater the number of blood vessels the fibroids exhibited, the greater the therapeutic doses that were required. The reason for this was that the increased blood flow allowed energy to be dissipated from the target area, resulting in a decrease in the treatment temperature at the target site. Experienced operators, therefore, need to increase the temperature at the target area by increasing the HIFU therapeutic dose and sonication time. The chance of sacral injury, however, is also increased.
The ultrasound beams may be absorbed, reflected or scattered by the tissues of the acoustic pathway when the focused ultrasound beams are used to ablate deep fibroids and this consequently results in the ultrasonic beam being attenuated. To achieve a high ablation efficiency, the ultrasonic intensity needs to be increased and when the target fibroid is relatively close to the sacrum, the risk of sacral injury increases. In the present study, multivariate analysis showed that the distance from the fibroid’s dorsal side to the sacrum was a protective factor with respect to sacral injury. To prevent such injury to the sacrum and adjacent lumbosacral nerves, operators must maintain a safe distance between the target fibroid’s dorsal side and the sacral surface.
Cho et al. [Citation1] showed that HIFU exerts a specific therapeutic response on uterine fibroids, especially when the diameter of the fibroid is less than 4 cm; large fibroids, however, require an increased acoustic intensity and sonication time to improve the effect. With respect to fibroid types, transmural fibroids are relatively large compared to other types and their blood supply is rich as they supply nutrients. Since blood flow is relatively fast in great vessels, the deposited thermal energy is easily lost along the bloodstream. Therefore, in comparison to intramural or subserosal types, the transmural type required more sonication time and therapeutic intensity to complete the same dimensions of necrotic tissue. As shown in our study, the univariate analyses revealed that the type of fibroids was independently significant with respect to sacral injury. To further demonstrate the relationship between sacral injury and the types of fibroid, we defined the dummy variables as multiple classification variables. These observations suggested that the intramural and subserosal types were protective factors and further verified the theoretical basis of previous studies.
In a recent study [Citation3], EEF and TD were depicted as easily affected by the degree of enhancement, the fibroid’s size, location and T2WI. In their fibroid ablations, Zhang et al. demonstrated a progressive enhancement, a markedly homogeneous hyperintense region (T2WI), a location near the sacrum, or a combination of these indices along with the need for a longer ultrasonic irradiation time, a greater TD and EEF, and a higher occurrence of sacral injury. We studied 5 therapeutic parameters in our study: TD, EEF, NPV, treatment efficiency and NPV ratio. Multivariate analysis demonstrated that EEF and TD were risk factors for sacral injury, showing that it is certainly necessary to reduce EEF and TD to combine different methods and produce synergistic effects. Yang et al. [Citation3] used high-intensity focused ultrasound combined with intratumoral ethanol to effectively reduce the therapeutic dose output, increasing overall ablation efficiency. Other investigators [Citation19,Citation20] used oxytocin to shrink the tumor’s nourishing vascular supply and reduce blood perfusion at the target site, increasing the overall efficiency of energy deposition and reducing total energy output. Furthermore, previous studies [Citation21,Citation22] have confirmed that ultrasound microbubble contrast agents could improve treatment efficiency due to the augmented number of cavitation nuclei at the target site. As the microbubbles burst, the pressure of the acoustic wave increases and the temperature at the target site instantaneously rises to a higher level and destroys tissues, resulting in a reduction in the therapeutic dose and the chance of sacral injury. Thus, we expect that through our study and other studies more effective and safe treatment measures will be provided to patients with symptomatic uterine fibroids who underwent USgHIFU ablation.
Safety is always a sensitive topic in the USgHIFU ablation of uterine fibroids. In accordance with the Society of Interventional Radiology (SIR) classification system for complications by outcome [Citation14], in a total of 305 AEs in 346 patients, 229 out of 305 (75.1%) AEs were classified as Class A, which recovered spontaneously within three days. In addition, 71 of 305 (23.3%) AEs subsided within 1 week without any specific treatment and thus these were classified as grade B. One patient with surgical scars reported second-degree burns in the group without injury; these were classified as Class C because the burnt area necessitated resection. Two patients reported leg pain after treatment because of a temporary sciatic nerve irritation. The pain lasted for 3 months and NSAIDs for pain control were suggested, so this was established as Class D. Sciatic nerve irritation was only observed in the injury group. This could be explained by fibroid features and the treatment parameters. The use of too much water with a degassed water balloon or overfilling the bladder may also have played a role in the sciatic nerve injury. With increased experience and a safe distance between the fibroid and the sacral surface, the rate of sciatic nerve injury could be reduced further. However, the incidence of other AEs, including fever, bowel injury, nausea or vomiting, bladder injury or urinary retention were rare when compared to previous studies [Citation16,Citation23]. Therefore, USgHIFU is considered to be a safe and promising treatment for uterine fibroids.
