Abstract
Background: Secondary hyperparathyroidism (SHPT) is a frequently encountered problem in patients with end-stage renal disease (ESRD). Some patients with severe SHPT could not be managed by medical treatment and are ineligible for surgical resection.
Purpose: Our objective was to evaluate the efficacy, safety of microwave ablation (MWA) on these patients.
Materials and Methods: Between 1 April 2015 and 28 February 2017, 35 patients (M/F 19/16, age 49.8 ± 12.9 years) were enrolled. All patients were treated with MWA. Levels of intact parathyroid hormone (iPTH) and of serum calcium and phosphorus were compared pre- and post-ablation. Repeated-measures ANOVA was used to compare treatment outcomes pre- and post-ablation.
Results: Complete ablation was achieved in all 63 glands in the 35 patients with SHPT. The mean follow-up time was 15.9 ± 2.2 months. The maximum gland diameter was 6–31 mm (mean, 14.9 ± 5.5 mm). The trends of the changes in iPTH and calcium levels showed a curve: the level of iPTH and calcium at 6 months post-ablation were lower than those pre-ablation (both p < .0001); after then iPTH remained relatively stable and the end of follow up, with no rebound (p < .0001), while instead of calcium at the end of follow up was not significantly lower than pre-ablation (p = .462). The trend in the change in phosphate levels showed a straight line; the level of phosphate at 6 months post-ablation and at the end of follow up both were significantly lower than pre-MWA (p < .001). There was no major complication.
Conclusions: In this series, MWA was used successfully to treat SHPT patients who are ineligible for surgical resection.
Introduction
Secondary hyperparathyroidism (SHPT) refers to excessive secretion of parathyroid hormone (PTH) by the parathyroid glands in response to hypocalcemia and associated hyperplasia of the glands. This disorder is seen especially in patients with end-stage renal disease (ESRD) [Citation1,Citation2]. Its serious clinical complications include renal osteodystrophy, neurotoxicity and vascular calcification. The condition has a high impact on fractures and mortality in ESRD patients [Citation3,Citation4]. The majority of patients with SHPT can be managed by medical treatment, such as phosphate binders [Citation5,Citation6], orally active vitamin D sterols [Citation7,Citation8], intravenous vitamin D analogs [Citation9,Citation10] and cinacalcet [Citation11,Citation12]. However, medical treatment does not always result in control of parathyroid disorders. Parathyroidectomy is recommended by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for patients with chronic kidney disease-related mineral and bone disorders [Citation13]. Parathyroidectomy is recommended in patients with severe hyperparathyroidism (persistent serum levels of intact PTH >800 pg/mL or 88.0 pmol/L) associated with hypercalcemia and/or hyperphosphatemia refractory to medical therapy. Parathyroidectomy can induce a marked improvement in SHPT: postoperative serum phosphorus and calcium levels are maintained readily within their target ranges, and quality of life and survival rates are improved. However, some patients are ineligible for surgical resection, such as those who are not suitable for highly invasive surgery under general anaesthesia, those whose parathyroid glands are located in an area that renders resection difficult, those in whom it is difficult to resect all affected parathyroid tissues, and those with recurrent SHPT (iPTH level <300 pg/mL within 6 months postoperatively, which then increased again >6 months postoperatively [Citation14,Citation15]), and those who have undergone surgery at least once before. Treatment would be a challenge for these patients and their clinicians. Unfortunately, little research has been reported regarding treatment recommendations for these patients at present. Based on our preliminary experience, microwave ablation (MWA) is safe and effective for destroying parathyroid gland tissue in patients with ESRD [Citation15,Citation16]. In this study, we report new data in terms of safety and efficacy at mid-term of parathyroid MWA in SHPT patients ineligible for surgical resection.
Materials and methods
Patients
The study protocol was approved by the Human Ethics Review Committee of the China-Japan Friendship Hospital. Written informed consent was obtained from each patient before MWA.
