Abstract
Purpose: This study aimed to assess the safety and technical feasibility of percutaneous ablation therapy for lymph node (LN) metastases of hepatocellular carcinoma (HCC).
Material and Methods: A total of 31 consecutive HCC patients with LN metastases who were treated with ablation were included in this retrospective study. Percutaneous ablation was performed under local anesthesia and computed tomography–guidance. The primary endpoint was technique success; secondary endpoints were overall survival (OS), progression-free survival (PFS), and local progression-free survival (LPFS). Survival curves were constructed using Kaplan-Meier method.
Results: The median diameter of metastatic LNs was 30 mm (range, 10–77 mm). The 1-, 3-, and 5-year OS rates were 74.6%, 50.3%, and 50.3%, respectively. The 1-, 3-, and 5-year PFS rates were 24.7%, 0%, and not available for calculation (NA), respectively. The 1-, 3-, and 5-year LPFS rates were 78.7%, 69.9%, and 69.9%, respectively. The technique success and technical effectiveness rates were 100% and 64.5%, respectively. The technical effectiveness rates were 65.4% (17/26) and 60% (3/5) in abdominal LN metastases and distant LN metastases, respectively. Only one patient (1/31, 3.2%) had major complications (massive pleural effusion and severe pneumonia) related to ablation. Minor complications related to ablation included mild abdominal pain (10/31, 32.3%) and self-limiting hematoma (2/31, 6.5%). No ablation-related death occurred.
Conclusion: Percutaneous ablation appears to be a safe and feasible method for treatment of metastatic LNs in patients with HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers, and the incidence continues to rise worldwide [Citation1]. After the lung, the lymph nodes (LNs) are the second common site of extrahepatic metastases in HCC. LN metastases have been found in 0.75–7.5% of HCC patients during surgery [Citation2,Citation3], and in 30.3% at autopsy [Citation4]. Spread to LNs has important clinical implications. The median survival of patients with LN metastases from HCC is 3 months in the absence of any treatment [Citation5]. Lymphadenectomy and radiation therapy have been reported to prolong median survival to 8.0–14.0 months and 7.0–13.0 months, respectively [Citation6]. However, treatment-related adverse events such as refractory ascites and gastrointestinal hemorrhage are common, and often necessitate treatment interruption [Citation2,Citation7].
Since advances of technology, such as multiple electrode radiofrequency system, multi-modality-ultrasound fusion imaging, new computed-tomography techniques, magnetic navigation system, and thermal-based ablation combined with 125I seed implant brachytherapy [Citation8–10], variety of minimally invasive techniques, including radiofrequency ablation (RFA), microwave ablation (MWA), and percutaneous ethanol injection (PEI), have been shown to be safe and effective in HCC patients [Citation11]. In patients with early-stage HCC, RFA can be a curative choice. Thermal ablation modalities such as MWA and RFA also improve outcomes in intermediate- or advanced-stage HCC [Citation11–13]. PEI using 95% alcohol can induce complete necrosis in 95% of tumors <2 cm in size, with major complications occurring in only 0–2% of patients [Citation14,Citation15].
Sethi et al. were the first to demonstrate the feasibility of endoscopic ultrasound–guided thermal ablation of LN metastases in a porcine model [Citation16]. Since then several researchers have reported the use of different modalities—including RFA [Citation17,Citation18], MWA [Citation19], and chemotherapy [Citation20]—for ablation of LN metastasis in humans. Local hematoma formation and abdominal pain are the most frequently reported adverse events; however, they are relatively uncommon and, when they do occur, are usually mild and self-limiting. Besides, percutaneous ablation therapy has several advantages: it is minimally invasive and well tolerated and can also be safely applied multiple times for treatment of recurrences. It is therefore becoming an important mode of treatment for advanced cancers with spread to LNs.
Most previous studies on the application of ablation therapy were limited by their retrospective nature and small sample size. The safety and effectiveness of ablation therapy for prolonging survival in patients having advanced cancer with spread to LNs remain to be established. In the present study we aimed to evaluate the safety and feasibility of percutaneous ablation for treatment of metastatic LNs in patients with advanced HCC.
Materials and methods
Patients
This retrospective study was performed with approval from institutional ethics committee, and written informed consent was obtained before treatment.
Between March 2011 and July 2016, 56 consecutive patients with LNs metastases of HCC were treated by percutaneous ablation in our institute. Twenty-five patients were excluded as LN metastases originating from other kinds of tumors instead of hepatocellular carcinoma (HCC). The remaining 31 patients meeting the inclusion criteria were included in this retrospective study.
