?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: To determine the safety and efficacy of cryoablation combined with sorafenib for the treatment of advanced renal cell carcinoma.
Material and methods: We conducted an observational study in 156 patients with advanced renal cell carcinoma unsuitable for surgical treatment. Participants received cryoablation + sorafenib (n = 67) or sorafenib only (n = 89). Objective response rate (ORR), disease control rate (DCR), progression-free survival time (PFS), overall survival (OS), change in immune function after treatment, rate of adverse events, and quality of life were compared between the two groups.
Results: In the cryoablation + sorafenib group, ORR and DCR were significantly higher and PFS and OS were significantly longer than in the sorafenib only group (both p < .05). Immune function-related indicators were significantly improved after treatment in the cryoablation + sorafenib group (p < .05), but no significant difference was found between before and after treatment in the sorafenib only group (p > .05). The incidence of targeted drug-related side effects was not significantly different between the groups (p > .05), and cryoablation did not increase the risk of side effects of targeted drugs.
Conclusion: Cryoablation combined with sorafenib had superior clinical efficacy compared with sorafenib-only for the treatment of advanced renal cell carcinoma unsuitable for surgical treatment. Moreover, this combined therapy may enhance the body’s anti-tumor immunity and effectively prolong PFS and OS without compromising patient quality of life, thus representing a new treatment strategy for advanced renal cell carcinoma.
Introduction
Kidney cancer is one of the most common malignancies affecting the urinary system and its incidence accounts for 2–3% of adult malignant tumors, ranking second in incidence after only bladder cancer [Citation1,Citation2]. The development of kidney cancer is relatively insidious. Early stage disease frequently presents no specific symptoms, and given the lack of an effective screening method for early-stage tumors, tumors have metastasized in 20–30% of new cases at the time of diagnosis [Citation3,Citation4]. Patients with advanced renal cell carcinoma (RCC) who are unable to undergo surgical resection have a poor prognosis, with a 2-year survival rate of only 10–20% and median survival rate of 6–12 months [Citation5]. Sorafenib is an oral multi-kinase inhibitor that targets tumor proliferation and angiogenesis and promotes apoptosis by inhibiting multiple intracellular kinases (CRAF, BRAF, and mutant BRAF) and cell surface kinases (KIT, FLT-3, RET, VEGFR-2, VEGFR-3, and PDGFR-B). The efficacy of sorafenib in RCC has been previously confirmed in phase II and phase III trials, leading to its approval by the US Food and Drug Administration in December 2005 as the first targeted agent to show clinical activity against RCC [Citation6]. Previous studies, including the pivotal TARGET trial, have shown promising evidence for sorafenib administered at 400 mg twice daily as both a first-line and second-line therapy for advanced RCC, primarily in Western populations [Citation7,Citation8]. These studies have shown various degrees of improvement in progression-free survival (PFS), overall response rate, overall survival (OS), tolerance, and quality of life compared with other investigational agents including interferon, IFNα2a, tivozanib, temsirolimus, AMG 386, and axitinib [Citation5,Citation9–13]. However, as for other molecular targeted drugs, sorafenib resistance inevitably develops and leads to tumor progression [Citation6]. Cryoablation has achieved similar efficacy to surgical resection in the treatment of early-stage RCC, and has been recommended as an alternative treatment for this stage of the disease [Citation14,Citation15]. Some researchers have explored the use of cryoablation for the treatment of advanced RCC and found that it can effectively control disease progression and prolong patient survival [Citation16,Citation17]. Cryoablation combined with sorafenib has also been shown to improve clinical efficacy in the treatment of liver cancer. However, little has been reported to date on cryoablation combined with sorafenib for the treatment of advanced RCC. The purpose of this study was to investigate the safety and efficacy of cryoablation combined with sorafenib for the treatment of advanced RCC by investigating whether the combined therapy could reduce tumor load, increase the disease control rate, prolong the effective duration of molecular targeted drugs, and prolong the survival period, with an acceptable safety profile, thus improving the prognosis of patients with this disease.