Of 249 patients with severe SHPT seen in the nephrology department between 1 April 2015 and 28 February 2017, 35 who were ineligible for surgical resection met the inclusion criteria for MWA. The inclusion criteria were (1) ESRD with SHPT and medical intolerance (uncontrolled SHPT despite adequate medical therapy), (2) iPTH level ≥600 pg/mL[1], (3) at least one enlarged parathyroid gland (maximum diameter of gland ≥6 mm) accessible to MWA treatment, (4) increased 99mTc sestamibi (MIBI) accumulation in the gland during both the early and delayed phases, (5) ineligible for surgical resection (such as patients who have undergone renal transplantation, coronary artery bypass surgery, cardiac valve replacement or coronary stent implantation; patients with thoracic deformities; patients whose parathyroid glands were located in a difficult-to-resect area (such as parathyroid glands located within the mediastinum, thymus or thyroid, as well as the tracheoesophageal groove, retroesophageal region and submandibular region), patients in whom it would be difficult to resect all affected parathyroid tissues, and patients with recurrent SHPT who have undergone one or more previous surgeries), (6) prothrombin time ≤25 s, activated partial thromboplastin time ≤45 s, and platelet count ≥100 × 109/L and (7) no intractable complication (such as cardiac insufficiency or hypertension that could not be controlled by drugs).
Exclusion criteria were (1) iPTH level <600 pg/mL, (2) medication effectiveness (SHPT controlled with adequate medical therapy), (3) ultrasound examination showing no hyperplastic parathyroid glands, (4) MIBI scan of the gland without radionuclide accumulation, (5) candidate for parathyroidectomy, (6) abnormal coagulation function tests (such as prothrombin time >25 s, activated partial thromboplastin time >45 s, and platelet count <100 × 109/L) and (7) intractable complications, such as intractable cardiac insufficiency or intractable hypertension, that could not be controlled by drugs.
Percutaneous MWA procedure
MWA was performed by an experienced radiologist. The ultrasound characteristics of the affected parathyroid glands (echo features on ultrasound, maximum diameter and position) were evaluated. A microwave generator and 17-gauge internally cooled antenna with a 0.4 cm tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China) were used for the ablation procedures. The antenna was inserted freehand into the parathyroid gland to ablate one-by-one under US guidance (Aplio 500, Toshiba Medical Systems). The detailed ablation procedures have been described previously [Citation16]. After ablation, a contrast-enhanced ultrasound (CEUS; SonoVue; Bracco, Italy) was performed to evaluate the extent of ablation of the parathyroid gland [Citation17]. The ablation was considered complete if the non-enhanced zone on CEUS covered the ablated gland. An additional ablation was performed immediately if there was nodular enhancement within a gland.
An anesthesiologist was present to assist the radiologist in cases of anesthesia, pain, vasovagal reactions or potentially uncontrollable hematoma pressing on the trachea during the procedure.
Mild compression was applied to the site of the needle insertion for 20 min and patients remained under observation for 2 h after the procedure.
Clinical data collection and follow-up
Clinical data were collected for all patients, including the iPTH, serum calcium and serum phosphate levels, before and 6 months after ablation and at the end of follow-up (cut-off 30 September 2017). All data were obtained in the same laboratory at the China-Japan Friendship Hospital. Ultrasound was performed and evaluated at each follow-up evaluation post-ablation (6 months after ablation and at the end of follow-up) to identify complications and recurrence (the parameters, such as size (maximum diameter), volume and echogenicity (homogeneous or heterogeneous) of parathyroid gland were used for evaluation, CEUS also was performed.). Major and minor complications were defined according to the criteria of the Society of Interventional Radiology [Citation18,Citation19].
Definition of local recurrence and new occurrence
Local recurrence implied the appearance of a new parathyroid gland at the ablative margin after local eradication of all gland cells by ablation [Citation20]. A new occurrence was a new parathyroid gland growing at another site.
Statistical analysis
Statistical analyses were performed using the JMP software (ver. 11.0; SAS, Institute Inc., Cary, NC). Continuous data were presented as means ± SD (normally distributed data) or medians and interquartile ranges (IQR, non-normally distributed data). Repeated-measures ANOVA was used to compare treatment outcomes pre- and post-ablation. Values of p < .05 were considered to indicate statistical significance.
