Abstract
Objective: To evaluate the long-term clinical effect of high-intensity focussed ultrasound (HIFU) as a non-invasive modality for ablation of abdominal wall endometriosis (AWE) foci.
Methods: All women who were diagnosed with cutaneous endometriosis and underwent HIFU ablation and 4-year follow-up were included. Patient symptoms, imaging performed, HIFU ablation, recurrence, lesion location, size and number were collected and analyzed.
Results: A total of 51 women with 57 painful abdominal wall masses with a median volume of 4.00 cm3 and a mean age of 30.5±2.12 years were treated with HIFU. The main symptoms were a palpable painful abdominal mass (93%), protrusion of the skin (28.1%, 16) or lack of protrusion of the skin (71.9%, 41). Ultrasound was initially performed in 100% (51) of women, whereas 6% (3) required MRI examinations to distinguish the features and range of the masses. Ablation was performed with a median 300 s of sonication time, 40 min treatment time, 150 W of power and 41800 J of total energy to treat lesions that were a median volume of 3.83 cm3. No severe complications occurred, except in one patient with a first-degree skin burn, during the 48-month follow-up period. The pooled recurrence of cutaneous endometriosis occurred in 3.9% (2) of women.
Conclusion: The diagnosis of AWE should be confirmed with imaging of the lesion number, location, size and features before HIFU ablation. HIFU should be the first choice for the treatment of AWE as it is a non-invasive method, with high efficiency and safety and rapid postoperative recovery.
Introduction
High-intensity focussed ultrasound (HIFU) is a non-invasive treatment method that induces coagulation necrosis of the target tissues in vivo via ultrasound waves without injuring the adjacent normal tissues [Citation1–3]. HIFU beams are precisely focussed on a small region of diseased tissue to locally deposit high levels of energy, which induce the focussed tissue temperature will rise to between 65 °C and 100 °C, through destroying the diseased tissue via coagulative necrosis. Anesthesia is not required, but sedation is generally recommended [Citation1,Citation2]. Recently, HIFU has been widely used for the treatment of uterine fibroids [Citation1,Citation2] and endometriosis [Citation1,Citation2,Citation4]. Moreover, although HIFU has been shown to be effective and safe during short-term clinical follow-up after ablation for abdominal wall endometriosis (AWE), until now, no studies with long-term follow-up evaluating recurrence after HIFU treatment of AWE have been performed [Citation5–8]. However, our study involves the largest number of patients with AWE who underwent ablation with HIFU at a gynecological center and has the longest follow-up period published to date. Moreover, we describe our 4-year experience with HIFU treatment and prognosis of AWE foci over 4 years.
AWE is a rare disease that usually has a unique characteristic of a periodically painful mass associated with the menstrual cycle; due to the increasing trend in cesarean section as one of the most common causes of extra-pelvic endometriosis in recent years [Citation9–14]. When this ectopic endometrial tissue arises, periodic bleeding and fibrosis occur in combination with changes in menstrual cycle hormones, which ultimately lead to the formation of a painful mass [Citation12,Citation13,Citation15]. However, due to the high rates (0–29%) of relapse and defects in the abdominal wall [Citation9,Citation10,Citation16], a safer non-invasive approach, such as HIFU, is necessary for the clinical setting. In this study, 51 patients with AWE treated with HIFU ablation were selected. Their demographic characteristics and relevant factors affecting prognosis were retrospectively analyzed in order to provide a significant reference on the study of AWE and to further elucidate potential treatment approaches for use in the clinical setting.
Materials and methods
A total of 51 patients with AWE who were treated at the Capital Medical University Affiliated Beijing Obstetrics and Gynecology Hospital from March 2014 to May 2018 were included. Their baseline clinical data following HIFU ablation were retrospectively analyzed. All patients provided written informed consent for surgery and ultrasound contrast administration. The criteria for inclusion and pre-procedural preparation were detailed in a prior study [Citation5].