The inclusion criteria were as follows: (a) patients were aged at least 18 years; (b) the diagnosis of HCC was confirmed by cross-sectional imaging or biopsy according to American Association for the Study of Liver Diseases guideline, and the tumor response of intrahepatic disease was evaluated as complete response, partial response or stable disease at the time of LN metastasis diagnosis; (c) the diagnosis of LN metastasis was based on findings of contrast-enhanced computed tomography (CECT) or contrast-enhanced magnetic resonance imaging (CEMRI): enlarged LN was detected at dynamic follow-up; the maximum diameter of enlarged LN was more than 10 mm; enhancement of LN was observed at arterial and/or venous phases; (d) preserved liver function was Child-Pugh class A or B; (e) no evidence of severe coagulopathy was detected (a prolonged prothrombin time <5 s, prothrombin activity >50% and platelet count >50,000/mm3); (f) patients were not eligible for surgical treatment or refused surgery; (g) LN metastases were not treated with other cancer therapies, such as resection, radiation therapy or Sorafenib; (h) Eastern Cooperative Oncology Group (ECOG) status was 0–2.
The exclusion criteria were as follows: (a) patients whose preserved liver function was Child-Pugh C; (b) patients who refused ablation therapy; (c) patients who had received other treatments than ablation for treatment of LN metastases; (d) patients who had severe coagulopathy.
Ablation equipment
The RFA system (RITA Medical Systems, Mountain View, CA) and microwave ablation system (FORSEA MTC-3CA; Qinghai Microwave Electronic Institute, Nanjing, China) were used in the current study. The RF generator with a frequency of 460 kHz provided maximum output power of 200 W. MWA was performed with a frequency of 2450 MHz and an output power of 0–120 W. A 22-gauge Chiba needle and a 21-gauge three-hole alcohol injection needle were used in procedures. Injectable drug was 99.9% absolute alcohol. A 16-slice CT scanner (Aquilion™, Toshiba Medical CO, Tokyo, Japan) was used to guide all procedures.
Ablation procedure
All procedures were under CT-guidance. MWA was performed for large nodule or lesions adjacent to vital vessel to decrease the impact of heat-sink effect; for metastatic LNs abutting visceral organs, RFA, or PEI was considered to avoid unintended injury. Procedures were performed by two radiologists specializing in tumor ablation. Patients were under local anesthesia with 2% lidocaine, and placed in an appropriate position (prone, supine, or lateral decubitus position) according to tumor location. Vital signs were continuously monitored during and for 24 h following procedures. Under CT-guidance, an appropriate approach of antenna/electrode insertion was determined. A 22-gauge Chiba needle was advanced into the target lesion and used to lead antenna/electrode to the target. A single ablation treatment was performed for tumor <2 cm, while multi-point overlapping ablations were carried out for treatment of lesions ≥2 cm. For patients who were treated by PEI, a 22 G Chiba needle was advanced into the target lesion. Subsequently, 4–20 mL of absolute alcohol based on the tumor volume was injected to obtain homogeneous perfusion. Intraoperative CT scan was necessary to ensure the correct position of antenna/electrode. At the end of procedures, the needle track was ablated to avoid bleeding along the electrode route. Postprocedural CECT was performed to evaluate immediate tumor response, and complications, such as hemorrhage, abscess, hematoma, and pneumothorax. showed the procedures of ablation for LN metastases.
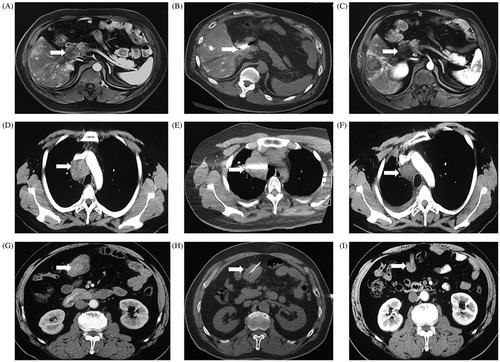
Figure 1. Ablation therapies for lymph node metastases originating from hepatocellular carcinoma. (A) The preoperative MR imaging showed a porta hepatic LN metastasis. (B) The microwave antenna was advanced into the target lesion. (C) The MR imaging at 5 days later showed partial response and mild heterogenous enhancement. (D) The preoperative computed tomography scan showed a superior vena caval LN metastasis. (E) The radiofrequency electrode was advanced into the lesion. (F) The CT scan 6 months later showed complete response and no enhancement. (G) The preoperative computed tomography scan showed a retroperitoneal LN metastasis. (H) Absolute alcohol (20ml) was slowly injected into the retroperitoneal LN metastasis. (I) The CT scan 3 months later showed significant tumor shrinkage, complete response and no enhancement.
