Abstract
Objective: The aim of this study was to introduce a management strategy for nerve damage occurring during radiofrequency ablation (RFA).
Methods: From January 2016 to October 2017, 17 patients who experienced the symptoms of nerve damage during RFA were enrolled in this study. If damage to nerves was suspected during RFA, ablation was stopped immediately, and a cold solution of 5% dextrose was injected directly into the space where the nerves were located until symptoms improved. Patients were followed up after the procedure until symptoms had resolved. The clinical data of patients who received a cold dextrose solution injection for nerve damage were compared with those who did not receive such an injection.
Results: Of 17 patients who experienced nerve damage, 12 received an injection of cold dextrose solution shortly after the emergence of symptoms. While resolution of symptoms was seen in all 17 patients, the mean time to recovery was significantly faster in the 12 patients who received treatment with an injection of cold dextrose solution than in those patients who did not receive such a treatment (p value = .041).
Conclusions: In the event of thermal damage to adjacent nerve structures during RFA, the direct injection of a cold dextrose solution is a simple and effective treatment that can result in rapid symptom resolution.
Introduction
Radiofrequency ablation (RFA) is a promising treatment modality for benign thyroid nodules [Citation1–8] and recurrent thyroid cancers [Citation9–15]. In benign thyroid nodules, RFA effectively ameliorates the symptoms and cosmetic problems by reducing the thyroid nodule volume; reported reduction rates are 33–58% at 1 month and 51–92% at 6 months [Citation1–8]. According to a recent meta-analysis, the therapeutic success rate of RFA for recurrent thyroid cancers is 100%, with a serum thyroglobulin reduction of 71.6% [Citation16]. By applying the new moving shot technique instead of a fixed technique and using a small-sized electrode tip (0.5–1 cm), RFA can be safely applied to small organs such as the thyroid gland, while avoiding damage to surrounding critical structures [Citation3,Citation7,Citation17,Citation18].
RFA is associated with several complications, including voice change, Horner syndrome, drooping of the shoulder, nodule rupture, skin burns, hematoma formation, and transient hyperthyroidism [Citation1,Citation12,Citation14,Citation15,Citation19–24]. Among the various possible complications, damage to adjacent nerves is the most serious [Citation22–24]. Voice change is caused by thermal damage to the recurrent laryngeal nerve, or infrequently to the vagus nerve. Therefore, the incidence of voice change after RFA is higher in patients with recurrent thyroid cancers because of the absence of a safety margin around the recurrent tumor [Citation23]. Horner syndrome and drooping of the shoulder are caused by thermal injury to the cervical sympathetic ganglion and spinal accessary nerve, respectively [Citation24]. Despite it being the most common and serious major complication of RFA [Citation22–24], no studies have reported a strategy for the management of nerve damage. Therefore, the aim of this study was to introduce an effective approach to the management of nerve damage occurring during RFA.
Materials and methods
The institutional review boards of our hospital approved this retrospective case control study. Informed consent for RFA was obtained from all patients prior to ablation. From January 2016 to October 2017, 362 thyroid RFAs were performed on 282 patients. Among these patients, 17 experienced the symptoms of nerve damage during RFA and were enrolled in this study.
RFA procedure
Prior to RFA, all nodules were pathologically confirmed as benign or malignant by fine-needle aspiration or core needle biopsy [Citation24–26]. Ultrasound-guided RFA was performed by three faculty radiologists (J.H.B., J.H.L., and Y.J.C., with 24, 20, and 9 years of experience in thyroid ultrasound, respectively). All ablations were performed with an RF generator (VIVA RF generator, STARmed, Goyang, Korea, and M-2004, RF Medical, Seoul, Korea) and 18-gauge thyroid-dedicated straight-type internally cooled electrodes with a 0.4, 0.5, 0.7, or 1 cm active tip (VIVA, STARmed, and RFT-0710, RF Medical), depending on the size of the nodule. For local anesthesia, the patients were injected with 2% lidocaine at the skin puncture site and in the perithyroidal soft tissue [Citation27]. The trans-isthmic approach with a moving shot technique was used under ultrasound guidance [Citation3,Citation7,Citation28]. To prevent complications during the procedure, careful observation of the adjacent structures was made, including nerves and blood vessels [Citation29]. A hydrodissection technique was applied if the nodule was located in close proximity to critical structures [Citation27]. Continuous injection of fluid and constant monitoring of the relationship between the nerves and tumor was required, as injected fluid gradually disappeared along the muscle plane. If a patient complained of pain during the ablation procedure, the RF power was reduced or turned off for several seconds. The procedure was terminated when the entire visualized area of the nodule became a transient hyperechoic zone. Complications occurring during and after the procedure were evaluated by monitoring for the appropriate clinical signs and symptoms. The patients were discharged after 30 min of observation in the hospital.
