Abstract
Purpose: The purpose of this study was to investigate the feasibility, safety and efficacy of intra-procedural contrast-enhanced ultrasound (CEUS) monitoring of the radiofrequency ablation (RFA) of liver cancers adjacent to gallbladder (GB) without GB isolation.
Materials and methods: From May 2016 to July 2017, patients with liver cancers adjacent to GB (≤10 mm) who intended to undergo ultrasound-guided RFA without GB isolation in our hospital were prospectively enrolled. During the RFA procedures, CEUS was employed to evaluate the therapeutic response and the perfusion of the intact GB wall. The outcomes of GB and liver cancers were followed up and recorded.
Results: 23 patients (18 male, 5 female) with 23 liver cancers (mean 18 mm, range 8–34 mm) adjacent to GB were enrolled. There were 12 tumors that abutted the GB while 11 tumors located within 10 mm of the GB. After the RFA procedures, intra-procedural CEUS evaluation demonstrated the perfusion of the GB wall was intact in all 23 patients and technical success rate of RFA was 100% (23/23). According to the contrast-enhanced CT/MR one month after RFA, the technical efficacy rate was 100% (23/23). During the follow-up period (range: 12–23 months, median: 17 months), no local tumor progression occurred and no major complications arised. Overall survival at 1-year was 100%. Thickening of GB wall was detected in 11 patients. The thickness of GB wall returned to the pre-ablation level in five patients.
Conclusion: CEUS-monitored RFA of liver cancers adjacent to GB without GB isolation was feasible, safe and effective.
1. Introduction
Radiofrequency ablation (RFA) has been recognized as a safe, effective and minimally invasive curative modality for early-stage hepatocellular carcinoma (HCC) [Citation1,Citation2]. Several reports have suggested that the efficacy of thermal ablation for early-stage HCC is comparable to that of liver resection [Citation3–5]. However, for tumors adjacent to critical extrahepatic organs, thermal ablation is considered relatively contraindicated because of the collateral thermal damage [Citation6,Citation7]. The gallbladder (GB) is one of the critical organs at risk for potential thermal damage [Citation8]. Some serious major complications such as perforation and cholecystitis may occur after RFA [Citation9,Citation10]. In addition, to avoid thermal damage of the GB, the ablation zone is usually insufficient, sometimes resulting in residual tumors or local tumor progression (LTP) [Citation8].
To improve the efficacy and reduce the risk, several measures have been reported to assist thermal ablation of liver tumors adjacent to the GB. The most frequently used technique is GB isolation using artificial ascites or needle decompression [Citation11–14]. On the other hand, thermometric needles inserted in the GB fossa or laparoscopy monitoring are used to predict potential thermal damage to the GB [Citation15,Citation16]. Moreover, cholecystectomy immediately after thermal ablation is another invasive method of preventing major complications due to potential thermal damage [Citation17]. However, these above additional invasive measures may increase the complexity, trauma and cost of RFA.
A few reports [Citation18,Citation19] further showed that thermal ablation of liver cancers adjacent to the GB without GB isolation and protection is also feasible and safe. However, due to the lack of assisted measures and effective monitoring techniques, the operators tend to restrict the ablation zone to avoid GB thermal damage, causing rates of residual and LTP were as high as 21.7%∼25% [Citation18,Citation19].
Contrast-enhanced ultrasound (CEUS) is recognized as an effective imaging modality for the immediate assessment of treatment response and has been utilized routinely during thermal ablation of the liver [Citation20]. It has also been reported to be useful in the evaluation of microvascular perfusion in non-liver organs [Citation21,Citation22], including GB. The perfusion or ischemia of the GB wall can also be assessed by CEUS. Therefore, we hypothesized that CEUS could be utilized to assess the treatment response and monitor the thermal damage of the GB wall during the RFA of liver cancer. In this way, the efficacy and safety of thermal ablation for liver cancers adjacent to the GB may be ensured without GB isolation.
In this study, we aimed to investigate the feasibility, safety and efficacy of RFA treatment of liver cancers adjacent to the GB without GB isolation under intra-procedural CEUS monitoring.
2. Materials and methods
2.1. Study population and lesions
From May 2016 to July 2017, patients with liver cancers adjacent to the GB who intended to undergo US-guided RFA in our hospital were consecutively included in this prospective study.
The inclusion criteria were as follows: (1) patients with malignant liver tumors had at least one lesion adjacent to GB; (2) the distance between the index lesion and the GB was less than 10 mm; and (3) all parameters agreed with the indication for liver tumor thermal ablation [Citation23].