Follow-up
CECT or CEMRI, and laboratory tests included serum a-fetoprotein (AFP, the recorded level prior to treatment for LN metastases was showed in ), liver function tests, blood biochemistry tests, and blood coagulation tests were routinely preformed on the day following treatment, at 1 month from initial discharge, every 3 months during the first years and every 6 months thereafter to evaluate the efficacy of ablation therapy in LN metastases. For residual LN metastases, up to 2 ablation sessions would be performed. The interval between 2 ablation sessions was 1 month. If intrahepatic disease recurrence or other extrahepatic recurrence was detected during the follow-up, multidisciplinary team would decide appropriate therapies, such as TACE (or TAE) and/or ablation, sorafenib, orchemotherapy, to control the disease.
Table 1. The major characteristics of included patients who had lymph node metastases originating from hepatocellular carcinoma.
Assessment of therapeutic efficacy
The primary endpoint was technique success which was defined as target lesion to be treated according to the preplanned protocol and covered completely [Citation21]. Technical effectiveness was that the target lesion achieved complete ablation within 4 sessions of ablation or a 3-month treatment window [Citation21].
The second endpoints were overall survival (OS), progression-free survival (PFS) and local PFS (LPFS). OS was defined as the interval between the time of initial ablation and the time of death or the last time of follow-up. PFS was defined as the time elapsed from the first ablation to first local or distant recurrence after ablation. LPFS was the interval between the initial ablation and first local recurrence or progression.
Tumor response was evaluated based on the Modified Response Evaluation Criteria in Solid Tumor (mRECIST) [Citation22]. Complete response (CR) was defined as no enhancement in the target lesion. Partial response (PR) was defined as a reduction of more than 30% in the maximal diameter of the enhancement area. Stable disease (SD) was between PR and PD. If there was a 20% increase in the maximal diameter of the enhanced area, the tumor response would be defined as progressive disease (PD). Local control rate (LCR) was calculated as (CR + PR)/total number of patients ×100%.
Minor complication and Major complication were categorized based on the criteria of the Society of Interventional Radiology Classifications [Citation23]. The definition of major complication was an event that required major therapy or resulted in substantial mortality or disability. Other complications were identified as minor complications.
Statistical analysis
Data were analyzed by SPSS 22.0 for Windows. The baseline and clinical characteristics were summarized with mean ± standard deviation (continuous variable with normal distribution), median ± range (continuous variable with non-normal distribution) or frequency (categorical variables). The survival rates and curves were calculated and depicted by the Kaplan–Meier method. Log-rank test was used to detect the difference among the three different techniques in terms of OS rate. All statistical tests were two-sided, and a significant difference was considered when p < .05.
Results
Clinicopathologic characteristics
and show the baseline characteristics of enrolled patients. A total of 31 patients (28 males and 3 female) were included in the study. The mean age of the patients was 57.26 ± 8.9 years (range, 41–80 years). Diagnosis of metastatic LN was based on imaging (CECT or CEMRI) or pathological findings. The median number of LN metastases per patient was 1 (range: 1–5). The median maximum diameter of lesions was 30 mm (range: 10–77 mm).
Table E1. The complete characteristics of included patients who had lymph node metastases originating from hepatocellular carcinoma.
Among the 31 patients, 9 patients had concurrent metastases to other sites including bone (1/31, 3.2%), lung (5/31, 16.1%), suprarenal gland (2/31, 6.5%), and pelvis (1/31, 3.2%). Vascular invasion was identified in 7 patients: 5 patients had portal vein invasion, 1 patient had cava invasion, and 1 patient had portal vein and hepatic vein invasion. A total of 30 patients (30/31, 96.8%) had received treatments for intrahepatic disease before the time of LN metastases diagnosis, including resection (6/31, 19.4%), RFA (22/31, 71.0%), MWA (11/31, 35.5%), cryoablation (5/31, 16.1%), PEI (1/31, 3.2%), TACE (29/31, 93.5%), and Sorafenib (2/31, 6.5%).
lists the sites of LN metastases. Abdominal LN metastases were detected in 26 patients (26/31, 83.9%), with a total of 55 metastases; the nodes involved were porta hepatic LNs (6/31, 19.4%), aortocaval LNs (2/31, 6.5%), portacaval LNs (2/31, 6.5%), gastric LNs (1/31, 3.2%), mesocolic LNs (2/31, 6.5%), retroperitoneal LNs (11/31, 35.5%), and inferior diaphragmatic LNs (2/31, 6.5%). Distant metastatic LNs were found in 5 patients (5/31, 16.1%); the nodes involved were the mediastinal LNs (3/31, 9.7%), superior vena caval LNs (1/31, 3.2%), and subcutaneous LNs (1/31, 3.2%).
