Abstract
Purpose: To investigate the clinical effectiveness and safety of ultrasound (US)-guided percutaneous microwave ablation (MWA) for colorectal liver metastasis (CRLM) and evaluate the influencing factors of local efficacy.
Methods: From January 2013 to January 2017, 137 CRLM patients accepting US-guided percutaneous MWA were included. The 2450-MHz microwave ablation system and a cooled-shaft antenna were used. All patients were regularly followed up for at least 6 months. Technical success, complete ablation, local tumor progression (LTP), complications and side effects were assessed. Logistic regression analysis was used to identify the independent prognostic factors for LTP.
Results: In total, 411 lesions (mean diameter 15.4 ± 7.2 mm, range 5–67 mm) were treated. Complete ablation was achieved in 99.27% (408/411) of lesions and 97.81% (134/137) of patients. LTP occurred in 5.35% (22/411) of lesions and 16.06% (22/137) of patients. LTP was more likely to occur in lesions larger than 3 cm in diameter (OR: 14.71; p < .001; 95% CI: 3.7 3–57.92), near a large vascular structure (OR: 7.04; p < .001; 95% CI: 2.41–20.60), near the diaphragm (OR: 4.02; p = .049; 95% CI: 1.05–16.11) and in patients with no response to chemotherapy before MWA (OR: 3.25; p = .032; 95% CI: 1.14–15.30). MWA was well tolerated, with a major complication rate of 3.65%, a minor complication rate of 8.03% and a mortality rate of 0%. Fever and pain were the most common side effects after MWA.
Conclusions: US-guided percutaneous MWA of CRLM is a safe and effective method that is expected to become a routine treatment for local tumor control of CRLM.
Introduction
Colorectal cancer (CRC) is a common malignancy worldwide [Citation1], for which liver metastasis presents a serious clinical problem and contributes to poor prognosis [Citation2]. Up to 25–50% of CRC patients will develop liver metastases [Citation3]. The median survival time for patients with colorectal liver metastasis (CRLM) without any treatment is 8–10 months, and the 5-year survival rate is less than 5% [Citation3]. Surgical resection is the gold-standard treatment modality for CRLM. The 5-year survival rate of selected patients after surgical resection may reach 31–58% [Citation4,Citation5]. However, approximately 80–90% of CRLM patients are not candidates for surgical resection, and thus, only non-surgical treatments are available for most of these patients [Citation3].
Radiofrequency ablation (RFA) and microwave ablation (MWA) are commonly employed in local treatment of malignant liver tumors. As thermal ablation modalities, both techniques induce cellular death via coagulation necrosis by local hyperthermia [Citation6]. Since MWA has not been widely applied in the treatment of CRLM, clinical data with long-term follow-up of CRLM ablation are based on experience with RFA. The 3- and 5-year survival rates of RFA are 37–77% and 17–51%, respectively [Citation7]. Some studies reported that RFA resulted in survival rates similar to surgical resection, especially for patients with CRLMs smaller than 3 cm in diameter and less than 5 lesions [Citation8,Citation9]. While some studies found that patients who received surgical resection had better survival rates and less local recurrence, there may be selection bias because ablation was mostly used for unresectable CRLM [Citation9,Citation10]. The local tumor progression (LTP) rate following RFA was reported to be between 6% and 51% [Citation1]. LTP after RFA can be influenced by various factors, such as the size and location of lesions, presence of vessels, ablative margin, chemotherapy regimens and physician experience [Citation1,Citation11–13]. The relatively high LTP rate is the main weakness limiting ablation as a curative modality for CRLM compared with surgical resection.
Despite similar outcomes for CRLM ablation between MWA and RFA (3-year survival rate of 43–73% and LTP rate of 6–51% [Citation14,Citation15]), MWA might present multiple advantages over RFA. These include the larger ablation zone, shorter ablation time, decreased susceptibility to the ‘heat-sink effect’ and avoidance of skin burns by the grounding pad [Citation3,Citation16]. This study summarized the experience of ultrasound (US)-guided percutaneous MWA of CRLM based on a relatively large sample size at a single center. The purpose of this study was to investigate the clinical effectiveness and safety of US-guided percutaneous MWA for CRLM and evaluate the factors influencing local therapeutic efficacy.