Material and methods
Patients
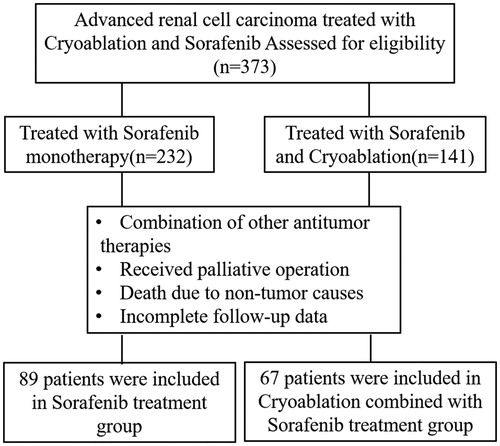
This study was approved by the Medical Ethics Committee of the Cancer Hospital of Tianjin Medical University, China. All included patients provided signed informed consent prior to participation in the study. Clinical data of 373 patients with advanced RCC received treatment at the Cancer Hospital of Tianjin Medical University, China between August 2010 and August 2017 were collected. The available treatment options were cryoablation combined with sorafenib and sorafenib only. The attending physician explained the potential benefits and risks of the treatment options, and the choice of treatment method was ultimately made by patients or their authorized representatives. According to the treatment protocol, these patients were divided into cryoablation + sorafenib and sorafenib only groups. Patients who met all of the following criteria were eligible for inclusion: (1) advanced RCC according to WHO classification; (2) no previous history of surgery, radiotherapy, chemotherapy, or biotherapy; (3) at least one evaluable primary lesion (diameter greater than or equal to 1 cm); (4) no severe basic diseases such as heart failure, renal failure, respiratory failure, and severe coagulation dysfunction; (5) Eastern Cooperative Oncology Group (ECOG) score less than or equal to 2 points; (6) no history of infectious disease; (7) expected survival greater than or equal to 3 months; (8) complete follow-up data, including imaging and hematology data; (9) treatment with oral sorafenib alone or in combination with cryoablation. Patients presenting with any of the following were excluded from the study: (1) death attributable to non-tumor causes during the treatment period; (2) combination with other therapies during the treatment period; (3) lost to follow up.
Treatments
Patients were treated with oral sorafenib using a continuous dosing regimen starting at 400 mg twice daily, at 12-h intervals, until disease progression or the appearance of unacceptable toxicity, in which case the dose was decreased to 400 mg once daily. Unacceptable toxicity included grade 3 or 4 hematologic toxicity, dermal toxicity, hypertension, and/or liver dysfunction, as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0.
Cryoablation was performed using the Cryocare Operative System (Endocare, Austin, TX, USA). After local anesthesia, a cryoprobe was inserted into the freezing target area along a predetermined route under computed tomography (CT) guidance. In accordance with 2008 cryoablation treatment guidelines of the American Urological Association, argon working pressure was maintained at 17 225 kPa (2500 PSI) and the output power was 100%, resulting in the maintenance of a freezing temperature of −140 °C to −160 °C for a period of 15–20 min, followed by a 5-min thawing period where the tissue temperature was restored to 38 °C. Each procedure was repeated twice, and a no-contrast CT examination was performed during the procedure at 3–5 min intervals to visualize the formation of an ice ball and changes in the surrounding tissues. Depending on the size and location of the primary tumor, the required number of cryoprobes (n = 3–6) was determined. Where the tumor diameter was less than 4 cm with no adjacent important organs, freezing was conducted until an ice ball extended 1 cm beyond the boundary of the tumor. Where a tumor was too large to be completely ablated, one further cryoablation treatment was performed 1–2 weeks later. Oral sorafenib treatment was initiated 1 week after the cryoablation procedure and continued until tumor progression or patient death.