Results
Patient demographics and clinical characteristics
shows the baseline clinical characteristics of the enrolled patients. In total, 35 patients (19 males, 16 females; median age 49.8 ± 12.9 (range, 20 − 84) years) underwent ultrasound-guided MWA from 1 April 2015 to 28 February 2017 at our center. All patients had ESRD. Thirty patients were on hemodialysis for 10.3 ± 4.1 years, one was on peritoneal dialysis for 5 years and four were not undergoing dialysis (patients with successful renal transplantation; eGFR (estimated glomerular filtration rate) of 82.8 ± 13.6 ml/min/1.73 m2). All patients had high serum iPTH levels; iPTH is the main bioactive product of PTH, and the iPTH level shows the best correlation with the production and biological activity of the hormone (620–3434 pg/mL; normal range, 15–88 pg/mL). This was associated with normal-to-high serum phosphorus (0.52–3.62 mmol/L; normal range, 0.81–1.78 mmol/L) and normal-to-high total calcium levels (2.16–3.06 mmol/L; normal range, 2.00–2.75 mmol/L). Of the patients, 18 had one hyperplastic parathyroid gland, 8 had two, 7 had three and 2 had four glands. The maximum gland diameters were 6–31 mm (mean 14.9 ± 5.5 mm). Reasons for ineligibility for surgery were recurrent SHPT with a history of one or more surgeries (n = 12), poor heart or lung function (n = 10), renal transplantation (n = 4), parathyroid glands located in a difficult-to-resect area (n = 3), thoracic deformities (n = 3), prior coronary stent implantation (n = 1), advanced age (≥80 years old) (n = 1) and underweight (weight = 24 kg; BMI = 12.2 kg/m2) (n = 1).
Table 1. Baseline characteristics of SHPT patients ineligible for surgical resection at the time of MWA (n = 35).
Technical outcomes after MWA
All 63 glands in 35 patients were treated by MWA in a single treatment session. All 63 glands showed hyperenhancement in the arterial phase and were isoechoic in the late phase on pre-ablation CEUS. This enhancement behavior was consistent with high parathyroid function. After ablation, all glands showed non-enhancement on CEUS, indicating complete ablation. shows a percutaneous MWA procedure. The maximum gland diameters were 6–31 mm (mean, 14.9 ± 5.5 mm) in these patients, whereas the size of the adenoma showed marked regression during the 6 months following ablation (3–25 mm, mean 10.8 ± 4.9 mm) and at the end of follow-up (0–23 mm, mean 7.7 ± 5.0 mm). At the end of follow-up, the local recurrence rate was 8.8% (3/34) and the new occurrence rate was 11.8% (4/34).
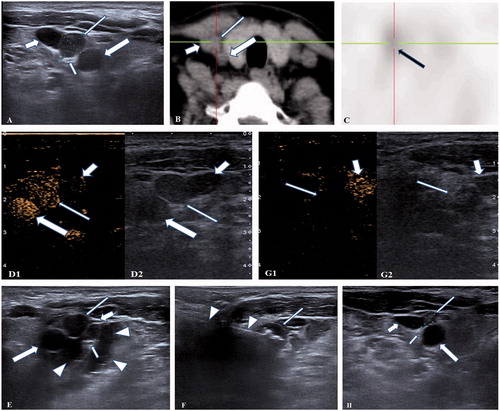
Figure 1. A 47-years-old female patient with a recurrent and ectopic SHPT nodule 2 years after parathyroidectomy was treated by microwave ablation (MWA). (A) A hypoechoic SHPT nodule (long thin arrow) closing to the right sympathetic nerve (short thin arrow) was disclosed to locate between the right carotid artery (long thick arrow) and jugular vein (short thick arrow) in ultrasound. (B) The CT scan showed that the SHPT nodule (long thin arrow) is between the right carotid artery (long thick arrow) and jugular vein (short thick arrow). (C) The SHPT nodule has radioactivity concentration (black arrow) in the late phase on MIBI scan. (D) A uniform hyperenhancement of the SHPT nodule (long thin arrow) between the right carotid artery (long thick arrow) and jugular vein (short thick arrow) was displayed in CEUS preablation. (E) The spacer fluid (white triangle) was injected around the right sympathetic nerve (short thin arrow) behind the right carotid artery (long thick arrow), jugular vein (short thick arrow) and SHPT nodule (long thin arrow) for preventing the nerve from thermal injury during ablation. (F) The hyperechoic signal emerging along the tip of antenna (white triangle) inside the SHPT nodule (long thin arrow) during ablation. (G) A non-enhancement area covered the SHPT nodule (long thin arrow) beside the jugular vein (short thick arrow) after MWA, suggesting complete ablation was achieved by MWA. (H) The SHPT nodule (long thin arrow) in front of right carotid artery (long thick arrow), jugular vein (short thick arrow) and sympathetic nerve (short thin arrow) was obviously shrinking 6 months after MWA in ultrasound.
