Abstract
Purpose: Patients with toxic adenomas (TAs) that are too large to undergo radioactive iodine (RAI) treatment aimed at resolving hyperthyroidism and/or relieving mechanical pressure symptoms are referred to surgery. This prospective study aimed to assess the outcomes of combining laser ablation (LA) plus RAI vs lobectomy to treat large TAs in terms of clinical efficacy and the health-related quality of life (HRQoL).
Patients and methods: Patients with TAs of volumes greater than 20 mL and a calculated therapeutic activity exceeding 600 Mbq were randomly assigned to undergo LA + RAI (Group A) or lobectomy (Group B). The HRQoL was assessed using 12-item Short Form Health Survey questionnaire before and 6 months after treatment.
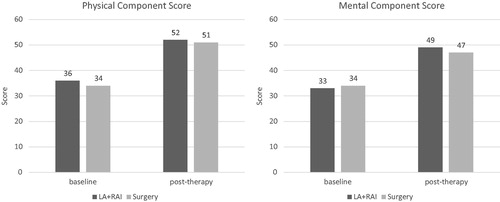
Results: Twenty-seven patients entered the study. After completing treatment, patients in Group A showed a TA reduction by a mean of 68% compared to baseline. Two of 14 patients (14.3%) in Group A and 2 of 13 (15.4%) in Group B became subclinically hypothyroid, whereas the remaining patients were euthyroid. HRQoL significantly improved in both groups after treatment.
Conclusions: For patients with large TAs, a combination of LA and RAI is a feasible alternative to surgery. Similar to surgery, LA + RAI resolves the mechanical discomfort induced by nodule pressure and effectively treats the hyperthyroidism. This procedure also avoids the potential complications associated with surgery while guaranteeing a similar HRQoL benefit.
Introduction
A toxic adenoma (TA) is a monoclonal, autonomously functioning thyroid nodule that produces supraphysiological amounts of thyroid hormones resulting in the suppression of serum thyroid-stimulating hormone (TSH). The function of the surrounding normal thyroid tissue is also suppressed. TAs are more frequent in women and in patients older than 60 years [Citation1,Citation2]. The typical presentation that leads to the diagnosis of large TAs is thyrotoxicosis, which causes compressive symptoms or manifests as a lump in the neck. Treatment options include surgical excision or radioactive iodine (RAI) treatment. Surgical excision carries the risks associated with general anesthesia as well as the potential complications of thyroid surgery; similarly to RAI, it is also associated with permanent hypothyroidism in 5–15% of patients [Citation3–5]. On the other hand, hyperthyroidism due to large TAs might not be definitely curable by RAI when the calculated therapeutic activity exceeds 600 Mbq (the allowed amount on an outpatient basis). In this setting, the idea of combining laser ablation (LA) with RAI appears to be an attractive option for patients with large TAs, as the debulking achieved with LA is combined with the functional impairment induced by RAI [Citation6]. An Italian study by Chianelli et al. demonstrated that combining LA and RAI induced faster and greater improvement of local and systemic symptoms than RAI alone; the authors therefore suggested that this combined treatment is a valid option for patients who refuse surgery or are at an elevated anesthesiological risk.
Moreover, individuals with thyroid disease experience a significant impairment in their quality of life; this aspect should also be considered when tending to these patients and devising treatment options. In fact, the health-related quality of life (HRQoL) is significantly impaired in patients with thyroid disease [Citation7,Citation8]. Data have shown that the HRQoL scores in patients with Graves' hyperthyroidism and toxic nodular goiter are significantly worse than those in the general population. While HRQoL scores improved significantly when treating both these diseases, significant disease-specific and generic HRQoL deficits persisted in multiple domains in both patient groups [Citation9]. A significant deterioration of HRQoL was also clearly demonstrated by the 12-item Short Form Health Survey (SF-12) in thyroidectomized patients with hypothyroidism induced by thyroid hormone withdrawal [Citation10]. In this study, we aimed to compare the clinical outcomes and HRQoL in patients diagnosed with large TAs who underwent LA + RAI vs those who underwent surgery.