The treatment equipment was produced by JC200D (ultrasound-guided HIFU therapeutic system, Chongqing Haifu Medical Corporation Ltd., Chongqing, China). The ultrasound-guiding device (Mylab79, Esaote, Italy), which was situated at the center of the tank, guided the procedure in real-time during treatment. The therapeutic probe was 20 cm in diameter with a frequency of 0.8 MHz and emitted an energy ranging from 24 W to 400 W. The sizes of the focal regions were 1.5 mm × 1.5 mm × 8 mm. Patients in prone position were immersed in chilled degassed water (temperature below 10 °C). Fentanyl (0.8–1 µg/kg) and midazolam hydrochloride (0.02–0.03 mg/kg) were injected intravenously to induce sedation and analgesia, and injections were repeated at 30–40-min intervals so that all patients could communicate with the doctor during the process. Ultrasonographic imaging was used to map the ablated area and the surrounding tissues, such as the skin and intestines in the abdominal cavity, in real-time. Sonication was limited to the range of the lesions with a safe distance of 8–10 mm from the center of the lesions to the skin or abdominal cavity. The entire therapeutic range was based on the left and right margins of the lesion as visualized on a longitudinal scan.
No bowel or any other foreign material was present in the acoustic pathway. The treatment plan was drafted using 3–5 mm sequence-by-sequence images to calculate the volume of the treated tissue, according to a protocol developed by the physician. Then, sequence-by-sequence sonication was started at a ratio of 1:2 or 1:3 with an energy of 100–300 W, a temperature of 65–100 °C for 10 min after an intravenous bolus injection of SonoVue contrast agent ( and ) [Citation1,Citation4,Citation5]. The rhythm of adjustment was based on the patient’s skin reaction to the thermal radiation and skin changes. The results were evaluated in the non-perfused range of contrast-enhanced ultrasound (CEUS) ( and ). CEUS and posttreatment observation and management procedures were similar to those described in prior studies [Citation1,Citation4,Citation5]. After HIFU, patients were followed up from 1 month to 48 months, and any adverse sides or complications that met the standard of the International Radiological Association were recorded [Citation4,Citation5].
Figure 1. (a) CEUS image of an AWE cyst before the HIFU procedure. (b) CEUS image of an AWE cyst immediately after the HIFU procedure. (c) Ultrasonic image of an AWE cyst 6 months after the HIFU procedure. (d) Ultrasonic image of an AWE cyst 12 months after the HIFU procedure.
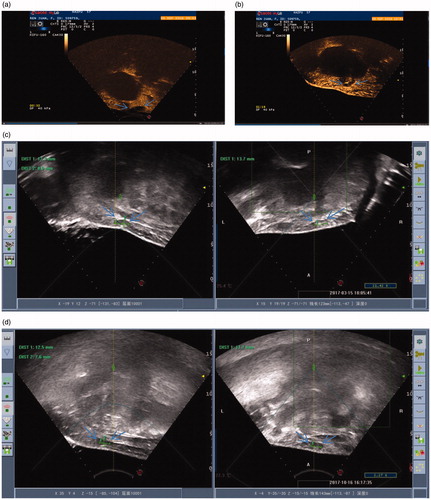
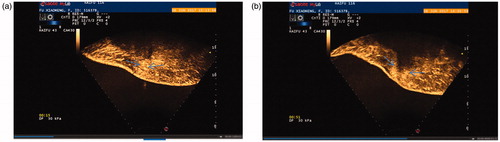
Figure 2. (a) CEUS image of an AWE mass before the HIFU procedure. (b) CEUS image of an AWE mass immediately after the HIFU procedure.
All data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using Student’s t-test SPSS software for self-contrast (version 21.0, http://spss.en.softonic.com/). p < .05 indicated significant differences.
Results
A total of 51 patients with 57 AWE lesions with the following characteristics were selected: average age of 30.5 ± 2.12 years; body mass index (BMI) of 25.67 ± 7.89 m2/kg; caesarean delivery time of 54 ± 8.49 months; interval from caesarean delivery to AWE diagnosis of 30 ± 8.49 months; and visual analogue scale (VAS) score of 5.5 ± 0.71 (). Of these 51 patients, 94.1% (48/51) underwent transverse cesarean sections, whereas 5.9% (3/51) underwent vertical cesarean sections. The texture of the lesion was hard in 100% of patients (57/57), and the degree of activity was high in 15.9% (9/57) of patients and low in 82.5% (47/57). Approximately 68.4% of patients (39/57) exhibited adhesions to the rectus or had lesions located in the rectus, whereas 26.3% (15/57) had lesions located in the subcutaneous fat layer, and 3.5% (2/57) had adhesions to the fascia. Pain on touch was experienced in 92.9% (53/57) of patients with a pain score of 2.00 ± 0.00, and the others experienced no pain. Skin protrusion was seen in 46.6% of patients (26/57).