Laboratory analyses
As of 30 September 2017, the mean follow-up time was 15.9 ± 2.2 months. The trends of the changes in iPTH and calcium levels showed a curve, the level of iPTH and calcium at 6 months post-ablation were lower than those pre-ablation (both p < .0001), after then, iPTH remained relatively stable and the end of follow up, with no rebound (p < .0001), but calcium at the end of follow up was not significantly lower than pre-ablation (p = .462). The trend in the change in phosphate levels showed a straight line; the level of phosphate at 6 months post-ablation and end of follow up were both significantly lower than pre-MWA (p < .001, ).
Table 2. Laboratory data of SHPT patients before and after MWA.
Survival following MWA
Nobody died during the 30-day post-MWA period. One died from cardiovascular diseases by the end of the follow-up (mean 15.9 ± 2.2 months). The mortality rate was thus 3.0% (1/33), and the survival rate was 97.0% (32/33) in these patients at the end of follow-up.
Complications
Complications related to MWA at our institution occurred in 28.6% of 35 patients, and no one was considered major. Minor complications included mild subcutaneous oedema in three patients, which resolved 2–4 days after MWA, prolonged vocal cord mobility impairment in two patients, which improved within 2–3 months after MWA, and slight bucking (mild cough) on swallowing water in five patients, all recovering within 3 days to 2 months after MWA. All ablation-related minor complications showed spontaneous remission with no medical intervention. However, no other complication was reported during follow-up.
Discussion
Although parathyroidectomy has potential complications, such as transient or permanent hypocalcemia and laryngeal nerve injury, patients with SHPT can gain many benefits from surgery. These may include improved serum phosphorus and calcium levels, bone mineral density, survival and alleviation of symptoms. Thus, parathyroidectomy is currently recommended in the KDIGO for severe SHPT patients who fail to respond to medical therapy [Citation13]. However, not all severe SHPT patients can be treated by parathyroidectomy. Some patients are not suitable for surgery for various reasons. Thus, such a treatment would be a challenge for these patients and their clinicians.
There is currently no guideline or recommendation for these patients as to what to do next. Fortunately, there have been many good studies on non-surgical treatments for hyperparathyroidism in recent years. Several case reports and case series have been published. Percutaneous injection of ethanol [Citation21,Citation22], acetic acid [Citation23] and calcitriol [Citation24] and thermal ablation, including radiofrequency (RF) [Citation25,Citation26], laser [Citation27] and high-intensity focussed ultrasound (HIFU) [Citation28,Citation29], have been assessed in patients with hyperparathyroidism. A preliminary study by our group also confirmed that ultrasound-guided MWA was safe and effective in destroying parathyroid gland tissue in patients with SHPT [Citation15,Citation16]. At the same time others have done the same jobs [Citation30,Citation31].