Follow-up
For patients in the sorafenib only group, one treatment cycle was defined as 4–6 weeks from the start of oral administration of sorafenib; for patients in the cryoablation + sorafenib group, one treatment cycle was defined as 4–6 weeks from the start of oral administration of sorafenib initiated 1 week after cryoablation. During the treatment period, all patients were followed up once every month, and once every 3 months after treatment discontinuation to identify unacceptable toxicity. Assessment items include complete medical history, physical examination, routine laboratory tests (complete blood count, serum electrolytes, liver and kidney function tests, and immunological indicators), and imaging examination (enhanced CT or enhanced magnetic resonance imaging [MRI]).
Efficacy and safety assessments
The primary endpoint in this study was objective response rate (ORR) from the start of oral sorafenib treatment to death or to the last follow up. The secondary endpoint was progression-free survival (PFS) from the start of oral sorafenib treatment to the first record of disease progression or to death from any cause. Safety was assessed as the rate of adverse events from the start of oral sorafenib treatment to the last follow up. After treatment, mRECIST criteria were used to evaluate efficacy, which was rated as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Overall response rate (ORR) was defined as the sum of the number of patients with CR and the number of patients with PR) divided by the total number of patients. The disease control rate (DCR) was defined as the sum of the number of patients with CR, the number of patients with PR, and the number of patients with SD divided by the total number of patients. Adverse drug reaction was rated as grade 0–IV according to the National Cancer Institute Common Toxicity Criteria (NCI CTC). Patient quality of life was evaluated using the Eastern Cooperative Oncology Group (ECOG) performance status.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA) and Prism 7 (GraphPad Software, San Diego, CA, USA). Life tables and Kaplan–Meier survival curves were used to estimate overall survival (OS) and PFS. The mean of all patient data was used as the cutoff value for analysis of the clinical impact of treatment. Measurement and numerical data were compared using t-tests and chi-square tests, respectively. The Cox proportional hazards model was used to evaluate the prognostic value of the investigated parameters. All p values were two-sided and were considered significant if <.05. The concordance index and the proportion of variance explained (R2) were calculated to assess the prediction performance for OS.
Results
Patient baseline data
A total of 373 patients with advanced RCC received treatment at the Cancer Hospital of Tianjin Medical University, China between August 2010 and August 2017. Of these, 156 patients who fulfilled the inclusion and exclusion criteria were enrolled in the study () in either a cryoablation + sorafenib (n = 67) or sorafenib only (n = 89) group. These 156 eligible patients, consisting of 87 males and 69 females, were 48–84 years old, with a median of 65 years old. A total of 184 tumors were recorded, with diameters ranging from 3.8–14.0 cm (average 7.27 ± 2.12 cm), and were classified pathologically as clear cell carcinoma (n = 145; 92.95%), papillary carcinoma (n = 8; 5.13%), and chromophobe RCC (n = 3; 1.92%) ().
Table 1. Clinical data of 156 patients with advanced renal cell carcinoma.
According to the 7th edition of the American Joint Committee on Cancer tumor node metastases staging, all patients presented with stage III (n = 31) or IV tumors (n = 125). Pulmonary metastasis occurred in 45 (28.84%) patients, bone metastasis in 37 (23.72%), adrenal metastasis in 25 (16.03%), liver metastasis in 25 (16.03%), inferior vena cava tumor thrombus in 43 (27.56%), renal venous tumor thrombus in 54 (34.62%), and inferior vena cava tumor thrombus and renal venous tumor thrombus in 23 (14.74%) patients. Clinical symptoms at the time of presentation at the hospital were hematuria in 67 (42.95%) patients, abdominal mass in 43 (27.56%) patients, and pain in 39 (25.00%) patients. An ECOG score of 0 point was recorded in 88 patients, 1 point in 42 patients, and 2 points in 26 patients (). The expected survival time was greater than 3 months in all included patients. Advanced RCC was diagnosed in all patients by imaging and puncture pathology. There were no significant differences in sex, age, size of lesion, pathological type, tumor node metastases staging, and ECOG score between the cryoablation + sorafenib and sorafenib only groups (p < .05; ).