The exclusion criteria were as follows: (1) patients were lost to follow up; (2) additional GB isolation or protection measures were employed (i.e., combined with ethanol injection, artificial ascites isolation or laparoscopy monitoring); and (3) patients were allergic to ultrasound contrast agents (UCAs).
The diagnosis of HCC [Citation2] was based on pathology for non-cirrhotic patients or typical imaging appearance on contrast-enhanced computed tomography/magnetic resonance imaging (CECT/CEMRI) combined with a history of cirrhosis. The diagnosis of other liver cancers was based on pathological results.
This study complied with the Declaration of Helsinki. Informed consent was obtained from each patient. This study was approved by the Institutional Review Board of the Third Affiliated Hospital, Sun Yat-sen University.
2.2. Equipment
RFA was performed using the Cool-tip RFA system (Covidien, Mansfield, MA, USA) with a 17-gauge, internally cooled-tip electrode with a 3-cm tip. Generally, the RF generator was set in the impedance mode with maximum output. Four to twelve minutes were used for each RF electrode insertion according to real-time US monitoring during ablation.
MyLab Twice ultrasound machine (Esoate, Genoa, Italy) with CnTI contrast-specific imaging was employed. The convex probe CA541 (frequency range from 1 to 8 MHz) was used for the ablation procedure.
2.3. Preablation assessment of the GB
The clinical symptoms and imaging findings of the GB were assessed before the RFA. Cholecystitis and cholecystolithiasis were recorded. The thickness of the GB walls on the liver side and the distance between the index lesion and the GB were measured.
2.4. RFA procedure
To avoid the difference of technical experience, the RFA procedure was performed by a single experienced interventional physician (X.E.J.) with more than 10 years of experiences in ablation procedures. The RFA procedure was performed under general anesthesia. For all lesions, single or overlapped multiple punctures were used to encompass the index tumor and an additional 0.5–1 cm of the normal liver parenchyma around the tumor to achieve a sufficient ablative margin, whenever possible (because the target tumor next to the GB fossa, the ablative margin of position in GB fossa was not always necessary. The ablation zone only needed to cover the target tumor completely at this position). The direction of the electrode insertion was parallel to the GB wall, and a minimum distance of 5 mm to the GB wall was maintained.
2.5. CEUS monitoring and evaluation
2.5.1. CEUS
CEUS was performed to monitor the ablation zone and perfusion of GB wall. SonoVue (Bracco, Milan, Italy) was injected as a rapid bolus of 1.0–2.0 ml via the antecubital vein. When necessary, SonoVue could be injected repeatedly.
2.5.2. Immediate evaluation of treatment response
Technical success was assessed by CEUS immediately after the ablation procedure. Technical success was defined as achieved if no-perfusion zone covered the tumor and the surrounding at least 5 mm normal liver parenchyma (except for the position next to the GB fossa).
2.5.3. Monitoring of the GB wall
The thickness of GB wall was monitored by US during and after the ablation procedure. Thickening of the GB wall was defined as an increase in the thickness of the GB wall of more than 2 mm after RFA.
Generally, CEUS was then employed to evaluate the perfusion of GB wall after each RFA cycle which was adjacent to the GB wall (≤10 mm) during the RFA procedure. If the RFA cycles were away from the GB wall, CEUS was not applied routinely. After the RFA procedure was complete, CEUS was carried out to confirm the intact perfusion of GB wall. Perfusion was considered to be intact when both the serosa and the mucosa were perfused continuously and uniformly. In contrast, perfusion was considered to not be intact when a perfusion defect was observed on the GB wall.
Cholecystectomy or drainage was suggested to perform subsequently to avoid GB perforation if the perfusion of the GB wall was not intact.
2.6. Follow-up
US examination was performed within 72 h after RFA to observe the thickness of the GB wall and to exclude early complications. Cholecystitis was diagnosed by the local signs of inflammation, systematic signs of inflammation and imaging finding [Citation24]. CECT/CEMRI was performed one month after RFA and was taken as the standard reference for the evaluation of technique efficacy. Follow-up generally required every three months. The overall survival (OS), LTP, intrahepatic distant recurrence (IDR), complications and side effects (especially those related to the GB) were monitored and recorded during the follow-up period.