Table 1. Baseline characteristics of patients with abdominal wall endometriosis.
As shown in , before treatment, the nodule volume was 2.70 ± 2.0 cm3, and after treatment for 55.50 ± 48.79 min of total treatment time and 346.00 ± 5.66 s of total sonication, the non-perfused volume was 1.10 ± 1.38 cm3, non-perfused volume rate was 3.04 ± 2.88%. The sonication time for 1 cm3 was 59.05 ± 8.56 s and the total sonication volume was 5.93 ± 0.96 cm3. The total energy was 46150 ± 7883.20 J, with an energy efficiency factor (EFF) of 7.99 ± 2.52 KJ/cm3 and the sonication intensity was 605.24 ± 525.95 s/h.
Table 2. Results of patients with abdominal wall endometriosis after high-intensity focussed ultrasound treatment.
As shown in , 12 patients experienced SIR Class A skin thermalgia, and 51 patients with the pain in the treatment area had SIR Class B skin thermalgia except for one who had a first-degree skin burn.
Table 3. Side effects or complications of patients with abdominal wall endometriosis after high-intensity focussed ultrasound treatment (n = 51).
At 48 months of follow-up, no patient had periodic pain. Their VAS scores significantly decreased from 1.00 ± 1.41 at 1 month and 1.00 ± 1.41 at 6 months to 0.5 ± 0.711 at 12 months and 0.00 ± 0.00 at 24, 36 and 48 months (). In addition, the rate of decrease in volume increased from 25.34 ± 18.83% at 1 month to 81.89 ± 15.69% at 6 months () 96.16 ± 5.44% at 12 months () to 85.25 ± 20.86% at 24 months 85.40 ± 20.60% at 36 months and to 100.00 ± 0.00% at 48 months. Pre-procedural AWE cystic and solid lesions showed different features on T1- and T2-weighted magnetic resonance imaging (MRI), as shown in and . The changes in preoperative and postoperative CEUS findings are shown in and . These changes included preoperative perfusion of cysts within a circumference of 3.3 cm × 2.5 cm × 1.5 cm in , and no blood perfusion of cysts within a circumference of 3.5 cm × 2.4 cm × 2.0 cm in . Furthermore, obvious blood flow was visible on preoperative CEUS but disappeared on postoperative CEUS.
Figure 3. (a) T2-weight MRI of an AWE cyst before the HIFU procedure. (b) T1-weighted MRI of an AWE cyst before the HIFU procedure.
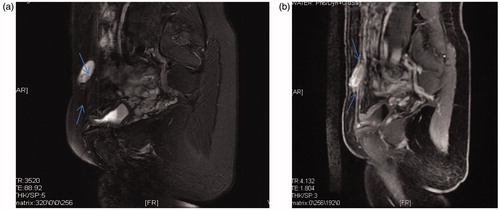
Figure 4. (a) T1-weighted MRI of an AWE mass before the HIFU procedure. (b) T2-weighted MRI of an AWE mass before the HIFU procedure.
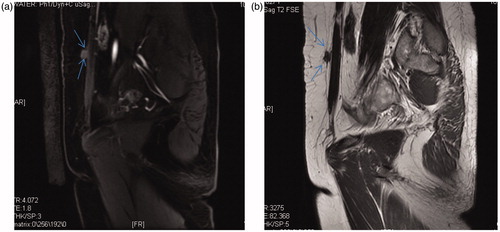
Table 4. Follow-up of patients with abdominal wall endometriosis after treatment with high-intensity focussed ultrasound ablation.