Patients and methods
Between 2015 and 2017, twenty-seven patients with the following characteristics were recruited: (1) a single or dominant solid nodule, (2) suppressed TSH levels with free thyroid hormone levels above the upper limit, (3) hyperactive appearance of the lesion on 99mTc thyroid scintigraphy, (4) nodule volume >20 ml with a calculated therapeutic activity exceeding 600 Mbq, and (5) negative thyroid antibodies (peroxidase and TSH receptor). Recruited patients were randomly assigned to two treatment groups: Group A patients received LA + RAI, while Group B patients underwent lobectomy.
Group A (LA + RAI) protocol
A total of 14 patients were randomized to LA + RAI treatment. Patients underwent treatment with anti-thyroid drugs (ATDs) and beta-blockers to restore normal thyroid function before undergoing LA. ATDs were suspended after LA and patients were periodically monitored and re-administered ATDs when necessary. Six months after LA, the treated thyroid nodule was monitored using ultrasonography, and the patients received RAI at a fixed dose (555 MBq); all ATDs were stopped 7 days before RAI and remained suspended after RAI therapy. Six months later (i.e., 12 months after LA), patients underwent follow-up thyroid function tests and ultrasonographic scans. The SF-12 was administered at the time of diagnosis (before starting ATD) and 6 months after RAI.
Group B (surgery) protocol
A total of 13 patients were randomized to surgery. Patients received ATDs and beta-blockers as necessary to restore normal thyroid function before undergoing lobectomy. ATDs were stopped after surgery, and thyroid function tests were performed 6 months later. The SF-12 was administered at the time of diagnosis (before starting ATD) and 6 months after surgery.
In both groups, the SF-12 was administered 6 months after the final treatment to obtain a reliable evaluation of the HRQoL once patients achieved a stable and definitive cure.
LA procedure
Light conscious sedation was achieved with intravenous midazolam (2–5 mg) in fractionated boli. After ultrasonographic examination of the neck and selection of needle entry points, local anesthesia (2% xylocaine) was injected deep into the thyroid capsule. LA was performed in a single session by inserting 21-gauge spinal needles into the target thyroid lesion under ultrasonographic guidance. After the free-hand positioning of the needle tips, a 300 µm-diameter plane-cut quartz optical fiber was introduced through the sheaths of the needles, and a fiber tip was placed in direct contact with the tissue. Optic fibers were connected to the laser source, and a continuous-wave Nd-YAG laser operating at 1064 µm with an optical beam splitting device (Elesta, Florence, Italy) and an output power of 3 W was delivered according to a previously described technique [Citation11]. Between 1 and 3 needles were placed manually along the longitudinal, craniocaudal, and major nodule axes at a distance of 10 mm each and in a manner best fitting the shape of the nodule. The procedure commenced with a deposition energy of 1200–1800 J per fiber in the caudal part of the nodule, 10 mm from the lower margin, trachea, and carotid. Using upward needle/fiber pullbacks of 10 mm, additional energy was administered until a distance of 5–10 mm from the upper part of the nodule was attained. Using ultrasonography, the treatment area was visualized as a hyperechoic zone enlarging over time due to the formation of gas microbubbles within the coagulated tissue. After treatment, the patients were administered an intravenous injection of ketoprofen.
HRQoL
The HRQoL was assessed using the SF-12 Health Survey, which is an abbreviated version of the more extensive SF-36 questionnaire but is reliable, simple, and requires only a few minutes to complete. The SF-12 comprises 12 questions that cover 8 health domains: physical functioning, role limitations due to physical health, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The results are produced as a physical component summary and a mental component summary. Higher scores indicate a better health status.
Laboratory evaluation
Serum TSH, free triiodothyronine (FT3), free thyroxine (FT4), and thyroglobulin (Tg) were measured using a third-generation electrochemiluminescence immunoassay. Reference values were 0.3–3.6 mIU/liter for TSH, 2.2–4.2 pg/mL for FT3, 0.8–1.7 ng/dL for FT4, and 0.2–70 ng/mL for Tg. Thyroperoxidase, Tg, and TSH receptor antibody levels were determined using a radioimmunoassay kit.
Thyroid ultrasonography
Thyroid sonographic evaluation was conducted using a commercially available US scanner (Esaote) equipped with a 7.5–13.0 MHz linear transducer. The nodule volume was calculated using the ellipsoid formula (mL).