2.7. Data analysis
SPSS 22.0 (SPSS, Chicago, Ill, USA) was employed, and the measurement data are presented as the average ± standard deviation if they displayed a normal distribution or as the median (range) if the data were not normally distributed. Comparison of the thickness of the GB wall pre- and post-ablation was performed by a t-test between two paired samples. The correlation between certain variables (the distance between the lesion and the GB, the distance between the electrode and the GB, and the WBC count) and the thickening of the GB wall was assessed by the use of Spearman’s rank test. Cumulative probability of survival and local tumor progression were estimated with Kaplan–Meier method. p values less than .05 indicated significant differences.
3. Results
3.1. Enrollment
From May 2016 to July 2017, there were 36 patients with 36 malignant liver tumors adjacent to the GB who underwent US-guided RFA and who satisfied the inclusion criteria. Among them, 12 patients were excluded because of the utilization of GB isolation (artificial ascites or needle decompression), and one patient was excluded due to being lost to follow-up. Finally, 23 patients with 23 liver cancers were enrolled in this study. The baseline characteristics of the study population are shown in .
Table 1. The baseline characteristics of the study population.
3.2. RFA procedure
These 23 lesions were all successfully ablated. The number of ablations cycles ranged from one to five (median: three times). The thickness of the GB walls during the ablation procedures was 5.74 ± 2.09 mm (2 ∼ 9 mm), which was thicker than that of before the ablation procedures (p = .001). The thickening of the GB wall was observed in 11 patients.
During the ablation procedures, CEUS was performed to evaluate the technical success of RFA and the perfusion of the GB wall. According to the CEUS evaluation and monitoring, the technical success rate for RFA was 100% (23/23). The perfusion of the GB wall was intact in all 23 patients. As a result, no emergency cholecystectomy or drainage was needed in any patient.
3.3. Follow up after RFA
3.3.1. Technique efficacy rate
According to the CECT/CEMR one month after RFA, no residual was detected in any of the 23 lesions, yielding a technical efficacy rate of 100% (23/23).
3.3.2. LTP and IDR
Until July 2018, the median follow-up period was 17 months (range: 12–23 months). During the follow-up period, no LTP occurred in any of the lesions. IDR in other segments occurred in 21.7% (5/23) of the enrolled patients.
3.4. Complications and side effects
Postablative syndrome was observed in nine patients, manifested by fever (five patients) and localized pain in the right upper abdomen (six patients). These symptoms resolved after conservative treatment.
The thickness of the GB wall on US examination was 5.61 ± 1.88 mm (2 ∼ 9 mm) within 72 h after RFA. This result was similar to that during the RFA procedure (p = .589). Thickening of the GB wall was also diagnosed in 11 patients. There were no correlations between the thickening of the GB wall and the distance between the lesion and the GB or the distance between the electrode and the GB (r= −1.96, p = .369 and r = 0.092, p = .675). No further treatment was needed for the thickening of the GB wall. The thickness of the GB wall returned to the preablation level in 45% (5/11) of patients within a follow-up period of 2–5 months.
No overt cholecystitis or perforation occurred in these 23 patients after RFA procedure or during the follow-up period. The white blood cell (WBC) count after RFA was 8.83 ± 4.61 (2.47–24.62) *109/L, which was higher than that before RFA (p < .001), and elevated WBCs were observed in nine patients. No correlation was found between WBC count elevation and the thickening of the GB wall (p = 1.000).
According to CECT/CEMR one month after RFA, the perfusion of the GB wall was intact in 23 patients, which was consistent with the results of the CEUS. No effusion in the GB fossa was observed in any of the 23 patients either on US examination or on CECT/CEMR during the follow-up period. () No cases of death were found in all the enrolled patients and the 1-year OS rate was 100% (23/23).
Figure 1. A 55-year-old male. (a, b, c) Contrast-enhanced ultrasound and MRI indicated a small liver tumor located in segment 5 abutting the gallbladder with a maximum diameter of 14 mm. (d, e) The electrode was inserted parallel to the gallbladder wall with a minimum distance of 10 mm. (f) Contrast-enhanced ultrasound demonstrated a complete ablation and intact perfusion of gallbladder wall. (g) Contrast-enhanced MRI one month after the ablation procedure confirmed the technical efficacy. (h) Normal gallbladder wall was showed in the follow-up period.