Discussion
The prevalence of AWE is low and is generally estimated to be between 0.03% and 1% [Citation12,Citation13,Citation16]. Leite et al. estimated that AWE occurred in 0.03–3.5% of patients after obstetric procedures [Citation9]. The incidence rates of AWE have increased with an increasing trend of cesarean deliveries corresponding to the two-baby policy [Citation11]. There are several factors that have influenced the increase in cesarean sections: nutrition excess during pregnancy, late marriage, late childbearing, etc. Our analysis showed that a total of 51 patients with AWE during reproductive stages (mean age, 32 years) had a previous history of cesarean sections; 88.2% (44/51) of patients had at least one cesarean section, and 11.8% (6/51) had two. Approximately 82.4% of patients had normal weights, and 0% was underweight. The safe distance of the thermal focus point to the skin surface or the abdominal wall cavity was at least 8–10 mm. Approximately 60% of patients wished to give birth again in the near future, whereas 28% did not want a second child. The median cesarean delivery time was 48 months, the median time from cesarean section to disease onset was 28 months, and the median time of onset was 12 months. In most patients with full-term pregnancies who undergo a cesarean section, the etiology of AWE is thought to be correlated to iatrogenic factors associated with endometrial debris contamination and dissemination during surgery.
A total of 51 patients were selected from our clinic with symptoms of painful masses with a median VAS score of 6 score (range from 2 to 10). Approximately 28.1% of patients had protrusion of skin, 94.6% had pain on palpation with a median VAS score of 3.5 (range from 0 to 10). All 57 lesions were confirmed, and the safety of ablation path was assessed with HIFU equipment before treatment. All lesions were confirmed with ultrasound, whereas 5.9% lesions were verified using MRI; 98.2% the lesions were solid nodules, except for one which was cystic. In addition, 92.2% of patients had single nodules, 5.9% had two nodules, and 1.9% had three nodules. Approximately 49.1% (26/57) of lesions were to the left of the scar, 17.5% (10/57) were in the middle and 33.3% (19/57) were to the right. In 84.2% (48/57) of lesions, the activity was low, whereas in 15.8% (9/57) it was high. In terms of adhesions to surrounding tissues, approximately 69.6% (39/57) of lesions had adhesions at the rectus, 26.8% (15/57) had adhesions to the subcutaneous fat tissue and 3.6% (3/57) had fascial adhesions. The median rectus thickness was 9.6 mm (range from 4.6 mm to 12.9 mm), and the median fatty tissue thickness was 24.9 mm (range from 5 mm to 43.9 mm). The median lesion diameter was 25.5 mm (range from 8 mm to 63 mm).
Ultrasound [Citation14,Citation15,Citation17–21] plays a vital role in verifying the presence of lesions, regardless of how small they are; moreover, ultrasound can be used to determine the location, size, texture, margins and number of AWE lesions and even differentiate cystic from solid neoplasms. The typical ultrasound features of an AWE lesion is a solid, hypoechoic and vascularized mass with irregular borders invading the surrounding tissue (fascia, muscle and fat). Color flow can show vascularity in the lesion; however, it is difficult to clearly identify color flow in the microvasculature of smaller lesions, especially those in the superficial tissue. The CEUS features provide a clearer depiction of the microcirculation in AWE lesions and in the surrounding tissue than color flow imaging. Before the thermal procedure, the borders and blood supply of these painful masses should be visualized to identify a focussed range for thermal energy treatment. In addition, CEUS can be used to assess the effect of HIFU ablation by evaluating the non-perfusion volume of lesions. Therefore, the use of ultrasound contrast to evaluate nodules preoperatively may be able to determine their blood perfusion. Similarly, it can be used intraoperatively to assess the effect of ablation and also to help to guide the therapeutic regimen.
MRI [Citation22–26] is typically considered the best modality for delineating the anatomy of soft tissue neoplasms and their surrounding structures. This modality can be used to evaluate small lesions and clearly differentiate muscles from abdominal subcutaneous tissues and from the invasion of the abdominal and pelvic wall structures. MRI can distinguish complicated cystic masses in the superficial tissues as hyper-intense heterogeneous masses on both T1 and T2-weighted images, with contrast enhancement only in the wall of lesion and can identify infiltration of rectus. According to our findings, the HIFU treatment of cysts should be focussed on the outline of the cysts to destroy the cystic wall cells. After 1 month of treatment, cyclic pain vanished, and the volumes of the lesions diminished. The combination of ultrasound with MRI can be used to determine the characteristics of nodules and their relationship to the adjacent tissues.