Statistical analysis
Results are expressed as the mean ± SD. Paired Student t-tests were used to compare data within the same group, while unpaired Student t-tests were used to compare data between groups.
Patients gave written and informed consent. The study was approved by the hospital ethical committee.
Results
Twenty-seven patients were recruited for the study, including 14 in Group A and 13 in Group B. The baseline characteristics of patients in these two groups were similar ().
Table 1. Characteristics of patients.
All 14 Group A patients received ATDs, while 10 (71.4%) received beta-blockers. The nodules of two patients (14.3%) had a cystic component that was aspirated before LA. Thyroid nodules were treated with a mean of 2.2 ± 0.4 fibers that delivered 555.6 ± 35 J/mL. One patient (7.1%) experienced transient dysphonia. Six months after LA, the mean thyroid volume was 19.2 ± 4 ml, with reduction achieved by a mean of 39% ().
Figure 1. Representative images of: baseline 99mTc thyroid scintiscan (A); ultrasound scan of toxic nodule before laser ablation (B); ultrasound scan 6 months after laser ablation (C); ultrasound scan 6 months after radioactive treatment and 12 months after laser ablation (D).
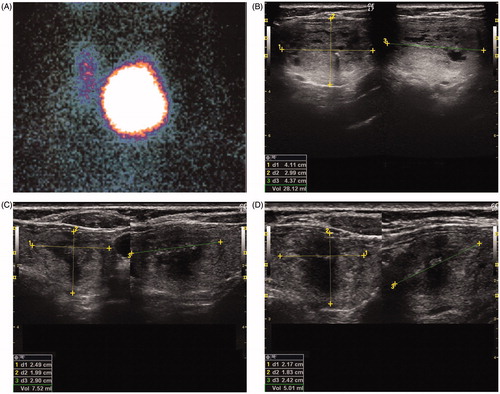
Before RAI, 6 of the 14 patients (42.9%) were subclinically hyperthyroid whereas the rest were overtly hyperthyroid (TSH: 0.2 ± 0.3 mIU/L; FT4: 2.1 ± 0.6 pg/mL). Six months after RAI, the mean thyroid volume was 9.9 ± 1.9 ml, which represented a mean 68% reduction compared to baseline. Two patients (14.3%) were subclinically hypothyroid, whereas the remainder were euthyroid (TSH: 2.2 ± 1.3 mIU/L; FT4: 1.0 ± 0.1 pg/mL).
All 13 Group B patients received ATDs, and 9 (69.2%) received beta-blockers. Six months after surgery, 2 of 13 patients (15.4%) were subclinically hypothyroid, whereas the remainder were euthyroid (TSH: 2.3 ± 1.4 mIU/L; FT4: 0.9 ± 0.1 pg/mL).
Patients in both groups showed similar HRQoL scores at baseline; these were significantly improved at the follow-up time (p < .01) without significant differences between the groups ().
Discussion
Non-randomized, randomized and multicenter retrospective studies have confirmed the effectiveness, safety and clinical efficacy of LA in the treatment of symptomatic cold thyroid nodules in terms of volume reduction and improvement in symptoms and cosmetic problems [Citation12,Citation13]. Once subjected to LA, nodules display a progressive reduction by almost 70% at 3 years, with local symptoms and cosmetic signs decreasing in 80% of cases [Citation12]. In skilled hands, LA is considered a safe procedure; complications (usually transient dysphonia) arise in approximately 1% of cases [Citation13].