Efficacy
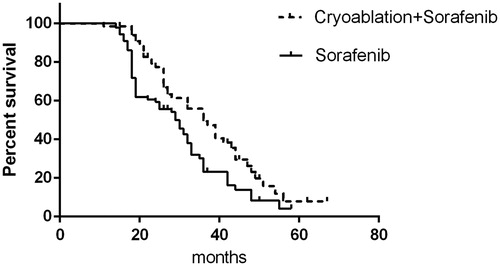
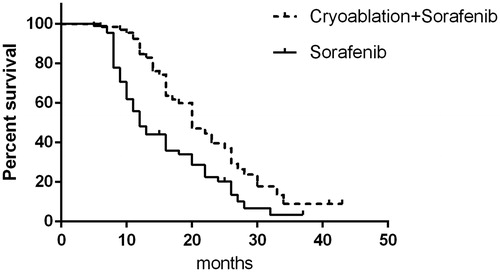
Follow-up data were complete for all patients, with a mean follow-up time of 28.8 months (range, 7–67 months). In the cryoablation + sorafenib group, PFS was 20 months and OS was 36 months, and the values were 12 and 29 months for PFS and OS, respectively, in the sorafenib only group. This finding indicates that cryoablation combined with sorafenib can more significantly prolong PFS and OS than sorafenib can (p < .05; and ).
Figure 2. Progression-free survival of patients in the cryoablation + sorafenib and sorafenib-only groups.
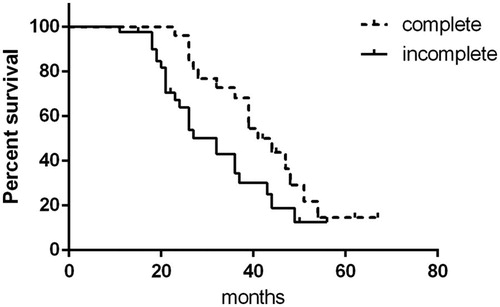
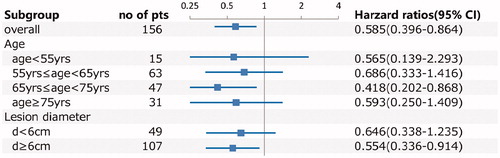
Stratification analysis showed that in the cryoablation + sorafenib group, 26 (38.81%) patients underwent complete cryoablation of the primary lesion, with a mean tumor diameter of 5.58 ± 0.86 cm, PFS of 23 months, and OS of 41 months; while 41 (61.19%) patients underwent incomplete cryoablation of the primary lesion, with mean tumor diameter of 8.74 ± 1.66 cm, PFS of 17 months, and OS of 32 months. Patients who underwent complete cryoablation had significantly longer PFS and OS than patients who underwent incomplete cryoablation (p < .05; and ). Typical cases of complete and incomplete cryoablation are shown in and . Prognostic factor analysis indicated that cryoablation combined with sorafenib had superior efficacy for OS in patients with lesion diameter ≥6 cm (HR: 0.554 [95%CI: 0.336–0.914]; p = .021) and in patients aged ≥65 years and <75 years (HR: 0.418 [95%CI: 0.202–0.868]; p = .019) (). Overall, these results indicate that cryoablation combined with sorafenib was effective for the treatment of advanced RCC.
Figure 4. Comparison of progression-free survival between complete cryoablation and incomplete cryoablation.
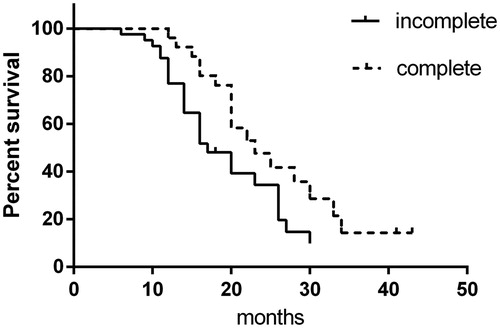
Figure 6. A 60-year-old man with a massive tumor on the right kidney accompanied by multiple (lung and bone) metastases who underwent cryoablation combined with sorafenib. (A, B) Preoperative CT scans show that the tumor was located in the subrenal pole and involved the renal hilum, with the largest tumor diameter of 6.8 cm. (C, D) CT scans show incomplete ablation of the renal hilum tumor during cryoablation. (E, F) CT scan at 1 month after cryoablation shows that most of the tumor body is completely necrotic and that there is residual tumor tissue in the renal hilum.