Table 2. The treatment efficacy assessed at 24 h after ablation for lymph node metastases in different sites.
Among the 31 patients, 22 patients underwent 1 session of ablation and 8 patients underwent 2 sessions of ablation; only 1 patient (having mediastinal LN metastases with maximal LN diameter of 20 mm; number of LNs involved, 5) underwent 3 sessions of ablation.
Treatment efficacy
presents the treatment efficacy as assessed at 24 h after ablation. There were total 31 patients who received the treatment, and the technique success rate was 100%. Among them, 20 (20/31, 64.5%) achieved technical effectiveness.
The technical effectiveness rate in abdominal LN metastases was 65.4% (17/26). The technical effectiveness rate was ≥50% in aortocaval (2/2, 100%), retroperitoneal (9/11, 81.8%), inferior diaphragmatic (2/2, 100%), portacaval (1/2, 50%), and mesocolic (1/2, 50%) LN metastases. For porta hepatic LN metastases, the technical effectiveness rate was 33.3% (2/6).
In 5 patients with distant LN metastases, the technical effectiveness rate was 60% (3/5). Two patients (2/5, 40%) failed to achieve effective ablation: one had subcutaneous LN metastasis and the other had mediastinal LN metastasis.
and show the tumor response at 1 month after ablation. Since 3 patients were lost to follow-up, only 28 patients were available for evaluation. The rates of CR, PR, SD, PR, and LCR were 60.7% (17/28), 17.9% (5/28), 17.9% (5/28), 3.6% (1/28), and 78.6% (22/28), respectively. One patient with PD and portacaval LN metastasis (with maximal lesion diameter of 31 mm) who was treated with RFA died due to multiple organ failure at 2 months after ablation.
Figure 2. Tumor response after percutaneous ablation therapy based on the Modified Response Evaluation Criteria in Solid Tumor. Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
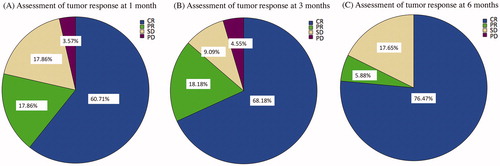
Table 3. The tumor response after ablation therapies for metastatic LNs based on mRECIST criteria.
At 3 months after ablation, 2 patients had died and 8 were lost follow-up. Tumor response was evaluated in the remaining 21 patients. The rates of CR, PR, SD, PD, and LCR were 71.4% (15/21), 19.0% (4/21), 9.5% (2/21), 0% (0/21), and 90.4% (19/21), respectively. Among the patients assessed as PR, 2 progressed to SD at 6 months after ablation.
At 6 months after ablation, 5 patients had died, and 8 patients were lost to follow-up. Thus, a total of 18 patients were available for evaluation. The rates of CR, PR, SD, PD, and LCR were 77.8% (14/18), 5.6% (1/18), 16.7% (3/18), 0% (0/18), and 83.3% (15/18), respectively.
Overall survival, local progression–free survival, and progression-free survival
and show the OS of the patients. In the entire sample, the 1-, 2-, 3-, and 5-year OS rates were 74.6%, 50.3%, 50.3%, and 50.3%, respectively. For patients treated with RFA (n = 14), after median follow-up of 12.51 months (range: 0.13–79.51 months), the 1-, 2-, 3-, and 5-year OS rates were 61.4%, 30.7%, 30.7%, and 30.7%, respectively. For patients treated with MWA (n = 5), after median follow-up of 13.47 months (range: 2.04–26.87 months), the 1-, 2-, 3-, and 5-year OS rates were 60%, 60%, NA, and NA, respectively. For patients treated with PEI (n = 12), after median follow-up of 11.73 months (range: 0.2–33.25), the 1-, 2-, 3-, and 5-year OS rates were 90%, 72%, NA, and NA, respectively. The difference in cumulative OS rates between patients receiving the three therapies was not statistically significant (p = .639).
Figure 3. Survival curves after ablation therapy for treatment of lymph node metastases in patients with hepatocellular carcinoma. (A) The overall survival (OS) rate of patients with lymph node metastases after percutaneous ablation therapy. (B) The local progression - free survival (LPFS) rate of patients with lymph node metastases after percutaneous ablation therapy. (C) The progression-free survival (PFS) rate of patients with lymph node metastases after percutaneous ablation therapy. (D) The OS rates after ablation therapies, including RFA, MWA, and PEI, in patients with lymph node metastases. (E) The LPFS rates after ablation therapies, including RFA, MWA, and PEI, in patients with lymph node metastases. (F) The PFS rates after ablation therapies, including RFA, MWA, and PEI, in patients with lymph node metastases. Abbreviations: RFA, radiofrequency ablation; MWA, microwave ablation; PEI, percutaneous ethanol injection; LNM, lymph node metastases.