Management of nerve damage during RFA
If the symptoms of nerve damage (such as voice change, palpitations, Horner syndrome, shoulder movement problems, or paresthesia) occurred during RFA, ablation was immediately stopped. Under the presumption that the nerve damage was caused by thermal damage to the adjacent nerve structures, a cold solution was injected directly into the space in which the nerves were located. A 5% dextrose solution at 0° was used, as normal saline is an anionic fluid and is therefore able to conduct electricity [Citation30]. As the recurrent laryngeal nerve cannot be clearly seen on ultrasound, voice change was treated by injecting cold dextrose solution into the tracheoesophageal groove (the anticipated location of the recurrent laryngeal nerve) using a 22 G spinal needle connected to a 10 ml syringe. Injection of the solution continued until symptoms resolved ( and ). Patients were followed up by telephone on the day after the procedure; if the patient’s symptoms had not improved at this point, follow-up calls were repeated regularly until the symptoms had resolved.
Figure 1. A 25-year-old woman presented with a recurrent tumor in the left neck operation bed close to the esophagus and tracheoesophageal groove (a). Before RFA, a hydrodissection technique was applied to obtain a safety margin from the esophagus and recurrent laryngeal nerve (b). An RF electrode with a 0.4 cm size active tip was inserted into the recurrent tumor and the ablation was started (c). During ablation, the patient complained that her voice had changed. Ablation was stopped immediately and a cold dextrose solution was injected directly around the ablated tumor (d). After injecting 15 ml of cold dextrose solution, the patient’s voice completely improved (e).
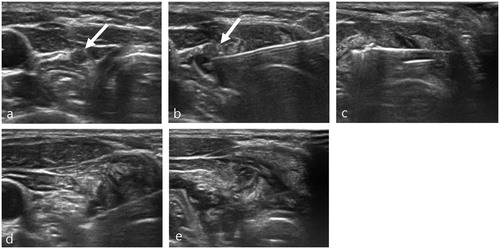
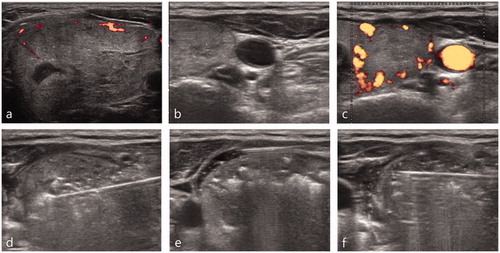
Figure 2. A 55-year-old woman who presented with a large nodule in the left thyroid gland (a). Pre-ablation US evaluation revealed a medial type middle cervical sympathetic ganglion located close to the thyroid nodule (b,c). An RF electrode with a 1 cm size active tip was used for ablation (d). During ablation, ipsilateral conjunctival injection was detected, which is one of the early symptoms of Horner syndrome. Ablation was stopped immediately and a cold dextrose solution was injected directly into the perithyroidal area (e). After injection of 7 ml of cold dextrose solution, the patient’s symptoms had completely improved (e).
Statistical analysis
The clinical data and recovery times after nerve damage were compared between those patients who received a cold dextrose solution injection for nerve damage and those who did not receive such an injection. Continuous variables are presented as mean ± standard deviation and are compared between the two groups using independent t-tests. The statistical significance of categorical variables was investigated using chi-square statistics. All statistical analyses were performed using commercially available software (PASW Statistics, version 17; SPSS, Inc., Chicago, IL). A p values<.05 was considered statistically significant.
Results
The 17 patients who experienced symptoms of nerve damage during RFA included 14 with voice change, one with both palpitation and voice change, one with Horner syndrome, and one with paresthesia in the peri-auricular area. These symptoms were caused by thermal damage to the recurrent laryngeal nerve, vagus nerve, middle cervical sympathetic ganglion, or greater auricular nerve, respectively (). The mean age of the 17 patients was 45.8 years (range, 15–90 years), with there being 15 women and two men. Six patients underwent RFA for a benign thyroid nodule, ten for recurrent papillary thyroid cancer, and one for a parathyroid lesion. All of the recurrent tumors were located within the thyroid bed, with the exception of two cases located in the right neck at levels 2 and 4. The mean recurrent tumor size was 0.7 cm (range, 0.4–2.2 cm), and the mean size of the benign nodules was 3.5 cm (range, 2.3–5.2 cm). All patients with recurrent tumors were treated using an RF electrode with a 0.4 or 0.5 cm active tip at a maximum power of 5–10 W. Symptoms of nerve damage occurred during RFA in 11 patients, while in the other six patients the symptoms emerged after the procedure had been completed, with the time interval ranging from just after RFA to 1 h post-RFA. compares the clinical data between patients who received a cold dextrose solution injection for nerve damage and those who did not receive such an injection. The mean age of patients, the use of a hydrodissection technique, and the recovery time from nerve damage were significantly different between the two groups. Although all 17 patients recovered from their symptoms, the mean time to recovery was significantly shorter in the 12 patients who received an injection of cold dextrose solution, with 10 of these patients making an almost immediate recovery during the injection. The voice of one of the other two treated patients improved just after the injection of the cold dextrose solution, but a voice change recurred when the patient returned home. After 2 months, the patient’s voice had completely recovered. The remaining patient showed an improvement in their voice during the injection, and complete recovery was achieved within 2 days.