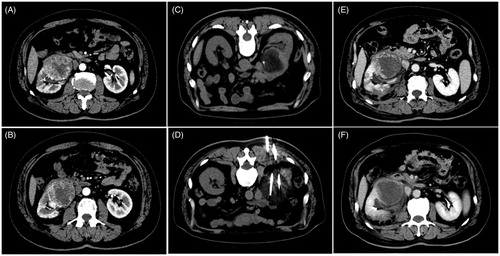
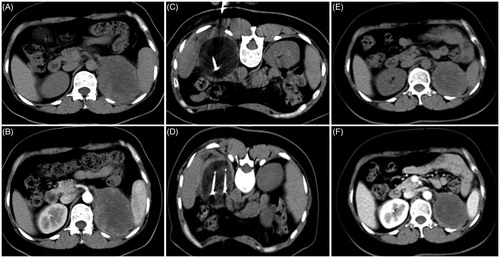
Figure 7. A 48-year-old woman with a tumor on the left kidney accompanied by bone metastasis who underwent cryoablation combined with sorafenib. (A, B) Preoperative CT scans show that the tumor was located in the lower pole of the left kidney, with the largest tumor diameter of 7 cm. (C, D) CT scans show complete ablation of the tumor during cryoablation. (E, F) CT scan at 1 month after cryoablation shows that the tumor is completely necrotic.
In the cryoablation + sorafenib group, CR was observed in 5 patients, PR in 43 patients, SD in 12 patients, and PD in 7 patients, with ORR of 71.64% (48/76) and DCR of 89.55 (60/67). In the sorafenib only group, CR was observed in 1 patient, PR in 16 patients, SD in 58 patients, and PD in 14 patients, with ORR of 19.10% (17/89) and DCR of 84.27% (75/89). ORR and DCR in the cryoablation + sorafenib group were significantly higher than in the sorafenib only group (both p < .05; ).
Table 2. Clinical efficacy evaluation.
Regarding immune function before and after treatment, the proportions of circulating CD8 + T and Treg cells were significantly decreased in the cryoablation + sorafenib group after 6–8 weeks of treatment compared with the proportions prior to treatment (p = .001, p = .032). In addition, the proportions of CD3 + T, CD4 + T, CD4 + T/CD8 + T, and NK cells were significantly increased after 6–8 weeks of treatment compared with the proportions prior to treatment (p = .03, p < .01, p < .01, and p = .041, respectively). There were no significant differences in immune function-related indicators before and after treatment in the sorafenib only group (p > .05; ).
Table 3. Immune-related indicators before and after treatment in each group.
Safety evaluation
Hepatic and renal function before and after treatment
There were no significant differences in liver and kidney function before and after treatment in each group (both p > .05; ).
Table 4. Hepatic and renal function-related indicators before and after treatment in each group (mean ± s).
Side effects of sorafenib
In the cryoablation + sorafenib group, hand–foot skin reactions occurred in 39 patients, nausea in 12 patients, hypertension in 22 patients, mild diarrhea in 26 patients, and oral mucositis in 15 patients. In the sorafenib only group, hand–foot skin reactions occurred in 55 patients, nausea in 19 patients, hypertension in 31 patients, mild diarrhea in 38 patients, and oral mucositis in 20 patients. Most patients reported Grade I or II adverse events, which were transient and resolved after symptomatic treatment. Few patients experienced severe adverse events, which were alleviated after dose adjustment or short-term interruption of sorafenib treatment. There was no significant difference in side effects between the cryoablation + sorafenib and sorafenib only groups (p < .05; ).
Table 5. Adverse events in cryoablation + sorafenib and sorafenib-only groups.