Materials and methods
Patients
All patients accepted US-guided percutaneous MWA following a multi-disciplinary team (MDT) discussion and written informed consent was obtained before ablation for each patient. Ethical approval was waived by the institutional research ethics board for this retrospective study. From January 2013 to January 2017, 137 patients (91 men and 46 women; mean age, 54.9 ± 12.7 years SD; range, 21–89 years) were retrospectively analyzed. The inclusion criteria were as follows: (1) CRC patients with liver metastases, (2) CRLM patients who underwent US-guided percutaneous MWA, and (3) no less than 6 months of regular follow-up. The diagnostic criteria of CRLM were as follows: (1) CRC patients with newly detected focal liver lesions showing typical imaging findings of CRLM on contrast-enhanced ultrasound (CEUS) and contrast-enhanced computed tomography (CECT) or contrast-enhanced magnetic resonance (CEMRI), (2) focal liver lesions showing increased size during regular follow-up of imaging examinations, and (3) biopsy performed in cases of atypical imaging features with confirmation of CRLM by pathology [Citation17]. The mean follow-up time was 17.62 ± 9.68 months (range, 6–48 months). Patients with <6 months of follow-up after MWA were excluded. The original tumor was colon cancer in 67 (48.91%) patients and rectal cancer in 70 (51.09%) patients, as confirmed by pathological assessment of samples obtained from endoscopic biopsy or surgical resection. There were 34 (24.82%) patients with extrahepatic metastasis at the time of ablation. Among them, only 3 (8.82%) patients accepted ablation or resection for the extrahepatic metastasis, while most patients (91.18%) accepted chemotherapy because of extensive extrahepatic metastases. A total of 49 (35.77%) patients received neoadjuvant chemotherapy previous to MWA. Response to chemotherapy was classified as partial response (PR), stable disease (SD), or progressive disease (PD) using the Cancer Response Evaluation Criteria In Solid Tumors (RECIST) [Citation18]. The characteristics of patients are shown in .
Table 1. Characteristics of Patient.
MWA procedure
The 2450-MHz microwave ablation system was used (KY2000; Nanjing Kangyou Biological Energy Co. Ltd, Nanjing, China) and included a microwave generator with a power output of 1–100 W, a flexible coaxial cable, and a cooled-shaft antenna (KY-2450-b; Nanjing Kangyou Biological Energy Co. Ltd, Nanjing, China). The dual channel’s antenna was 18 cm long and 1.9 mm in diameter (15 G), through which cold saline was circulated by a peristaltic pump to continuously cool the shaft. During the procedure, the temperature of each antenna was kept below 40 °C by adjusting the flow of the cold water [Citation19]. Preoperative sedation was performed with intramuscular injection of 50 mg Dilantin (Chaohui; Shanghai Pharmaceuticals, Shanghai, China) 30 min before ablation and another 50 mg of Dilantin on demand during ablation. Next, 2% lidocaine (Chaohui; Shanghai Pharmaceuticals, Shanghai, China) was used for local anesthesia before puncture, and a 24-G needle 10 cm in length (Kangdelai; Shanghai Medical Instruments, Shanghai, China) was used to ensure that lidocaine was injected into the liver capsule. The antenna was inserted into the lesion 5 mm beyond the deep margin of the lesion in the center of the maximum section under US guidance (LOGIQ E9; GE Healthcare, Milwaukee, WI, USA). The MWA procedure used in our center is described as follows. For lesions 2.0 cm or less in diameter, a single antenna and a single insertion were used; for lesions 2.0–3.0 cm, 1-2 antennas and 1-2 insertions were used; and for lesions more than 3.0 cm, two antennas and multiple insertions were used. The distance between the two antennas was 1.0–1.5 cm. The energy output was adjusted between 45–60 W over 5–15 min to ensure that the ablation zone covered the lesion and at least 5 mm of the surrounding liver parenchyma. The needle track was ablated at the end of the procedure to prevent bleeding and tumor seeding. All procedures were performed by the same radiologist, who has over 10 years of experience in liver cancer ablation.