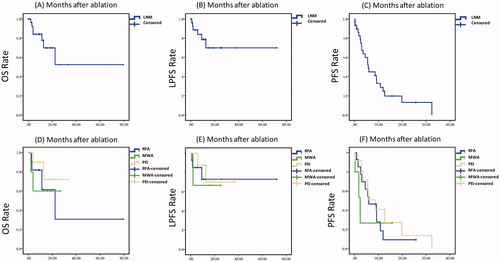
Table 4. The 1-, 2-, 3-, 4- and 5-year OS, PFS, LPFS rates of metastatic lymph nodes after ablation.
During the period of follow-up, a total of 8 patients (25.8%) died, and 6 of them died within 1 year: 2 died due to extrahepatic metastases and multiple organ failure, while 4 out-of-hospital patients died of PD after refusing treatment for recurrent lesions. Of the 6 patients, 3 patients had retroperitoneal LN metastases (the nodules treated in the current study); 1 patient had 3 aortocaval LN metastases (the nodules treated in the current study), and 2 patients had porta hepatic LN metastases (the nodules treated in the current study). The mean diameter of metastatic LNs in these 6 patients was 27.67 ± 16.16 mm (range: 10–47 mm). The maximum diameter of 47 mm was measured in a porta hepatic LN. Two patients (one treated with MWA and the other with PEI) did not achieve CR. In the remaining 4 patients, complete necrosis could be achieved through salvage ablation.
and show the progression-free survival (PFS) and local progression–free survival (LPFS) curves and rates. The 1-, 3-, and 5-year PFS rates in the whole sample were 24.7%, 0%, and NA, respectively. For patients treated with RFA, the 1-, 3-, and 5-year PFS rates were 9.3%, NA, and NA, respectively. For patients treated with MWA, 1-, 3-, and 5-year PFS rates were 26.7%, NA, and NA, respectively. For patients treated with PEI, the 1-, 3-, and 5-year PFS rates were 40.7%, NA, and NA, respectively. The difference between the PFS rates of patients treated with different modalities was not statistically significant (p = .678). Twenty-two patients occurred intrahepatic recurrence during the follow-up, and 11 of the 22 patients were treated with ablation combined with or without TACE/TAE.
The 1-, 3-, and 5-year LPFS rates in the whole sample were 78.7%, 69.9%, and 69.9%, respectively. For patients treated with RFA, the 1-, 3-, and 5-year LPFS rates were 73%, 73%, and 73%, respectively. For patients treated with MWA, 1-, 3-, and 5-year LPFS rates were 66.7%, NA, and NA. For patients treated with PEI, the 1-, 3-, and 5-year LPFS rates were 87.5%, 70%, and NA. The difference in LPFS rates between patients treated with the different modalities was not statistically significant (p = .882).
Safety of ablation therapy and complications
Among the 31 patients, major complications occurred in only 1 patient (1/31, 3.2%). This patient developed massive pleural effusion and severe pneumonia, and required chest tube placement and anti-infection treatment. Minor complications occurred in 12 patients (12/31, 38.7%); these complications included mild abdominal pain (10/31, 32.3%), and self-limiting hematoma (2/31, 6.5%). No ablation-related deaths occurred.
Median duration of hospitalization was 6 days (range: 2–27 days). A total of 3 patients needed hospitalization for >7 days. One patient who had paracaval LN metastasis developed severe pneumonia and massive pleural effusion after PEI, and needed hospitalization for 14 days for anti-infection treatment and chest tube drainage. Another patient with mediastinal LN metastasis remained in hospital for 15 days for receiving additional chemotherapy. The third patient required hospitalization for 22 days for performance of salvage RFA for the primary HCC.