MWA is an energy-based ablation technique; it creates a uniform electromagnetic field surrounding the antenna, causing rapid volume tissue heating via the oscillation of polar water molecules. However, chemical ablation (injection of ethanol, acetic acid or calcitriol) has the risk of solute leakage into the surrounding tissue, which could be harmful [Citation32]. The incidence of injection-related complications with chemical ablation, such as hemorrhage, recurrent laryngeal nerve palsy caused by ethanol leakage, and adhesions to surrounding tissue, increases with the number of injections[Citation32,Citation33]. Our previous and other researcher’s studies [Citation16,Citation34,Citation35] also mentioned that MWA devices could create a uniform electromagnetic field surrounding the antenna. By the oscillation of polar water molecules, it can make volume tissue rapidly heating. Because MWA does not rely on the conduction of an electrical current, tissues temperatures can increase markedly. Compared with other ablation techniques (such as RF, laser and HIFU), MWA may translate into larger, faster and more consistent ablation zones. Thus, MWA maybe is more suitable for SHPT patients who are ineligible for surgical resection.
Because of high surgical or anesthetic risk, such as after renal transplantation, thoracic deformity, parathyroid glands in ectopic locations, advanced age, underweight and recurrent SHPT, these patients are not suitable for parathyroidectomy. First, regarding the treatment effect, we show a marked improvement in SHPT patients. A marked decrease in serum iPTH levels were observed post-ablation, and serum calcium and phosphorus levels also improved. At 6 months post-ablation and at the end of follow-up, neck US revealed complete ablation or almost 50% reduction in the size of the treated parathyroid lesions, compared with the size pre-ablation in all patients. Thus, ultrasound-guided MWA can be used effectively for SHPT treatment.
In clinical applications of parathyroid MWA, we have improved the technology, including (1) Before ablation, injection more spacer fluid than those in previous study (Normal saline: 40–60ml vs 20 ml) surrounding SHPT nodule to fully separate the SHPT nodule from vital structure; (2) Provoking the SHPT nodule away from important structure like recurrent laryngeal nerve, carotid artery during ablation to avoid thermal damage. So, the incidence of complications has decreased significantly compared to our preliminary study [Citation16]. In this study, MWA caused little trauma and few severe complications. There were no severe complications during ablation or the follow-up period. This suggests that ultrasound-guided percutaneous MWA is safe for ESRD patients.
According to literature reports, the recurrent and persistent hyperparathyroidism rates after parathyroidectomy were 0.83–26% and 0.4–15% [Citation36–39], respectively. In our study, despite the poor physical conditions of and difficulties in managing these patients, the local recurrence rate was 8.8% (3/34) and the new occurrence rate 11.8% (4/34). Certainly, ablation differed from surgical treatment, but the long-term efficacy of MWA was not inferior to that of parathyroidectomy. Moreover, compared with other studies (the survival rate after parathyroidectomy was 90.4–97.1% at 1 year [Citation40–42], and the 30-day postoperative mortality rate following parathyroidectomy was 3.1% [Citation43]), the survival rate (97.0%) and the 30-day post-ablation mortality rate (0%) following MWA were not worse in our study. Despite the incidence of complications in this study was 28.6%, they were all spontaneous remission, with no medical intervention, including mild subcutaneous edema, prolonged vocal cord mobility impairment and slight bucking (mild cough) on swallowing water. There was no incidence of temporary or permanent recurrent laryngeal nerve palsy and with no incidence of wound hematoma or infection. Meanwhile, whether conventional parathyroid surgery or minimally invasive parathyroidectomy (MIP), the incidence of surgical severe complications was 1.1–3.1% [Citation44–47], including wound hemorrhage or hematoma, recurrent nerve injury and so on. Moreover, the hospital stay was 1–3 days in our population. This suggests that MWA has many advantages such as fewer risks, faster recovery and fewer side effects compared with surgical treatment.
There are several limitations to this study. First, MIBI was not repeated after treatment to confirm the reduction/disappearance of activity. Second, longer-term follow-up is needed after MWA.