Follow-up
First, CEUS was performed at 30 min and 24 h post-MWA. Next, CEUS and CECT/MRI were performed at 1-month post-MWA. Afterward, CEUS was performed every 3 months, and CECT (Aquilion One, Toshiba Medical System, Tokyo, Japan) or CEMRI (Optima MR360, GE Healthcare, Milwaukee, WI, USA) was performed every 3 months for half a year and every 6 months thereafter for follow-up. Technical success, complete ablation, LTP, complications and side effects were assessed in accordance with the standardization of terminology and reporting criteria of image-guided tumor ablation [Citation20]. ‘Technical success’ was defined as treatment of the tumor according to protocol and complete coverage by hyper-echoic gas at the end of the procedure. ‘Complete ablation’ was achieved when the entire ablated area presented no enhancement on follow-up CECT/MRI at 1-month post-ablation. LTP was defined as the appearance of a hyper-enhanced nodule with washout located at the marginal area of the ablated zone after the tumor had been documented as having achieved complete ablation. Complications were reported using the most recent version of the SIR Classification standard table. A major complication is an event that leads to substantial morbidity and disability that increases the level of care, results in hospital admission, or substantially lengthens the hospital stay (SIR classifications C–E). All other complications are considered minor complications (SIR classifications A–B). Side effects are expected undesired consequences of the procedure that commonly occur but rarely result in substantial morbidity. The Common Terminology Criteria for Adverse Events (CTCAE) v4.0 of the National Cancer Institute was adopted for the reporting of pain [Citation20]. For the patients who developed LTP, further ablation was offered. Surgical resection was performed for the lesions that could not be completely ablated under US guidance. For the patients with LTP who could not receive ablation or resection due to the location or other reasons, systemic chemotherapy was administered whenever possible [Citation17].
Statistical analyses
Descriptive data were summarized as the number and percentage or mean ± standard deviation. For qualitative data, the chi-squared test or Fisher’s exact probability test was used. For quantitative data satisfying the normal distribution, Student’s t-test was used. The accumulative LTP and overall survival (OS) rate were analyzed using the Kaplan–Meier method. Univariate analysis was carried out to assess the influence of various factors, including size, number and location of CRLM, synchronous or metachronous CRLM, type of primary tumor, extrahepatic metastasis, time of chemotherapy and response to chemotherapy. Logistic regression analysis was used to identify the independent prognostic factors for LTP. p < .05 was considered a statistically significant difference. SPSS 22.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis.
Results
Lesion characteristics
In total, 411 lesions were treated (mean diameter, 15.4 ± 7.2 mm; range, 5–67 mm). Regarding the size of the lesions, 398 (96.84%) lesions were <30 mm in diameter, 12 (2.92%) lesions were between 30 mm and 50 mm in diameter, and only 1 (0.24%) lesion was >50 mm in diameter. A lesion located ‘near’ an important structure (such as the large vessels, gastrointestinal tract, gallbladder, diaphragm and liver capsule) meant that the lesion was less than 10 mm from these structures. There were 35 (8.52%) lesions located near large vessels ≥3 mm in diameter, 22 (5.35%) lesions located close to the gastrointestinal tract, 27 (6.57%) lesions adjacent to the diaphragm, 44 (10.71%) lesions located near the liver capsule and 9 (2.19%) lesions located near the gallbladder. The characteristics of CRLM are shown in .
Efficacy of MWA
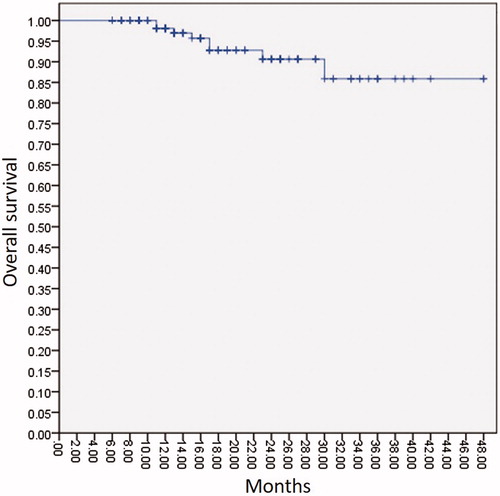
The total number of antenna insertions was 443, and the average number of insertions per lesion was 1.08 (range, 1–3). The technical success rate was 100.00%. Complete ablation was achieved in 99.27% of lesions and 97.81% of patients. The 1-year, 2-year, and 3-year OS rates were 98.1%, 90.6% and 85.9%, respectively ().