LA is not the first-choice treatment for hyperfunctioning thyroid nodules and multinodular toxic goiter. While several studies have shown significant reductions in nodule volumes with LA, hyperthyroidism persists in approximately 50% of patients [Citation14,Citation15]. The rate of patients with persistent hyperthyroidism after LA treatment correlates with the baseline volume; the larger the nodule, the higher the risk of hyperfunction [Citation16]. A recent Italian study by Gambelunghe et al. showed that the rate of unsuccessful LA treatment in terms of thyroid function is elevated when the nodule size exceeds 25 ml [Citation17]. Compared to their study, our cohort showed a lower reduction rate (39% vs. 57%) at 6 months; as the structures of the TAs and energy delivered were similar, this difference appears to be attributable to a significant difference in baseline volumes (31.5 ml vs 12 ml). This finding provides further evidence that a single LA exerts a remarkable but not decisive effect on large TAs. Highly positive results were obtained in terms of volume reduction and restoration of euthyroidism in another Italian study of large TAs (>40 ml): the investigators obtained a normal TSH concentration in 88.5% of patients, but used a high amount of energy in multiple (up to 3) treatment cycles with multiple LA sessions (up to 3 per cycle) required [Citation18]. These previous findings clearly show that large TAs cannot be successfully treated with thermal procedures because: (1) a single session is usually insufficient to restore euthyroidism within a short-term period, and (2) even though euthyroidism can be restored following multiple sessions, the untreated component of the nodule will tend to re-grow over the long term owing to its monoclonal nature, thus producing a proportionally excessive amount of thyroid hormones.
Chianelli et al.’s intriguing idea of combining LA (to shrink the nodule) with RAI (to destroy hyperfunctioning thyroid cells) introduced a new option for treating large TAs that would otherwise be referred to surgery [Citation6]. As surgery is the standard treatment for large TAs, our study aimed to compare the clinical outcomes of LA + RAI with those of surgery specifically in TAs exceeding 20 ml in volume. Our findings confirmed that the combined treatment was effective in terms of volume reduction and restoration of euthyroidism, indicating that this combination procedure represents a valid alternative to surgery.
We also considered the HRQoL, which (as expected) was significantly impaired in newly diagnosed patients with TA. The HRQoL significantly improved after both surgical treatment and LA + RAI, with no significant difference between the improvement rates of both.
In a 2-year follow-up study of patients who underwent radiofrequency ablation (RFA) for nodular goiter, both the mental and physical components of their HRQoL scores significantly improved after treatment [Citation19]. At the same time, patients who underwent surgery for nodular goiter also experienced significant improvements in HRQoL after thyroidectomy [Citation20]. In a large cohort of patients with benign nontoxic goiter, those who underwent RAI therapy, hemithyroidectomy, total thyroidectomy, or percutaneous ethanol injection had greater HRQoL impairment at baseline on the ‘Goiter Symptoms and Anxiety’ scales, and also experienced the greatest post-treatment improvements [Citation21]. Consistent with our study, Chinese investigators found patients undergoing RFA had significantly better HRQoL scores than those undergoing open thyroidectomies in term of general health, vitality, and mental health [Citation22]. In addition to the burden of mechanical symptoms induced by nodular goiter, our patients also experienced the burdens associated with hyperthyroidism, which were resolved in both treatment groups. Insofar as mechanical symptoms, Gulseren et al. showed that worsened physical and mental HRQoL scores in hyperthyroid patients were ameliorated upon restoration of normal thyroid function [Citation23]. Notably, Graves’ disease patients continued to show diminished vital and mental quality of life aspects many years after treatment with ATDs, RAI, or surgery [Citation9,Citation24]. A recently published telephone survey-based study in Italy by Bernardi et al. found that patients who underwent surgery or RFA for a single benign thyroid nodule were equally satisfied in terms of symptoms resolution when these nodules were nonfunctioning, whereas patients with TA were not as satisfied with RFA as those who were treated with surgery [Citation25]. The same study showed that, independent of thyroid nodule function, surgery was the preferred modality for resolving nodule-related symptoms, whereas RFA was preferred in terms of cosmetic results. The study by Bernardi et al. indirectly shows that, when the TA is large, thermal ablation procedures are partially effective in curing hyperthyroidism and resolving volume-related symptoms [Citation17,Citation25]. Our present study demonstrates that the combination of LA + RAI is a viable alternative to surgery for treating large TAs [Citation26]. As with surgery, LA + RAI is able to both resolve the mechanical discomfort induced by nodule pressure and cure thyroid hyperfunction. This procedure avoids the potential complications associated with surgery while guaranteeing a similar HRQoL benefit.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hamburger JI. Evolution of toxicity in solitary nontoxic autonomously functioning thyroid nodules. J Clin Endocrinol Metab. 1980;50:1089–1093.