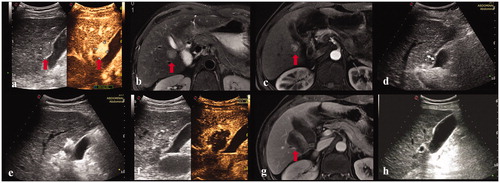
Figure 2. A 28-year-old female. (a, b) Contrast-enhanced ultrasound and MRI indicated a liver tumor located in segment 5 abutting the gallbladder with a maximum diameter of 27 mm. The thickness of gallbladder wall was 4 mm. (c) The electrode was inserted parallel to the gallbladder wall with a minimum distance of 8 mm. (d) The observation of gallbladder wall was affected by the gas produced by ablation. (e) Contrast-enhanced ultrasound showed that the perfusion of gallbladder wall was intact and the index tumor had been completely ablated. (f) Three day after ablation, the thickness of gallbladder wall increased to 9 mm. (g) Contrast-enhanced MRI one month after the ablation procedure confirmed the complete ablation of the index tumor. (h) Four months after ablation, the thickness of gallbladder wall was restored to 4 mm.
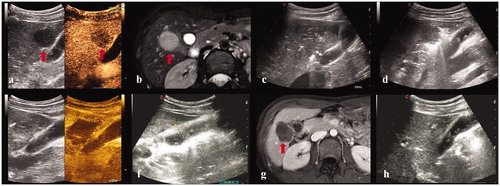
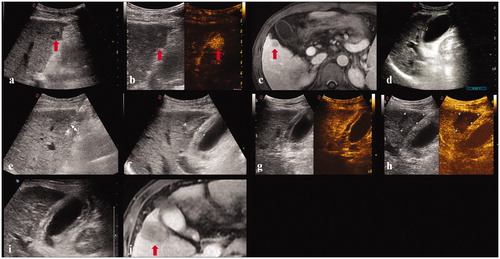
Figure 3. A 45-year-old male. (a, b, c). Contrast-enhanced ultrasound and MRI indicated a liver tumor located in segment 5 adjacent to the gallbladder with a maximum diameter of 18 mm. (d) Before ablation, the thickness of gallbladder wall was 5 mm. (e) The electrode was inserted parallel to the gallbladder wall with a minimum distance of 6 mm. (f) The thickness of gallbladder wall increased to 10 mm immediately after ablation. (g, h). Contrast-enhanced ultrasound showed that the perfusion of gallbladder wall was intact and the index tumor had been completely ablated. (i) The thickness of gallbladder wall was 9 mm five days after ablation. (j) Contrast-enhanced MRI one month after the ablation procedure confirmed the technical efficacy and the thickness of gallbladder wall was restored to 5 mm.
4. Discussion
In the present study, the technical efficacy rate was 100%, and no LTP was observed after the RFA procedure. In addition, no overt cholecystitis or perforation were observed in the follow-up period. These results indicated that RFA of lesions adjacent to the GB was feasible, effective and safe without GB isolation measures when the RFA was performed with CEUS monitoring. The strategies used to achieve these results in our study were as follows. First, only RFA with single straight electrode was employed for the ablation procedure. It was reported to produce a limited and predictable heat zone around the electrode [Citation25,Citation26], which made the thermal ablation procedure become feasible and safe in this high-risk location. Second, the electrode was inserted parallel to the GB wall with a distance of no less than 5 mm (6–11 mm) in the present study. It has been reported that inserting the electrode parallel to the GB wall with a distance of more than 10 mm was safer and more efficient [Citation19,Citation27]. In our study, a distance of 5 mm seemed to be the minimum cutoff value, which might be related to the shortened ablative time (approximately 4–6 min) when the electrode was close to the GB. Third, CEUS evaluation of the perfusion of the GB wall was performed as a routine monitoring method during the RFA of the lesions adjacent to the GB, which was accompanied by the immediate CEUS evaluation of tumor necrosis after RFA. The CEUS monitoring allowed the RFA procedures to be more aggressive to achieve the complete necrosis of these lesions, and the efficacy of this technique in one session and the LTP in this study were significantly improved compared with those in previous studies [Citation18,Citation19]. Even if thermal damage of the GB wall was found by the CEUS assessment, an emergency cholecystectomy or drainage could be carried out in time to avoid perforation. Meanwhile, unnecessary cholecystectomy or drainage could be avoided. Therefore, the RFA strategy in the present study was successfully applied for lesions adjacent to the GB without GB isolation.