Ultrasound ablation is a non-invasive procedure that induces coagulative necrosis by increasing temperature of a lesion to 65 °C; it is currently considered for treatment of benign and malignant tumors of the uterus [Citation1,Citation2,Citation4], pancreas [Citation3] and other organs and has become an indispensable therapeutic modality in the clinical setting [Citation27,Citation28].
Although this modality is restricted by the lesion number, size and location, it can conformally focus, and it does not have any adverse effects on the target tissue [Citation1–8,Citation27,Citation28]. In our retrospective study, we selected 51 patients with AWE. Fifty-seven lesions were subjected to HIFU treatment, and the follow-up time was between 1 and 48 months. The median treatment time was 40 min (12–113 min), the median ablation time was 300 s (105–1200 s), the median ablation volume was 3.83 cm3 (1.35–12.83 cm3) and the median EFF median was 10094.45 J/cm3 (6082.67–22231.58 J/cm3). The focus volume was 3.83 cm3 (1.73–12.8 cm3), and the deposition energy of unit volume ablation was low.
One month after surgery, the periodic pain in all patients faded away, and the median palpable sensitivity as assessed using VAS scores decreased from 3.5 to 1, and the rate of decrease in lesion volume was 25.34 ± 18.83%, as the adjacent tissues affected by thermal radiation swell after the treatment. From 6 months to 12 months of follow-up, the palpable sensitivity as assessed by VAS scores improved from 1 to 0, whereas the rate of decrease in lesion volume increased from 81.89 ± 15.69% to 96.16 ± 5.44%. With the passage of time, the rate of decrease in the lesion volume gradually increased until the lesions completely disappeared at the 48th month. Otherwise, at 24 months, 36 months and 48 months of follow-up, there was no palpable pain, and the masses reduced gradually and faded away eventually. Before and after thermal ablation, nodules were treated only with focussed HIFU ablation without any medical or other treatment regimens. Two patients with AWE had complications of uterine fibroids or adenomyosis, both of which were ablated simultaneously. First, we focussed on the uterine neoplasms and then the superficial nodules, which reminded us that HIFU as a non-invasive procedure has the advantageous feature of being able to simultaneously eliminate lesions located in different organs or at different sites in the treatment path. In addition, this technique does not induce iatrogenic dissemination of ectopic endometrium and has no effect on childbearing, especially for patients with uterine lesions.
With regard to the use of an ultrasound contrast agent, no adverse reactions were documented except in one patient who had first-degree skin burns before, during, and after surgery. The skin area had an ‘orange peel view’ in the middle of lesion with ‘dotty waxy change’ for less than 10 mm of the diameter of the lesion. Microvascular perfusion of superficial skin can be visualized with CEUS. The skin in the ablated area can be kept dry and intact without special techniques. The skin was normal until 15 days after treatment based on telephone and photo follow-up. In fact, the use of contrast can be used to guide the procedure or assess the effects of HIFU ablation. In addition, it can provide significant information for dealing with thermal skin damage. In general, with the exception of skin burns and pain in the treatment region during treatment, patients were able to walk freely and resume their normal life and work soon after treatment.
Compared with surgical incisions for AWE lesions, the advantages of HIFU ablation include a significantly shorter hospitalization time, lack of bleeding and dissemination, and non-invasive nature; its disadvantages include non-pathological results and residual lesions in the abdominal wall. Otherwise, this procedure can be repeated because it is non-invasive, even if the lesion should relapse. Although 4-year follow-up has clearly delineated the risk of malignancy, longer-term observations are needed to confirm the results regarding hyperthermia.
A prior systematic review on surgical excision of AWE lesions reported the rates of recurrence ranged from 0% to 29% [Citation8,Citation9,Citation16]. Postoperative recurrence rates of AWE lesions in China ranged from 1.5% (2/29) to 9.9% (10/101) [Citation10]. Recently, one study that compared HIFU treatment with surgery demonstrated that there was a 4.4% (1/23) recurrence rate of the AWE in patients in the HIFU group after one year, and these patients required wider surgical excision [Citation6].