- Bransom CJ, Talbot CH, Henry L, et al. Solitary toxic adenoma of the thyroid gland. Br J Surg. 1979;66:592–595.
- Eyre-Brook IA, Talbot CH. The treatment of autonomous functioning thyroid nodules. Br J Surg. 1982;69:577–579.
- Mariotti S, Martino E, Francesconi M, et al. Serum thyroid autoantibodies as a risk factor for development of hypothyroidism after radioactive iodine therapy for single thyroid 'hot' nodule. Acta Endocrinol (Copenh). 1986;113:500–507.
- Bolusani H, Okosieme OE, Velagapudi M, et al. Determinants of long-term outcome after radioiodine therapy for solitary autonomous thyroid nodules. Endocr Pract. 2008;14:543–549.
- Chianelli M, Bizzarri G, Todino V, et al. Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab. 2014;99:E1283–E1286.
- Watt T, Groenvold M, Rasmussen AK, et al. Quality of life in patients with benign thyroid disorders. A review. Eur J Endocrinol. 2006;154:501–510.
- Watt T, Cramon P, Hegedüs L, et al. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J Clin Endocrinol Metab. 2014;99:3708–3717.
- Cramon P, Winther KH, Watt T, et al. Quality-of-life impairments persist six months after treatment of graves' hyperthyroidism and toxic nodular goiter: a prospective cohort study. Thyroid. 2016;26:1010–1018.
- Shin YW, Choi YM, Kim HS, et al. Diminished quality of life and increased brain functional connectivity in patients with hypothyroidism after total thyroidectomy. Thyroid. 2016;26:641–649.
- Pacella CM, Bizzarri G, Guglielmi R, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology. 2000;217:673–677.
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules: results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99:3653–3659.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Døssing H, Bennedbaek FN, Bonnema SJ, et al. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol. 2007;157:95–100.
- Bernardi S, Stacul F, Michelli A, et al. 12-Month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine. 2017;57:402–408.
- Cesareo R, Naciu AM, Iozzino M, et al. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. Int J Hyperthermia. 2018;34:617–623.
- Gambelunghe G, Stefanetti E, Colella R, et al. A single session of laser ablation for toxic thyroid nodules: three-year follow-up results. Int J Hyperthermia. 2018;34:631–635.
- Amabile G, Rotondi M, Pirali B, et al. Interstitial laser photocoagulation for benign thyroid nodules: time to treat large nodules. Lasers Surg Med. 2011;43:797–803.
- Valcavi R, Tsamatropoulos P. Health-related quality of life after percutaneous radiofrequency ablation of cold, solid, benign nodules: a 2-year follow-up study in 40 patients. Endocr Pract. 2015;21:887–896.
- Mishra A, Sabaretnam M, Chand G, et al. Quality of life (QoL) in patients with benign thyroid goiters (pre- and post-thyroidectomy): a prospective study. World J Surg. 2013;37:2322–2329.
- Cramon P, Bonnema SJ, Bjorner JB, et al. Quality of life in patients with benign nontoxic goiter: impact of disease and treatment response, and comparison with the general population. Thyroid. 2015;25:284–291.
- Yue WW, Wang SR, Li XL, et al. Quality of life and cost-effectiveness of radiofrequency ablation versus open surgery for benign thyroid nodules: a retrospective cohort study. Sci Rep. 2016;6:37838.
- Gulseren S, Gulseren L, Hekimsoy Z, et al. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch Med Res. 2006;37:133–139.
- Abraham-Nordling M, Törring O, Hamberger B, et al. Graves' disease: a long-term quality-of-life follow up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery. Thyroid. 2005;15:1279–1286.
- Bernardi S, Dobrinja C, Carere A, et al. Patient satisfaction after thyroid RFA versus surgery for benign thyroid nodules: a telephone survey. Int J Hyperthermia. 2018 [Aug 15]:[1–9]. doi:10.1080/02656736.2018.1487590
- Pacella CM, Mauri G. Is there a role for minimally invasive thermal ablations in the treatment of autonomously functioning thyroid nodules? Int J Hyperthermia. 2018;34:636–638.