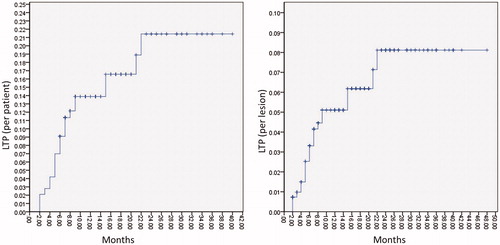
LTP occurred in 22 (5.35%) lesions and 22 (16.06%) patients (mean time, 7.9 ± 6.4 months; range, 2–23 months). The 1-year accumulative LTP rate was 14.0% in patients and 5.1% in lesions. The 2-year accumulative LTP rate was 21.0% in patients and 8.1% in lesions (). LTP was associated with the size of CRLM, location of CRLM and response to chemotherapy prior to MWA (). LTP was more likely to occur in lesions larger than 3 cm in diameter (OR: 14.71; p < .001; 95% CI: 3.73–57.92), near a large vascular structure (OR: 7.04; p < .001; 95% CI: 2.41–20.60), near the diaphragm (OR: 4.02; p = .049; 95% CI: 1.0 5–16.11) and in those with no response to chemotherapy prior to MWA (OR: 3.25; p = .032; 95% CI: 1.1 4–15.30) (). The LTP rate was significantly higher in tumors ≥3.0 cm than in those <3.0 cm (38.46% vs. 4.27%, p < .001). Among the lesions near the large vascular, there were 26 (74.29%, 26/35) lesions located near large vessels ≥5 mm in diameter. Among them, 23.08% (6/26) of lesions exhibited LTP, while lesions located near vessels <5 mm in diameter presented no LTP (p = .046; 95% CI: 0.62–0.95). The LTP rate was 11.11% (3/27) in tumors near the diaphragm. Five lesions near the diaphragm in 4 patients were completely ablated after artificial hydrothorax and presented no LTP (). However, the LTP rate was much higher in patients without artificial hydrothorax (13.64% vs. 0%, p = .526; 95% CI: 0.98–1.37). MWA was performed in 39 patients (79.59%) with PR after chemotherapy and resulted in significantly lower LTP rates compared to those in the non-PR group following chemotherapy (12.82% vs.50.00%, p = .020).
Figure 3. A 51-year-old female patient with four CRLM. (A) After artificial hydrothorax injection of 600 ml of saline (*), percutaneous US clearly showed one lesion 11 mm in diameter (thick arrow) under the diaphragm. (B) CEUS showed the lesion (thick arrow) with homogeneous hyper-enhancement in the arterial phase (30 s). (C) The lesion (thick arrow) presented hypo-enhancement in the late phase (180 s). (D) The antenna (thin arrow) was inserted into the lesion 5 mm beyond the deep margin of the lesion (thick arrow) under US guidance, and then the MWA procedure was initiated (50 W, 10 min). (E) US showed that the lesion (thick arrow) was completely covered by hyperechoic gas during the ablation procedure. (F–G) The ablation zone (thick arrow) showed non-enhancement in the arterial and venous phases of CEMRI 1 month after ablation. (H–I) The ablation zone (thick arrow) was smaller and showed non-enhancement in the arterial and venous phases of CECT 30 months after ablation.
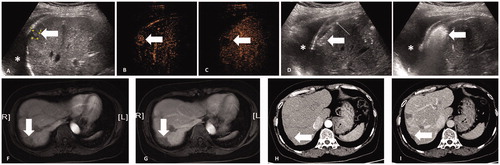
Table 2. Factors associated with local tumor progression.
Table 3. Factors to predict local progression.