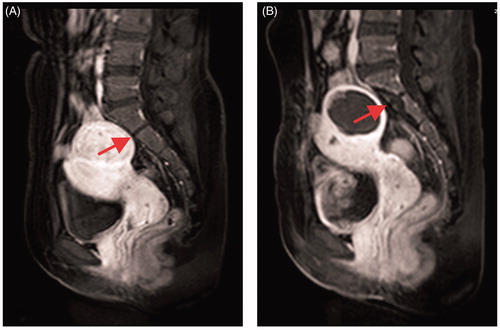
In the present study we observed immediate thermal injury to the sacrum using post-treatment CE-MRI. The MRI signal changes of the sacrum were as follows: the intensity of T1WI was lower than before treatment at the front edge of the sacral vertebral body, while the intensity of T2WI was increased. Additionally, a non-perfused region was observed upon CE-MRI (). This observation may be explained by thermal deposition and by the occlusion of small blood vessels during the procedure and compression of blood vessels by edema.
Figure 1. CE-MRI before and after USgHIFU ablation: (A) sacrum shows normal before treatment; (B) sacrum shows non-perfused upon CE-MRI immediately after treatment.
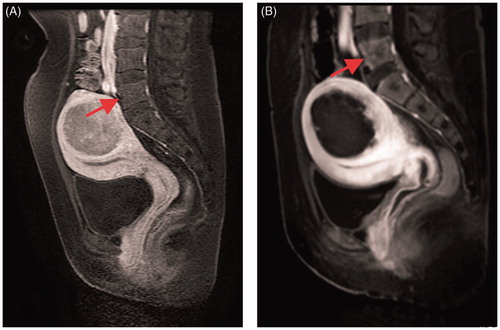
In addition, our study found that very few patients were injured in the lumbar region (), where the patient’s fibroids may be bulky, numerous or close to the lumbar spine. The lumbar spine is the load-bearing vertebral body and it may cause compression fractures after injury. This has not been reported thus far and long-term clinical follow-up studies are needed to confirm the ultimate responses to this injury.
Figure 2. CE-MRI before and after USgHIFU ablation: (A) the fifth lumbar spine shows normal before treatment; (B) the front edge of the fifth lumbar spine shows non-perfused upon CE-MRI immediately after treatment.
Although we found injury to the sacrum after USgHIFU treatment, the normal morphology and structure of the vertebral body were maintained. During follow-up, some patients showed gradual reduction in the non-perfused region of the vertebral body, and this finding demonstrated that the injury may recover spontaneously. However, it is necessary to avoid sacral injury because the MR changes of sacral injury are associated with sacral nerve irritation. In addition, our study entailed a small sample size and is a single-center retrospective analysis, thus requiring high-quality, multicenter-based future studies that have larger samples so as to support definitive conclusions.
In conclusion EEF and TD as quantitative indices indirectly reflect the relationship between the MR changes of sacral injury and the features of fibroids. Based upon the multivariable binary logistic regression model, we showed that ‘degree of enhancement’, ‘distance from fibroid dorsal side to sacrum’ and ‘types of fibroid’ were the important factors for therapeutic dose delivery. In order to further reduce the occurrence of AEs, influencing factors must be fully considered.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cho JY, Kim SH, Kim SY, et al. Efficacy and safety of daily repeated sonographically guided high-intensity focused ultrasound treatment of uterine fibroids: preliminary study. J Ultrasound Med. 2013;32:397–406.
- Zhang W, He M, Huang G, et al. A comparison of ultrasound-guided high intensity focused ultrasound for the treatment of uterine fibroids in patients with an anteverted uterus and a retroverted uterus. Int J Hyperthermia. 2016;32:623–629.