Table 1. Summary of the basic characteristics and clinical manifestations of the 17 patients.
Table 2. Comparison of clinical data between patients who received a cold dextrose solution injection for nerve damage and those who did not.
Discussion
In this study, we introduce a management strategy that can be followed in the event of thermal damage to adjacent nerves during RFA. To our knowledge, this is the first study to describe a treatment approach for this clinical situation. When nerve symptoms develop, we recommend the immediate injection of a cold dextrose solution directly into the space in which the nerves are located. Of the 17 patients in this study who experienced symptoms of nerve damage during RFA, this approach was undertaken in 12. In these patients, the symptoms improved significantly faster than in those patients who did not receive a cold dextrose injection. Thus, in the event of thermal damage to adjacent nerve structures during RFA, direct injection of a cold dextrose solution is a simple, safe, and effective treatment that can result in rapid symptom resolution.
Nerve damage is the most common and serious major complication of RFA [Citation22–24]. The recurrent laryngeal and vagus nerves are most commonly affected, with damage to the brachial plexus (n = 1), cervical sympathetic ganglion (n = 1), and spinal accessory nerve (n = 3) having also been reported [Citation22–24,Citation31]. Nerve injury during RFA is thought to be primarily caused by thermal damage. In this study, all cases of nerve damage during the treatment of benign thyroid nodules were attributed to recurrent laryngeal nerve injury. The nerve damage that occurred during the treatment of recurrent tumors varied, depending on the location of the lesion, with damage occurring to the nerve adjacent to the tumor in each case. For benign thyroid nodules, under-treatment of the danger triangle using a moving shot technique and trans-isthmic approach can minimize recurrent laryngeal nerve injury [Citation27]. By contrast, because recurrent tumors are usually small and there is no safety margin of normal thyroid parenchyma around the lesion, the incidence of nerve damage is significantly higher than that seen in benign thyroid nodules [Citation23]. To minimize nerve damage during ablation of a recurrent lesion, the use of a small active tip (0.4 cm) and the maintenance of a safety margin through the use of a continuous hydrodissection technique is recommended [Citation23,Citation27]. However, it is inevitable that injury will sometimes occur when the course of the recurrent laryngeal nerve is subject to anatomical change after surgery [Citation32], and when hydrodissection cannot be used to dissect the nerve from the recurrent tumor because of the presence of adhesions [Citation33]. It is, therefore, important to have an appropriate management strategy in place, so that nerve damage sequelae can be minimized.
The five patients who did not receive an injection of cold dextrose solution underwent RFA for recurrent cancer; thus the patients’ ages were higher and the hydrodissection technique was more frequently used than in the patients who received a cold dextrose solution. Because a hydrodissection technique was used, we considered the safety margin from adjacent critical structures to be sufficient to prevent damage, and that the symptoms were caused by the injected lidocaine, not by thermal damage. However, considering the fact that the recovery time of the symptoms in those patients was significantly slower, an injection of cold dextrose solution is recommended, regardless of the use of a hydrodissection technique. If the symptoms of nerve damage occur during RFA, the ablation should be stopped immediately, and cold water should be directly injected into the space in which the nerves are located. In cases of suspected recurrent laryngeal nerve damage during the treatment of benign nodules, we recommend injection of cold dextrose solution directly into the tracheoesophageal groove. The injected fluid then spreads into the posterior aspect of the perithyroidal area where the nerve is expected to pass. A cold 5% dextrose solution is recommended for the injection solution, as normal saline is an anionic fluid and is therefore able to conduct electricity [Citation27,Citation30]. As a result of its iso-osmolarity (252 mOsmol/L) and nonionic composition, 5% dextrose does not conduct electricity, and therefore potentially provides a thermal barrier when it surrounds the target organ [Citation27,Citation30]. Furthermore, we recommend injecting an additional 10–20 ml of fluid, even if the symptoms immediately resolve, because the injected fluid spreads to the surrounding structures, rather than remaining at the injection site [Citation27]. We also suggest avoiding the exertion of any pressure on the ablation area after the procedure, and to press only lightly at the needle insertion area. This is recommended because when the ablation area is compressed, the heat remaining inside the ablated lesion is transmitted to the surrounding structures and may cause additional thermal damage. In addition, an oral corticosteroid can be administered for approximately 1 month; some surgeons advise the use of steroids during thyroid surgery to reduce postoperative neural edema and promote recovery of nerve function in cases of nerve paralysis [Citation34,Citation35]. In this way, rapid symptom improvement can be expected when RFA-induced nerve damage occurs. Furthermore, these treatment approaches can be adopted for other thermal ablation techniques such as laser ablation or microwave ablation. Voice change is the most common major complication after laser ablation, with it being reported in 0.5% of cases, and having a recovery time varying from 2 to 84 days [Citation36–39]. Considering that nerve damage after laser ablation or microwave ablation is also caused by thermal damage to adjacent nerve structures, injection of a cold dextrose solution may be effective in reducing the recovery time.