Complications of MWA
There were no deaths that correlated with ablation. Major and minor complications occurred in 5 cases (3.65%) and 11 cases (8.03%), respectively (). The mean size of the lesion was 17.81 ± 8.20 mm in diameter for patients with complications and 15.02 ± 6.91 mm for those without complications (p = .101; 95% CI: −0.9 1 to 9.33). The treatment of 11 lesions near important structures resulted in complications, which were significantly more common than in those not located near important structures (p = .016; 95% CI: 1.35–10.29). Biloma occurred in 25.00% (2/8) of lesions near the intrahepatic bile duct and pleural effusion occurred in 11.11% (3/27) of lesions near the diaphragm. Fever and pain were the most common side effects after MWA. Seventeen (12.41%) patients showed a range in temperature of 37.2–39.9 °C, which occurred 24–48 h after MWA and persisted for 1–7 days. Seventy-three (53.28%) patients experienced local pain following MWA ().
Table 4. Major and minor complications of MWA.
Table 5. The degree of pain after MWA.
Discussion
Thermal ablation therapy has been recommended to treat CRLM alone or in combination with resection before or after systemic chemotherapy [Citation21]. At present, most literature concerning ablation of CRLM is based on experience using RFA. Only limited results using MWA for treatment of CRLM were reported in previous studies, with a relatively high complete ablation rate (97.6%) and low LTP rate (9.6%) [Citation22,Citation23]. MWA may have higher thermal efficacy and a larger ablation zone due to its physical advantages over RFA [Citation6]. In the present study, we summarized our experience using MWA in the treatment of CRLM and evaluated the influencing factors of treatment efficiency. Overall, we achieved satisfactory local tumor control with complete ablation rates of 99.27% of lesions and 97.81% of patients; LTP occurred in 5.35% of lesions and 16.06% of patients. We believe that our results benefited from relatively smaller lesion size (96.84% were less than 3 cm in diameter) and fewer total lesions (98.06% had less than 5 lesions) compared to the literature.
It has been acknowledged that the ablation margin is an independent factor affecting LTP after thermal ablation [Citation7]. Achieving a safety margin of 5–10 mm beyond the borders of the tumor in all directions is necessary for complete tumor destruction [Citation1,Citation12,Citation20]. In the liver, MWA may produce a larger ablation zone and more tissue contraction than RFA due to its physical advantages [Citation24]. Therefore, MWA could produce a relatively more adequate safety margin compared with RFA under the same conditions. Nevertheless, it is still difficult to achieve a sufficient ablation margin following MWA of large lesions and lesions near an important structure. We found that LTP after MWA was more likely to occur in lesions larger than 3 cm in diameter, near a large vascular structure and near the diaphragm.
Tumor size is an important factor for LTP following thermal ablation. Prior studies using RFA show an advantage for small CRLM, and the most common cut-off point of tumor size is 3 cm [Citation3,Citation25]. It has been reported that the LTP rate after RFA is 3% for tumors <3 cm, 4% for selected tumors of 3–5 cm and 27–45% for tumors >5 cm [Citation25,Citation26]. Similar results were observed in our study, which showed that LTP was significantly higher in tumors ≥3.0 cm than in those <3.0 cm.
The high LTP rate of lesions near large vessels is mainly due to the ‘heat sink’ effect. The ‘heat sink’ effect may vary depending on the vessel size, tumor size, and distance between the tumor and vessel [Citation1,Citation11]. RFA relies on the passive conduction of heat and is considered less suitable for lesions near large vessels [Citation27]. MWA has an active heating mechanism and is theoretically less affected by the ‘heat sink’ effect [Citation14,Citation27]. However, one study reported that RFA and MWA were equally effective in the treatment of perivascular CRLM [Citation27]. We found that LTP after MWA was also influenced by the ‘heat sink’ effect when the diameter of vessels was large enough. In our study, all the lesions with LTP were located near larger vessels of at least 5 mm in diameter.