Our study indicated that the relapse rate of patients with AWE lesions treated with HIFU was 3.9% (2/51). Two patients with AWE located in the rectus relapsed and then underwent surgical excision; these two patients had mesh implants removed after 11 and 47 months after HIFU, respectively. Analysis of the two patients showed that one had a focus volume of 12.99 cm3 before surgery, with no change of the focus area with an average power of 117 W (100–200 W). The treatment time was 92 min, the treatment ablation time was 448 s, the ablation volume was 8.7 cm3, the non-perfused volume was 12.55 cm3 and the percentage of non-perfused volume (NPV%) was 96.56%. At the 1-month follow-up, the hypogastrium was occasionally painful, and the lesion had a focus volume of 8.88 cm3 and shrinkage of 32%. At the 3-month follow-up, the patient’s VAS score for palpable pain was 6 points, without a change in the focus volume. Until 11 months postoperatively, the patient’s palpable pain was not relieved, and surgery with a mesh implant was performed because the lesion was larger.
In another patient, the ablated volume was 1.31 cm3 before HIFU with no change in the focus. The treatment time was 90 min, the ablation time was 350 s, the ablation volume was 1.31 cm3, the non-perfused volume was 1.30 cm3 and the NPV% 99.94%. At the 1-month follow-up, the hypogastrium was occasionally painful with a focus of 1.15 cm3 and shrinkage of 12%. At the 3-month follow-up, the patient’s palpable pain score relapsed to the same value as before, and the lesion had a focus volume of 0.38 cm3 and shrinkage of 71%. At 47 months of follow-up, surgery was performed.
A superficial lesion measuring 3.8 cm3 in size and another measuring 0.7 cm3 in size required an average power of 100 W (100 W) and 200 W (200 W), respectively, a treatment time of 40 min and 27 min, respectively, and an ablation time of 303 s and 113 s, respectively; the ablation volume was 3.6 cm3 and 1.73 cm3, respectively, and the non-perfusion volume was 3.39 cm3 and 1.79 cm3, respectively. Palpable pain was completely reduced, and pain around the lesion that occurred not during the menstrual cycle appeared 6 months after the procedure; otherwise, the size of lesions ablated remained unchanged. Currently, these patients are undergoing continued monitoring without any treatment.
In our retrospective study, until 4 years of follow-up, periodic pain occurred during the menstrual cycle associated with shrinkage of lesions located in the rectus or the fascia in two patients. Surrounding tissue pain that occurred not during the menstrual cycle without any changes in lesion size in the surrounding fat was identified in two other patients. The following influencing factors were considered: nodules infiltrating the muscle or fascia near the peritoneum of the abdominal cavity, pain during the HIFU procedure and low thermal energy to prevent damage to the intestines. Otherwise, the lesions that were ablated often were larger in size and widely invaded the surrounding tissues, especially the rectus. To protect deep structures from thermal ablation, the range of the ablation was maintained in the superficial skin for nodules located there. Until now, all studies on AWE have been retrospective studies, and the incidence of AWE is rare and low; thus, there may have been bias and error in the information collected regarding the recurrence rates of AWE. Overall, our study is retrospective; thus, we cannot draw a significant conclusion about the recurrence rate of AWE after HIFU ablation on AWE due to bias, error or lack of consistent follow-up.
Conclusion
Overall, HIFU treatment noninvasively ameliorated the periodic pain and palpable sensitivity of patients with AWE with superior efficacy and safety [Citation6–8]; finally, the lesions ablated disappeared. HIFU has the advantage of being able to destroy lesions located in different sites under ultrasonic guidance in real-time in a non-invasive manner. In addition, it is a simple, convenient and rapid technique that is accepted by patients. However, there are some limitations in this study due to the small sample size and retrospective nature of the study. Future work should focus on improving the treatment process, regulating treatment and developing personalized treatment strategies based on the characteristics of the masses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Feng Y, Hu L, Chen W, et al. Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: a retrospective cohort study. Ultrason Sonochem. 2017;36:139–145.
- Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med. 2018; [Epub ahead of print].
- Zhang XY, Guo YS, Chen JM, et al. Effect of pre-treatment with gonadotropin-releasing hormone analogue GnRH-α on high-intensity focussed ultrasound ablation for diffuse adenomyosis: a preliminary study. Int J Hyperthermia. 2018;2:1–9.
- Xiaoying ZH, Hua D. Effect of high-intensity focused ultrasound ablation on endometriosis of the abdominal wall. Int J Clin Exp Pathol. 2018;11:2118–2124.