The present study is subject to a number of limitations. First, because there is no quantitative and objective method of grading voice change, the degree of improvement was determined according to the patient interview. As patients are sensitive to changes in their voice, this was considered to be a suitable approach for assessing abnormalities and improvement. Secondly, as nerve damage is an uncommon complication during RFA, the number of patients enrolled in this study was small. Finally, a standard protocol for the treatment of nerve damage has not yet been established, and a larger prospective study evaluating further applications of our proposed protocol is required.
In conclusion, we suggest a management strategy that can be adopted in the event of thermal damage to adjacent nerve structures during RFA. In the event of thermal damage to adjacent nerve structures during RFA, direct injection of a cold dextrose solution is a simple and effective treatment that can result in rapid symptom resolution.
Disclosure statement
The authors declare that they have no relevant conflicts of interest to disclose.
References
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100:460–466.
- Faggiano A, Ramundo V, Assanti AP, et al. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab. 2012;97:4439–4445.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18:1244–1250.
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009;19:219–225.
- Ugurlu MU, Uprak K, Akpinar IN, et al. Radiofrequency ablation of benign symptomatic thyroid nodules: prospective safety and efficacy study. World J Surg. 2015;39:961–968.
- Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34:784–791.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194:1137–1142.
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16:361–367.
- Baek JH, Kim YS, Sung JY, et al. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011;197:W331–W336.
- Park KW, Shin JH, Han BK, et al. Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol. 2011;18:2564–2568.
- Guenette JP, Monchik JM, Dupuy DE. Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J Vasc Interv Radiol. 2013;24:672–679.
- Lee SJ, Jung SL, Kim BS, et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol. 2014;15:817–826.
- Wang L, Ge M, Xu D, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Cancer Res Ther. 2014;10 Suppl:C144–C149. Suppl:
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276:909–918.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25:163–170.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26:420–428.
- Ha EJ, Baek JH, Lee JH. The efficacy and complications of radiofrequency ablation of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. 2011;18:310–314.
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36:1321–1325.
- Aysan E, Idiz UO, Akbulut H, et al. Single-session radiofrequency ablation on benign thyroid nodules: a prospective single center study : Radiofrequency ablation on thyroid. Langenbecks Arch Surg. 2016;401:357–363.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262:335–342.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33:920–930.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27:3128–3137.
- Na DG, Baek JH, Jung SL, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017;18:217–237.
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016;17:370–395.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18:615–623.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–125.
- Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015;16:749–766.
- Laeseke PF, Sampson LA, Brace CL, et al. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR Am J Roentgenol. 2006;186:S249–S254.
- Wang J-F, Wu T, Hu K-P, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J. 2017;130:1361–1370.
- Chiang FY, Lu IC, Chen HC, et al. Anatomical variations of recurrent laryngeal nerve during thyroid surgery: how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci. 2010;26:575–583.
- Jiang Y, Gao B, Zhang X, et al. Prevention and treatment of recurrent laryngeal nerve injury in thyroid surgery. Int J Clin Exp Med. 2014;7:101–107.
- Wang LF, Lee KW, Kuo WR, et al. The efficacy of intraoperative corticosteroids in recurrent laryngeal nerve palsy after thyroid surgery. World J Surg. 2006;30:299–303.
- Emre A, Karadeniz Cakmak G, Karakaya Arpaci D, et al. The efficacy of intraoperative single dose methylprednisolone on recurrent laryngeal nerve function after thyroidectomy. Int Surg. 2016;101:116–120.
- Mainini AP, Monaco C, Pescatori LC, et al. Comment to article: [corrected] image-guided thermal ablation of benign thyroid nodules . J Ultrasound. 2017;20:11–22.
- Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100:3903–3910.
- Pacella CM, Mauri G, Cesareo R, et al. A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperthermia. 2017;33:911–919.
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia. [cited 2016 Nov 15]; [5 p.]. DOI:10.1080/02656736.2016.1244707