It is difficult to clearly display lesions close to the diaphragm when using trans-abdominal US because of gas in the lungs, which can result in an insufficient ablation margin. Moreover, tumors near the diaphragm are often undertreated for fear of damaging the diaphragm and lungs, and the intolerable pain during the ablation procedure [Citation20,Citation28]. In our study, these factors led to a higher LTP rate for lesions near the diaphragm. Artificial hydrothorax is an effective method to improve the visibility of lesions adjacent to the diaphragm. The fluid collection can simultaneously protect the lung from thermal injury and reduce pain during the procedure [Citation29]. In our study, there were 5 lesions in 4 patients who underwent artificial hydrothorax, and they presented no complications or LTP. The lack of a significant difference may be due to the small number of cases; thus, a larger sample size is needed.
In theory, it is difficult to achieve sufficient ablation margins in lesions near the liver capsule, gastrointestinal tract or gallbladder. Lesions located near the liver capsule may be undertreated for fear of burning the abdominal wall and causing intolerable pain when performing thermal ablation using the percutaneous approach under local anesthesia with or without sedation [Citation30,Citation31]. However, some studies found no difference in LTP or major complications between subcapsular and non-subcapsular lesions that underwent US-guided percutaneous RFA [Citation32,Citation33]. In our study, a location near the liver capsule also proved not to be a significant prognostic factor for LTP after MWA. First, lesions near the capsule can be clearly shown, especially under US guidance using a high-frequency linear probe [Citation34]. Second, the long needle used for local anesthesia in the present study helped to improve the infiltration of lidocaine into the tissue surrounding the liver capsule and produced an isolation strip between the liver capsule and abdominal wall. As a result, ablation could be successfully delivered with sufficient power and duration without causing intolerable pain during the treatment. Our results did not show that the location near the gastrointestinal tract and gallbladder significantly influenced LTP. The lack of a significant difference may also be due to the small number of cases, and further investigation is necessary.
With the development of drugs, chemotherapy plays an increasingly important role in the treatment of CRLM. Some literature suggests that ablation in combination with chemotherapy can help to prolong the median progression-free survival and OS [Citation7]. A randomized study reported that RFA plus chemotherapy resulted in significantly lower rates of tumor progression (including both LTP and distant recurrence) compared to chemotherapy alone (45% vs. 76.3%) [Citation35]. In the present study, chemotherapy itself presented no positive role in reducing the rate of LTP, but the response to chemotherapy was a strong independent predictor for LTP. Similar results have been reported in the literature on RFA [Citation36]. CRLM with PR may present with smaller sizes and relatively little blood supply, which is conducive to achieving adequate safety margins for ablation. Meanwhile, chemotherapy may eradicate micrometastases surrounding CRLM [Citation7,Citation36], which may help to prevent LTP following ablation.
In our study, MWA was well tolerated, with a major complication rate of 3.65%, a minor complication rate of 8.03% and a mortality rate of 0%, which are rates similar to previous reports. MWA of lesions near important structures was associated with a higher rate of complications. In our study, MWA of lesions located near the intrahepatic bile duct caused biloma [Citation37], and MWA of lesions located near the diaphragm caused pleural effusion. Some patients who previously underwent RFA reported less pain during MWA [Citation31]. In our study, 73 (53.28%) patients experienced local pain after MWA, which was less than that reported in the literature (80.1%) [Citation38]. We believe that using a long needle to ensure adequate anesthesia is an effective method to relieve pain for percutaneous ablation.
There were some limitations to this study. First, this study was a single-center retrospective study in which selection bias is unavoidable. Second, the various chemotherapy regimens used, as well as genetic differences between patients, may have had variable effects on LTP. However, these effects could not be measured nor the degree of their effect determined. Third, this study only reported on CRLM treated with one particular MWA device and thus limits extrapolation of these data to other types of liver metastases or devices.
Conclusion
US-guided percutaneous MWA of CRLM is a safe and effective method, with good local therapeutic efficacy and low complication rates, and is expected to become routine for the local treatment of CRLM. LTP was more likely to occur in lesions ≥3 cm, located near large vessels and the diaphragm, as well as in CRLM that presented with no response to chemotherapy before MWA. Further studies including prospective multicenter trials are needed to confirm the efficacy of MWA on CRLM and the factors influencing the effects of treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Napoleone M, Kielar AZ, Hibbert R, et al. Local tumor progression patterns after radiofrequency ablation of colorectal cancer liver metastases. Diagn Interv Radiol. 2016;22:548–554.