- Yang Z, Zhang Y, Zhang R, et al. A case-control study of high-intensity focused ultrasound combined with sonographically guided intratumoral ethanol injection in the treatment of uterine fibroids. J Ultrasound Med. 2014;33:657–665.
- Zhao WP, Chen JY, Chen WZ. Effect of biological characteristics of different types of uterine fibroids, as assessed with T2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablation. Ultrasound Med Biol. 2015;41:423–431.
- Chen Y, Jiang J, Zeng Y, et al. Effects of a microbubble ultrasound contrast agent on high-intensity focused ultrasound for uterine fibroids: a randomised controlled trial. Int J Hyperthermia. 2018;1–5.
- Lee JS, Hong GY, Lee KH, et al. Changes in anti-müllerian hormone levels as a biomarker for ovarian reserve after ultrasound-guided high-intensity focused ultrasound treatment of adenomyosis and uterine fibroid. BJOG: Int J Obstet Gy. 2017;124:18–22.
- Kim YS, Kim TJ, Lim HK, et al. Preservation of the endometrial enhancement after magnetic resonance imaging-guided high-intensity focused ultrasound ablation of submucosal uterine fibroids. Eur Radiol. 2017;27:3956–3965.
- Xiong Y, Yue Y, Shui L, et al. Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia. 2015;31:777–783.
- Kim YS, Lim HK, Rhim H. Magnetic resonance imaging-guided high-intensity focused ultrasound ablation of uterine fibroids: effect of bowel interposition on procedure feasibility and a unique bowel displacement technique. PLoS One. 2016;11:e0155670.
- Peng S, Zhang L, Hu L, et al. Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine. 2015;94:e650.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32:496–503.
- Wang X, Qin J, Wang L, et al. Effect of high-intensity focused ultrasound on sexual function in the treatment of uterine fibroids: comparison to conventional myomectomy. Arch Gynecol Obstet. 2013;288:851–858.
- Zhao WP, Chen JY, Zhang L, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82:e43–e49.
- Cardella JF, Kundu S, Miller DL, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S191.
- Leung JH, Yu SC, Cheung EC, et al. Safety and efficacy of sonographically guided high-intensity focused ultrasound for symptomatic uterine fibroids: preliminary study of a modified protocol. J Ultrasound Med. 2014;33:1811–1818.
- Feng Y, Hu L, Chen W, et al. Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: a retrospective cohort study. Ultrason Sonochem. 2017;36:139–145.
- Chen R, Keserci B, Bi H, et al. The safety and effectiveness of volumetric magnetic resonance-guided high-intensity focused ultrasound treatment of symptomatic uterine fibroids: early clinical experience in China. J Ther Ultrasound. 2016;4:27.
- Keserci B, Duc NM. The role of T1 perfusion-based classification in predicting the outcome of magnetic resonance-guided high-intensity focused ultrasound treatment of adenomyosis. Int J Hyperthermia. 2018;34:306–314.
- Jeong JH, Hong GP, Kim YR, et al. Clinical consideration of treatment to ablate uterine fibroids with magnetic resonance imaging-guided high intensity focused ultrasound (MRgFUS): sonalleve. J Menopausal Med. 2016;22:94–107.
- Zhang X, Zou M, Zhang C, et al. Effects of oxytocin on high intensity focused ultrasound (HIFU) ablation of adenomysis: a prospective study. Eur J Radiol. 2014;83:1607–1611.
- Jiang N, Xie B, Zhang X, et al. Enhancing ablation effects of a microbubble-enhancing contrast agent ("SonoVue") in the treatment of uterine fibroids with high-intensity focused ultrasound: a randomized controlled trial. Cardiovasc Intervent Radiol. 2014;37:1321–1328.
- Isern J, Pessarrodona A, Rodriguez J, et al. Using microbubble sonographic contrast agent to enhance the effect of high intensity focused ultrasound for the treatment of uterine fibroids. Ultrason Sonochem. 2015;27:688–693.
- Wang X, Qin J, Chen J, et al. The effect of high-intensity focused ultrasound treatment on immune function in patients with uterine fibroids: preliminary study. Int J Hyperthermia. 2013;29:225–233.