- Zhu X, Chen L, Deng X, et al. A comparison between high-intensity focused ultrasound and surgical treatment for the management of abdominal wall endometriosis. BJOG: Int J Obstet Gy. 2017;124:53–58.
- Luo S, Zhang C, Huang JP, et al. Ultrasound-guided high-intensity focused ultrasound treatment for abdominal wall endometriosis: a retrospective study. BJOG: Int J Obstet Gy. 2017;124:59–63.
- Stehouwer BL, Braat MNG, Veersema S. Magnetic resonance imaging-guided high-intensity focused ultrasound is a noninvasive treatment modality for patients with abdominal wall endometriosis. J Minim Invasive Gynecol. 2018; [Epub ahead of print].
- Leite GK, De Carvalho LF, Korkes H, et al. Scar endometrioma following obstetric surgical incisions: retrospective study on 33 cases and review of the literature. Sao Paulo Med J. 2009;127:270–277.
- Nominato NS, Prates LF, Lauar I, et al. Caesarean section greatly increases risk of scar endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;152:83–85.
- Ding Y, Zhu J. A retrospective review of abdominal wall endometriosisin Shanghai, China. Int J Gynaecol Obstet. 2013;121:41–44.
- Khamechian T, Alizargar J, Mazoochi T. 5-Year data analysis of patients following abdominal wall endometrioma surgery. BMC Womens Health. 2014;14:151–154.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014; 211:363.e1–365.
- Zhang J, Liu X. Clinicopathological features of endometriosis in abdominal wall–clinical analysis of 151 cases. Clin Exp Obstet Gynecol. 2016;43:379–383.
- Mui J, Allaire C, Williams C, et al. Abdominal wall pain in women with chronic pelvic pain. J Obstet Gynaecol Can. 2016; 38:154–159.
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, et al. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. 2018;221:58–63.
- Koninckx PR, Ussia A, Wattiez A, et al. Risk factors, clinical presentation, and outcomes for abdominal wall endometriosis. J Minim Invasive Gynecol. 2018;25:342–343.
- Gidwaney R, Badler RL, Yam BL, et al. Endometriosis of abdominal and pelvic wall scars: multimodality imaging findings, pathologic correlation, and radiologic mimics. Radiographics. 2012;32:2031–2043.
- Ozel L, Sagiroglu J, Unal A, et al. Abdominal wall endometriosis in the cesarean section surgical scar: a potential diagnostic pitfall. J Obstet Gynaecol Res. 2012;38:526–530.
- Solak A, Genç B, Yalaz S, et al. Abdominal wall endometrioma: ultrasonographic features and correlation with clinical findings. Balkan Med J. 2013;30:155–160.
- Coccia ME, Rizzello F, Nannini S, et al. Ultrasound-guided excision of rectus abdominis muscle endometriosis. J Obstet Gynaecol Res. 2015;41:149–152.
- Grigore M, Socolov D, Pavaleanu I, et al. Abdominal wall endometriosis: an update in clinical, imagistic features, and management options. Med Ultrason. 2017;19:430–437.
- Rindos NB, Mansuria S. Diagnosis and management of abdominal wall endometriosis: a systematic review and clinical recommendations. Obstet Gynecol Surv. 2017;72:116–122.
- Vellido-Cotelo R, Muñoz-González JL, Oliver-Pérez MR, et al. Endometriosis node in gynaecologic scars: a study of 17 patients and the diagnostic considerations in clinical experience in tertiary care center. BMC Womens Health. 2015;15:13
- Khan Z, Zanfagnin V, El-Nashar SA, et al. Risk factors, clinical presentation, and outcomes for abdominal wall endometriosis. J Minim Invasive Gynecol. 2017;24:478–484.
- Genç B, Solak A, Sahin N, et al. Diffusion-weighted imaging in the evaluation of hormonal cyclic changes in abdominal wall endometriomas. Clin Radiol. 2014;69:130–136.
- Hsiao YH, Kuo SJ, Tsai HD, et al. Clinical application of high-intensity focused ultrasound in cancer therapy. J Cancer. 2016;7:225–231.
- Ter Haar G. HIFU tissue ablation: concept and devices. Adv Exp Med Biol. 2016;880:3–20.