- Shibata T, Niinobu T, Ogata N, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276–284.
- Munireddy S, Katz S, Somasundar P, et al. Thermal tumor ablation therapy for colorectal cancer hepatic metastasis. J Gastrointest Oncol. 2012;3:69–77.
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766.
- Cauchy F, Faivre S, Belghiti J. Surgical results after downstaging of initially marginal or non-resectable liver metastases. Dig Dis. 2012;30:143–149.
- Liu Y, Li S, Wan X, et al. Efficacy and safety of thermal ablation in patients with liver metastases. Eur J Gastroenterol Hepatol. 2013;25:442–446.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438–3454.
- Lee WS, Yun SH, Chun HK, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 2008;42:945–949.
- Minami Y, Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver. 2013;7:1–6.
- Reuter NP, Woodall CE, Scoggins CR, et al. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg. 2009;13:486–491.
- Zorbas G, Samaras T. A study of the sink effect by blood vessels in radiofrequency ablation. Comput Biol Med. 2015;57:182–186.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the european organisation for research and treatment of cancer #40004 and #40983. Eur J Cancer. 2014;50:912–919.
- Boutros C, Somasundar P, Garrean S, et al. Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol. 2010;19:e22–e32.
- Rocha FG, D'Angelica M. Treatment of liver colorectal metastases: role of laparoscopy, radiofrequency ablation, and microwave coagulation. J Surg Oncol. 2010;102:968–974.
- Gravante G, Ong SL, Metcalfe MS, et al. Hepatic microwave ablation: a review of the histological changes following thermal damage. Liver Int. 2008;28:911–921.
- Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206–1213.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Sun Y, Zhang G, Yu J, et al. Evaluation of percutaneous microwave coagulation therapy for hepatic artery injury. Heliyon. 2015;1:e30.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422.
- Martin RC, Scoggins CR, Mcmasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178.
- Lorentzen T, Skjoldbye BO, Nolsoe CP. Microwave ablation of liver metastases guided by contrast-enhanced ultrasound: experience with 125 metastases in 39 patients. Ultraschall Med. 2011;32:492–496.
- Brace CL, Diaz TA, Hinshaw JL, et al. Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol. 2010;21:1280–1286.
- Hammill CW, Billingsley KG, Cassera MA, et al. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol. 2011;18:1947–1954.
- Nielsen K, van Tilborg AA, Meijerink MR, et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg. 2013;37:1340–1347.
- van Tilborg AA, Scheffer HJ, de Jong MC, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39:1438–1446.
- Hakime A, Tselikas L, Otmezguine Y, et al. Artificial ascites for pain relief during microwave ablation of subcapsular liver tumors. Cardiovasc Intervent Radiol. 2015;38:1557–1562.
- Han Y, Yu L, Hao YZ, et al. Percutaneous radiofrequency ablation with artificial hydrothorax for liver cancer in the hepatic dome. Zhonghua Zhong Liu Za Zhi. 2012;34:846–849.
- Yang B, Zou J, Xia J, et al. Risk factors for recurrence of small hepatocellular carcinoma after long-term follow-up of percutaneous radiofrequency ablation. Eur J Radiol. 2011;79:196–200.
- Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171.
- Kang TW, Lim HK, Lee MW, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016;280:300–312.
- Francica G, Meloni MF, de Sio I, et al. Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: safety and efficacy. Eur J Radiol. 2016;85:739–743.
- Qin S, Chen Y, Liu XY, et al. Clinical application of contrast-enhanced ultrasound using high-frequency linear probe in the detection of small colorectal liver metastases. Ultrasound Med Biol. 2017;43:2765–2773.
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23:2619–2626.
- Stang A, Donati M, Weilert H, et al. Impact of systemic therapy and recurrence pattern on survival outcome after radiofrequency ablation for colorectal liver metastases. J Cancer. 2016;7:1939–1949.
- Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol. 2012;35:868–874.
- Liang P, Wang Y, Yu X, et al. Malignant liver tumors: treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology. 2009;251:933–940.