In a preliminary study [Citation16], we have confirmed that MWA was safe and effective for destroying parathyroid gland tissue in patients with ESRD. In this study, we applied MWA to treat SHPT patients who are ineligible for surgical resection. In these patients, MWA is equally safe and effective, and there is no increase in complications due to prolonged follow-up. So, our study provides a new option for SHPT patients who are ineligible for surgical resection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109.
- Drueke TB. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol. 2000;11:1141–1152.
- Goldsmith D, Kothawala P, Chalian A, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of fracture and need for parathyroidectomy in CKD. Am J Kidney Dis. 2009;53:1002–1013.
- Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955.
- Ossareh S. Clinical and economic aspects of sevelamer therapy in end-stage renal disease patients. Int J Nephrol Renovasc Dis. 2014;7:161–168.
- Lloret MJ, Ruiz-Garcia C, Dasilva I, et al. Lanthanum carbonate for the control of hyperphosphatemia in chronic renal failure patients: a new oral powder formulation - safety, efficacy, and patient adherence. Patient Prefer Adherence. 2013;7:1147–1156.
- Pandey R, Zella J, Clagett-Dame M, et al. Use of 2MD, a novel oral calcitriol analog, in hemodialysis patients with secondary hyperparathyroidism. Am J Nephrol. 2016;43:213–220.
- Rauscher S, Lafrance JP, Pichette V, et al. Conversion of oral alfacalcidol to oral calcitriol in the treatment of secondary hyperparathyroidism in chronic hemodialysis patients. Int Urol Nephrol. 2017;49:325–328.
- Akizawa T, Akiba T, Hirakata H, et al. Comparison of paricalcitol with maxacalcitol injection in Japanese hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial. 2015;19:225–234.
- Adachi M, Miyoshi T, Shiraishi N, et al. A study of maintenance therapy after intravenous maxacalcitol for secondary hyperparathyroidism. CN. 2011;76:266–272.
- Akizawa T, Kurita N, Mizobuchi M, et al. PTH-dependence of the effectiveness of cinacalcet in hemodialysis patients with secondary hyperparathyroidism. Sci Rep. 2016;6:19612.
- Kuczera P, Adamczak M, Wiecek A. Treatment with cinacalcet increases plasma sclerostin concentration in hemodialysis patients with secondary hyperparathyroidism. BMC Nephrol. 2016;17:176.
- Moe SM, Drüeke TB, Block GA, et al. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;S1–S130.
- Chen HH, Lin CJ, Wu CJ, et al. Chemical ablation of recurrent and persistent secondary hyperparathyroidism after subtotal parathyroidectomy. Ann Surg. 2011;253:786–790.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia. 2016;32:180–186.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282:576–584.
- Machi J. Radiofrequency ablation for hyperparathyroidism: can it be a new treatment?. Surg Laparosc Endosc Percutan Tech. 2006;16:116–16773016.
- Saad WE, Wallace MJ, Wojak JC, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21:789–795.
- Lewis CA, Allen TE, Burke DR, et al. Quality improvement guidelines for central venous access. The Standards of Practice Committee of the Society of Cardiovascular & Interventional Radiology. J Vasc Interv Radiol. 1997;8:475–479.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Singh Ospina N, Thompson GB, Lee RA, et al. Safety and efficacy of percutaneous parathyroid ethanol ablation in patients with recurrent primary hyperparathyroidism and multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2009;113:S1–S130.
- Alherabi AZ, Marglani OA, Alfiky MG, et al. Percutaneous ultrasound-guided alcohol ablation of solitary parathyroid adenoma in a patient with primary hyperparathyroidism. Am J Otolaryngol. 2015;36:701–703.
- Onoda N, Kurihara S, Sakurai Y, et al. A case of secondary hyperparathyroidism whose high turnover bone improved after the direct injection of acetic acid into the parathyroid glands. CN. 2004;61:68–73.
- Junik R, Polańska M, Manitius J, et al. Local calcitriol injections as a suppressive treatment of secondary hyperparathyroidism in chronic dialysis patients. Ren Fail. 2007;29:941–945.
- Sormaz IC, Poyanli A, Acar S, et al. The results of ultrasonography-guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol. 2017;40:596–602.