Discussion
The current study investigated the clinically relevant outcomes of percutaneous ablation therapy for treatment of LN metastases in patients with HCC. Previous in vivo study has indicated that the application of RFA could be carried out for lymph nodes [Citation24]. Our research demonstrated that the RFA procedure achieved a technique success of 100% for LN metastases from HCC, and the 6-month LCR of 83.3% was promising. In Pan and colleagues’ study, the technique success of RFA for LN metastases from HCC was 100%. These findings indicate that ablation therapy seems to be an alternative treatment with high practicability for LN metastases originating from HCC. Besides, several published literature reported that ablation is able to provide an encouraging survival in HCC patients with lymph node metastases, with preferable LCRs (6 months) ranging from 68.3% to 73.3% [Citation17–19]. The LCR showed by the current study (83.3%) was comparable to the rate reported previously. Besides, some researchers suggested that preferable LCRs were associated with better survival [Citation25]. It may be because of a reduction rate of LN-related death. Moreover, minimally invasive ablation for LN metastases is helpful to palliate manifestations, such as pain, duodenal obstruction, jaundice, ascites or pleural effusion [Citation17,Citation26], and able to improve the quality of life. An encouraging technical effectiveness rate of 50–100% was seen in this study. Gao et al. [Citation18] investigated the clinical outcomes of patients undergoing RFA for treatment of retroperitoneal LN metastasis, and reported a technical effectiveness rate of 78.9%. This was similar to the technical effectiveness rate of 81.8% for retroperitoneal LNs seen in our study. However, a high technical effectiveness rate of ablation appears to be difficult to obtain in portohepatic, gastric, and subcutaneous LN metastases. There could be several possible causes. First, “heat sink effect” has been proposed as the reason for incomplete ablation of target lesions near major vessels [Citation27–29]. Heat generated in metastatic LN at the hepatic hilum tends to get conducted away from the site by the blood flow. Second, there is a potential risk of unintended injury in metastatic LNs adjacent to vital structures. To mitigate the risk of unintended injury, the protocol includes setting mild ablation energy, and shortening duration of procedure, which may correlate with incomplete necrosis.
In the current study, no significant difference was found concerning accumulated OS rates among the three different ablation techniques (RFA, PEI, and MWA). This may indicate that these three ablation modalities could provide similar survival outcomes. However, debate continues about whether different ablation techniques could induce different outcomes, specially whether MWA is superior to RFA. RFA is considered the standard of care for treatment of HCC of 3 cm or smaller [Citation30]. Compared with RFA, MWA is associated with increased ablation temperatures, increased ablation zones, and a reduction in the heat-sink effect for HCC [Citation31,Citation32]. Besides, MWA is reported to induce lower risk of unintended injury for lesions abutting visceral organs [Citation33]. However, recent randomized, controlled, phase 2 trial, comparing the efficacy and safety between RFA and MWA, demonstrated that microwave ablation was not superior to radiofrequency ablation in terms of procedure time, local tumor progression, frequency of complications, or overall survival [Citation34]; and some researchers demonstrated that RFA could be considered for HCC lesions adjacent to visceral organs without compromising the efficacy of the procedure or increasing the frequency of complications [Citation35]. It should be noted that the comparison between MWA and RFA for lymph node metastases has not been established yet. Several studies reported RFA to be a safe and feasible technique for lymph node metastases [Citation17,Citation36], while only a few studies shown the application of MWA for LNs. It is a pity that the comparison among different ablation techniques has not been established in the current study due to the limited sample size; and a selection bias may exist because the application of RFA is carried out earlier than MWA in the institution which may lead radiologists to be more specializing in RF procedures. Thus, further RCTs focused on the difference of clinically relevant outcomes among various ablation techniques are needed.
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, HCC with spread to LNs is classified as BCLC stage C, for which the currently preferred treatment is sorafenib. In the phase III Sorafenib Asia–Pacific Trial [Citation37], patients undergoing sorafenib therapy for LN metastases had 1-year OS rate of 20.6%, with a median OS of 5.6 months (range: 3.9–7.2 months). In comparison, patients receiving placebo had 1-year OS rate of only 6.14%, with a median OS of 3.2 months (range: 2.4–4.7 months). In our study the 1-year OS rate was 74.6%, suggesting that ablation therapy might offer considerable survival for advanced-stage HCC patients with LN metastases.
Lymphadenectomy and radiation therapy are considered effective treatments for patients with LN metastases [Citation36,Citation38–40]. In a previous study [Citation25] the 1-year OS rate after lymphadenectomy was 76.9%. Kyubo et al. [Citation41] examined the clinical outcomes of HCC patients with LN metastases after radiotherapy and reported 1-year survival rates ranging from 0% to 77.1% in different subgroups. As our study shows, percutaneous thermal ablation, especially RFA, provides short-term outcomes comparable to that with lymphadenectomy and radiation therapy. In our study, the 1-year OS rate of patients treated with RFA was 61.4%, with technique success being achieved in all patients (100%). Pan et al. [Citation17] reported the survival rate at 1 year after RFA to be 58.3% in HCC patients with metastatic LNs. Although the 1 year OS is relatively low, the 5-year OS rate after ablation is encouraging. In the present study, the 5-year OS rate was 50.3% vs. the 5-year OS rate of 22% reported after lymphadenectomy [Citation25]. Thus, for HCC patients with LN metastases, ablation appears to be as an effective treatment as lymphadenectomy or radiotherapy and, in fact, long-term survival after ablation therapy may even be more favorable. The survival benefit after ablation therapy may be because it is generally well tolerated by patients and can be repeated if necessary for treatment of recurrence.