However, a thermal effect on the GB wall was inevitable after the RFA procedure was performed on liver tumors adjacent to the GB. Although no perfusion defects of the GB wall were observed by CEUS assessment during RFA, thickening of the GB wall was demonstrated in 47.8% (11/23) of the enrolled patients soon after RFA. This thickening was slightly higher than that reported previously (2 6 ∼ 41%) [Citation13–15,Citation17–19,Citation27–29]. More cases abutting the GB (52%, 12/23), parallel insertion of the electrode and closer proximity to the GB (no less than 5 mm) might be considered causes of these differences. During the postoperative follow-up process, there was no progression of thickening of the GB wall in any of the cases. Moreover, 45% (5/11) of the cases with thickening of the GB wall recovered within 5 months. These results suggested that RFA near the GB could cause reversible thermal injury of the GB wall due to the thermal conduction. However, no overt cholecystitis or perforation occurred in any of these patients. The bile in the GB might act as a source of heat convection [Citation30]. The intra-procedural CEUS monitoring provided greater confidence in the maintenance of the intact state of the GB wall according to the wall perfusion. Therefore, even if the GB wall was obviously thickened, emergency cholecystectomy or drainage was not immediately necessary.
There were some limitations to this research. First, the present study was a single-arm study, and no comparative group was available for comparison. Second, the sample size was not sufficiently large. Third, the follow-up period was relatively short. Fourth, there were no cases with poor perfusion revealed by CEUS monitoring who required cholecystectomies in this study. As a result, the correlation between the CEUS findings and pathological changes could not be proved. Further research is needed to prove the reliability of this study.
In conclusion, RFA of liver cancers adjacent to the GB without GB isolation measures is feasible, safe and effective when performed with intra-procedural CEUS monitoring.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;10:761–780.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
- Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic Resection-Propensity Score analyses of long-term outcomes. Radiology. 2015;275:908–919.
- Kim GA, Shim JH, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg. 2016;103:126–135.
- Buscarini L, Buscarini E, Di SM, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921.
- Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound. 2001;13:159–166.
- de la Serna S, Vilana R, Sánchez-Cabús S, et al. Results of laparoscopic radiofrequency ablation for HCC. Could the location of the tumour influence a complete response to treatment? A single European centre experience. HPB. 2015;17:387–393.
- Akahane M, Koga H, Kato N, et al. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25:S57–S68.
- Chopra S, Dodd GD, Chanin MP, et al. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003;180:697–701.
- Kim JW, Shin SS, Heo SH, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol. 2015;16:1226–1239.
- Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33:428–436.
- Levit E, Bruners P, Gunther RW, et al. Bile aspiration and hydrodissection to prevent complications in hepatic RFA close to the gallbladder. Acta Radiol. 2012;53:1045–1048.
- Fernandes DD, Shyn PB, Silverman SG. Gallbladder needle decompression during radiofrequency ablation of an adjacent liver tumour. Can Assoc Radiol J. 2012;63:S37–S40.
- Li M, Yu X, Liang P, et al. Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperth. 2015;31:579–587.
- Jiang K, Su M, Zhao X, et al. “One-off” complete radiofrequency ablation of hepatocellular carcinoma adjacent to the gallbladder by a novel laparoscopic technique without gallbladder isolation. Cell Biochem Biophys. 2014;68:547–554.
- Pan WD, Zheng RQ, Nan L, et al. Ultrasound-guided percutaneous microwave coagulation therapy with a “cooled-tip needle” for the treatment of hepatocellular carcinoma adjacent to the gallbladder. Dig Dis Sci. 2010;55:2664–2669.
- Orlacchio A, Chegai F, Del Giudice C, et al. Radiofrequency thermoablation of HCC larger than 3 cm and less than 5 cm proximal to the gallbladder without gallbladder isolation: a single center experience. Biomed Res Int. 2014;2014:1.
- Kim SW, Rhim H, Park M, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:366–376.
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012. Ultraschall in Med. 2012;34:11–29.
- Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall in Med. 2018;39:e2–44.
- Salvatore V, Borghi A, Piscaglia F. Contrast-enhanced ultrasound for liver imaging: recent advances. CPD. 2012;18:2236–2252.
- The Ministry of Health of The People's Republic of China. Diagnosis and therapy criterion for hepatocellular carcinoma (version 2011). Chin Clin Oncol. 2011;2011:929–946.
- Yokoe M, Hata J, Takada T, et al. Tokyo guideline 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54.
- Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: an updated review. WJGPT. 2016;7:477–489.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208.
- Lee J, Rhim H, Jeon YH, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: histopathologic changes of gallbladder wall in a pig model. AJR Am J Roentgenol. 2008;190:418–425.
- Hao Z, Wan C, Han Q, et al. Comparison of the gallbladder damage caused by microwave ablation and cryoablation in vivo porcine livers. Indian J Cancer. 2015;52:84–90.
- Huang H, Liang P, Yu XL, et al. Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperth. 2015;31:40–47.
- Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–418.