- Wang R, Jiang T, Chen Z, et al. Regression of calcinosis following treatment with radiofrequency thermoablation for severe secondary hyperparathyroidism in a hemodialysis patient. Intern Med. 2013;52:583–587.
- Adda G, Scillitani A, Epaminonda P, et al. Ultrasound-guided laser thermal ablation for parathyroid adenomas: analysis of three cases with a three-year follow-up. Horm Res Paediatr. 2006;65:231–234.
- Ambrosini CE, Cianferotti L, Picone A, et al. High-intensity focused ultrasound as an alternative to the surgical approach in primary hyperparathyroidism: a preliminary experience. J Endocrinol Invest. 2011;34:655–659.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24:2052–2058.
- Wang G, Liu S, Liu X, et al. Microwave ablation: an effective treatment for mild-to-moderate secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2017;33:946–952.
- Diao Z, Wang L, Li D, et al. Efficacy of microwave ablation for severe secondary hyperparathyroidism in subjects undergoing hemodialysis. Ren Fail. 2017;39:140–145.
- Fletcher S, Kanagasundaram NS, Rayner HC, et al. Assessment of ultrasound guided percutaneous ethanol injection and parathyroidectomy in patients with tertiary hyperparathyroidism. Nephrol Dial Transplant. 1998;13:3111–3117.
- Stratigis S, Stylianou K, Mamalaki E, et al. Percutaneous ethanol injection therapy: a surgery-sparing treatment for primary hyperparathyroidism. Clin Endocrinol (Oxf). 2008;69:542–548.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37:2967–2973.
- Lubner MG, Hinshaw JL, Andreano A, et al. High-powered microwave ablation with a small-gauge, gas-cooled antenna: initial ex vivo and in vivo results. J Vasc Interv Radiol. 2012;23:405–411.
- Parikh PP, Farra JC, Allan BJ, et al. Long-term effectiveness of localization studies and intraoperative parathormone monitoring in patients undergoing reoperative parathyroidectomy for persistent or recurrent hyperparathyroidism. Am J Surg. 2015;210:117–122.
- Norlen O, Wang KC, Tay YK, et al. No need to abandon focused parathyroidectomy: a multicenter study of long-term outcome after surgery for primary hyperparathyroidism. Ann Surg. 2015;261:991–996.
- Schlosser K, Bartsch DK, Diener MK, et al. Total parathyroidectomy with routine thymectomy and autotransplantation versus total parathyroidectomy alone for secondary hyperparathyroidism: results of a nonconfirmatory multicenter prospective randomized controlled pilot trial. Ann Surg. 2016;264:745–753.
- Stracke S, Keller F, Steinbach G, et al. Long-term outcome after total parathyroidectomy for the management of secondary hyperparathyroidism. Nephron Clin Pract. 2009;111:c102–c109.
- Li W, Zhang M, Du S, et al. Impact of parathyroidectomy on survival among haemodialysis patients: a prospective cohort study. Nephrology. 2016;21:133–138.
- Chuang CH, Wang JJ, Weng SF, et al. Epidemiology and mortality among dialysis patients with parathyroidectomy: Taiwan National Cohort Study. JN. 2013;26:1143–1150.
- Iwamoto N, Sato N, Nishida M, et al. Total parathyroidectomy improves survival of hemodialysis patients with secondary hyperparathyroidism. JN. 2012;25:755–763.
- Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66:2010–2016.
- Rajeev P, Stechman MJ, Kirk H, et al. Safety and efficacy of minimally-invasive parathyroidectomy (MIP) under local anaesthesia without intra-operative PTH measurement. Int J Surg. 2013;11:275–277.
- Tominaga Y, Kakuta T, Yasunaga C, et al. Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the parathyroid surgeons' society of Japan. Ther Apher Dial. 2016;20:6–11.
- Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253:585–591.
- Richmond BK, Eads K, Flaherty S, et al. Complications of thyroidectomy and parathyroidectomy in the rural community hospital setting. Am Surg. 2007;73:332–336.