A few earlier reports have demonstrated the safety of percutaneous ablation therapy for LN metastases and reported low rates of major complications [Citation17,Citation18]. In our study the rate of major complications was only 3.2%. Although the rate of minor complications was 38.7%, all of these complications were mild and self-limiting, or subsided with only conservative treatment. No ablation-related death occurred. Significantly, Yoon et al. [Citation42] have suggested that there are potential risks of rupture of the treated LN and tumor seeding or dissemination during ablation therapy. However, these risks have not been detected in previous studies and were also not observed in the present study. Future studies with large sample size and randomized design are necessary to confirm the safety of ablation therapy for LN metastases.
There are several limitations in this study. First, this was a retrospective study with a nonrandomized design and a selection bias was unavoidable. Second, the strength of the evidence is limited by the small sample size. Third, because the statistical power to detect significant difference was low, insignificant differences should be interpreted carefully. Nevertheless, our findings indicate that survival rates might be improved with ablation therapy.
In conclusion, percutaneous ablation seems to be a safe and feasible therapy for HCC patients with LN metastases, with promising technique success rate. The 1-year OS rate after ablation is comparable to that obtained with conventional treatments such as sorafenib therapy, lymphadenectomy, and radiotherapy. The 5-year OS rate after ablation is also favorable. Further large-scale multicenter RCTs are required to investigate the usefulness of percutaneous thermal ablation for treatment of LN metastases from HCC.
Acknowledgments
The institutional review board of You’an Hospital Ethics Committee has approved this study. Informed consent was obtained from all individual participants included in the study. Human experimentation guidelines of China were followed in the conduct of this clinical research. The work described has not been published or accepted elsewhere, in whole or in part. All the authors listed have seen and approved the manuscript that is enclosed, contributed significantly to the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262.
- Ercolani G, Grazi GL, Ravaioli M, et al. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202–209. doi: 10.1097/01.sla.0000109154.00020.e0.
- Hasegawa K, Makuuchi M, Kokudo N, et al. Impact of histologically confirmed lymph node metastases on patient survival after surgical resection for hepatocellular carcinoma: report of a Japanese Nationwide Survey. Ann Surg. 2014;259:166–170. doi: 10.1097/SLA.0b013e31828d4960.
- Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on lymph node metastasis of hepatocellular carcinoma: a retrospective study of 660 consecutive autopsy cases. Jpn J Clin Oncol. 1994;24(1):37–41.
- Xia F, Wu L, Lau WY, et al. Positive lymph node metastasis has a marked impact on the long-term survival of patients with hepatocellular carcinoma with extrahepatic metastasis. PLoS One. 2014;9:e95889. doi: 10.1371/journal.pone.0095889.
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44.
- Yamashita H, Nakagawa K, Shiraishi K, et al. Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: retrospective study. J Gastroenterol Hepatol. 2007;22:523–527. doi: 10.1111/j.1440-1746.2006.04450.x.
- Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015;4:176–187. doi: 10.1159/000367740.
- Lee DH, Lee JM. Recent advances in the image-guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, real-time multimodality fusion imaging, and new energy sources. Korean J Radiol. 2018;19:545–559. doi: 10.3348/kjr.2018.19.4.545.
- Shi Y, Zhai B. A recent advance in image-guided locoregional therapy for hepatocellular carcinoma. Gastrointest Tumors. 2016;3:90–102. doi: 10.1159/000445888.
- Chinnaratha MA, Chuang MY, Fraser RJ, et al. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294–301. doi: 10.1111/jgh.13028.
- Li W, Man W, Guo H, et al. Clinical study of transcatheter arterial chemoembolization combined with microwave ablation in the treatment of advanced hepatocellular carcinoma. J Can Res Ther. 2016;12:C217–C220. doi: 10.4103/0973-1482.200598.
- Nouso K, Kariyama K, Nakamura S, et al. Application of radiofrequency ablation for the treatment of intermediate-stage hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32:695–700. doi: 10.1111/jgh.13586.
- Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:461–476. doi: 10.1111/j.1365-2036.2009.04200.x.
- Pompili M, Nicolardi E, Abbate V, et al. Ethanol injection is highly effective for hepatocellular carcinoma smaller than 2 cm. World J Gastroenterol. 2011;17:3126–3132. doi: 10.3748/wjg.v17.i26.3126.
- Sethi A, Ellrichmann M, Dhar S, et al. Endoscopic ultrasound-guided lymph node ablation with a novel radiofrequency ablation probe: feasibility study in an acute porcine model. Endoscopy. 2014;46:411–415. doi: 10.1055/s-0034-1364933.
- Pan T, Xie QK, Lv N, et al. Percutaneous CT-guided radiofrequency ablation for lymph node oligometastases from hepatocellular carcinoma: a propensity score-matching analysis. Radiology. 2017;282:259–270. doi: 10.1148/radiol.2016151807.
- Gao F, Gu Y, Huang J, et al. Radiofrequency ablation of retroperitoneal metastatic lymph nodes from hepatocellular carcinoma. Acad Radiol. 2012;19:1035–1040. doi: 10.1016/j.acra.2012.04.003.
- Shao H, Arellano RS. Percutaneous microwave ablation of hepatocellular carcinoma metastatic to a mesocolic lymph node. J Vasc Interv Radiol. 2017;28:1281–1283. doi: 10.1016/j.jvir.2017.06.001.
- Liu SR, Xiao YY, Le Pivert PJ, et al. CT-guided percutaneous chemoablation using an ethanol-ethiodol-doxorubicin emulsion for the treatment of metastatic lymph node carcinoma: a comparative study. Technol Cancer Res Treat. 2013;12:165–172. doi: 10.7785/tcrt.2012.500254.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. PubMed PMID: 24927329; PubMed Central PMCID: PMCPMC4263618.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132.
- Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2003;14:S293–S295.
- Yoon WJ, Daglilar ES, Kamionek M, et al. Evaluation of radiofrequency ablation using a 1-Fr wire electrode in porcine pancreas, liver, gallbladder, spleen, kidney, stomach, and lymph nodes: a pilot study. Digestive Endoscopy. 2016;28:465–468.
- Awazu M, Fukumoto T, Takebe A, et al. Lymphadenectomy combined with locoregional treatment for multiple advanced hepatocellular carcinoma with lymph node metastases. Kobe J Med Sci. 2013;59:E17–E27.
- Zeng ZC, Tang ZY, Fan J, et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys. 2005;63:1067–1076. doi: 10.1016/j.ijrobp.2005.03.058.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761.
- Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171.
- Pearson AS, Izzo F, Fleming RY, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592–599.
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344. doi: 10.3109/02656736.2015.1127434.
- Shiina S, Sato K, Tateishi R, et al. Percutaneous ablation for hepatocellular carcinoma: comparison of various ablation techniques and surgery. Can J Gastroenterol Hepatol. 2018;2018:1. doi: 10.1155/2018/4756147.
- Filippiadis DK, Spiliopoulos S, Konstantos C, et al. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia. 2018;34:863–869. doi: 10.1080/02656736.2017.1370728.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325. doi: 10.1016/S2468-1253(18)30029-3.
- Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108. doi: 10.1002/hep.21164.
- Wang L, Ge M, Xu D, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Can Res Ther. 2014;10:144–149. doi: 10.4103/0973-1482.145844.
- Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48:1452–1465. doi: 10.1016/j.ejca.2011.12.006.
- Keyver-Paik MD, Arden JM, Luders C, et al. Impact of chemotherapy on retroperitoneal lymph nodes in ovarian cancer. Anticancer Res. 2016;36:1815–1824.
- Ishihara S, Kawai K, Tanaka T, et al. Oncological outcomes of lateral pelvic lymph node metastasis in rectal cancer treated with preoperative chemoradiotherapy. Dis Colon Rectum. 2017;60:469–476. doi: 10.1097/DCR.0000000000000752.
- Watanabe M, Mine S, Yamada K, et al. Outcomes of lymphadenectomy for lymph node recurrence after esophagectomy or definitive chemoradiotherapy for squamous cell carcinoma of the esophagus. Gen Thorac Cardiovasc Surg. 2014;62:685–692. doi: 10.1007/s11748-014-0444-4.
- Kim K, Chie EK, Kim W, et al. Absence of symptom and intact liver function are positive prognosticators for patients undergoing radiotherapy for lymph node metastasis from hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;78:729–734. 1 doi: 10.1016/j.ijrobp.2009.08.047.
- Yoon WJ, Daglilar ES, Kamionek M, et al. Evaluation of radiofrequency ablation using the 1-Fr wire electrode in the porcine pancreas, liver, gallbladder, spleen, kidney, stomach, and lymph nodes: a pilot study. Dig Endosc. 2016;28:465–468. 1doi: 10.1111/den.12575.