Cryoablation-related complications
No serious complications such as renal failure, tumor rupture, cold shock, and skin frostbite were reported after cryoablation. However, 35 patients reported lumbago and back pain, 13 patients had low-grade fever, and 7 patients had transient hematuria. These symptoms resolved after symptomatic treatment.
Quality of life scoring
In the cryoablation + sorafenib group, 33, 21, and 13 patients had an ECOG score before treatment of 0, 1, and 2 points, respectively; after treatment, 30, 28, and 9 patients had an ECOG score of 0, 1, and 2 points, respectively. In the sorafenib only group, 55, 21, and 13 patients had an ECOG score before treatment, of 0, 1, and 2 points, respectively; after treatment, 52, 30, and 7 patients had an ECOG score of 0, 1 and 2 points, respectively. In each group, the ECOG score after treatment changed slightly compared with the score before treatment in each group, although the change was not statistically significant (p > .05).
Discussion
Sorafenib, as an oral multi-target anti-tumor drug, has achieved remarkable efficacy in the treatment of advanced RCC, effectively prolonging PFS and OS, and has become the recommended first-line treatment for advanced RCC [Citation7,Citation18–20]. Strong evidence exists that sorafenib treatment for advanced results in longer RCC, PFS, and OS in Asian populations than in European and US populations, indicating that sorafenib is particularity advantageous in the treatment of advanced RCC in Asian patients [Citation6,Citation21,Citation22]. Similar to other targeted drugs for tumor treatment, however, patients develop resistance to sorafenib, leading to disease progression and death. Whether sorafenib combined with other therapies can increase DCR, delay sorafenib resistance, and prolong PFS and OS in patients with advanced RCC is therefore worthy of further investigation.
In recent years, with the widespread application of cryoablation in the field of cancer therapy, multiple studies have shown that cryoablation has demonstrable efficacy with mild side effects in the treatment of various malignant tumors including early-stage liver cancer, kidney cancer, and prostate cancer; can achieve the same efficacy as surgical resection in the treatment of early-stage liver cancer, kidney cancer, and prostate cancer; and can effectively reduce tumor load, improve quality of life, and prolong survival [Citation16,Citation23–28]. Previous studies have demonstrated that, compared with sorafenib-only treatment, cryoablation combined with sorafenib can strengthen the clinical effect of cryotherapy. The first trial of hepatocellular carcinoma (HCC) showed that median TTP in the cryoablation + sorafenib group was longer than that in the sorafenib-only group [9.5 (8.4–13.5) months vs. 5.3 (3.8–6.9) months, p = .02) .Median OS in the cryoablation + sorafenib group was longer than that in the sorafenib-only group [12.5 (95% CI: 10.6–16.4) months vs. 8.6 (7.3–10.4) months, p = .01][Citation29]. Another clinical trial in HCC demonstrated that cryoablation combined with sorafenib prolonged TTP and OS to 6.6 months and 12.2 months, respectively [Citation30]. Moreover, a previous study showed that sorafenib in combination with local therapy (transarterial chemoembolization with/without cryoablation) was independently associated with longer OS and PFS in patients with advanced HCC [Citation31]. However, there are not any prior publications on the topic of renal cell cancer. The present study was therefore conducted to investigate the safety and efficacy of cryoablation combined with sorafenib for the treatment of advanced RCC, and to investigate whether the combined therapy could increase DCR, delay drug resistance, and prolong PFS and OS to a greater extent than sorafenib alone.
Our results demonstrated that ORR (CR, PR) and DCR (CR, PR, and SD) in the cryoablation + sorafenib group were 71.64% and 89.55%, respectively, and were 19.55% and 84.27%, respectively, in the sorafenib-only group. DCR and ORR were significantly higher in the cryoablation + sorafenib group than in the sorafenib-only group (p < .05). The combined therapy had a clear advantage in terms of efficacy, and possible reasons for this advantage were as follows: (1) anti-tumor efficacy was evaluated based on mRECIST criteria, which uses imaging techniques to define tumor diameter; and (2) cryoablation leads directly to coagulative necrosis of tumor cells. The combined therapy exhibited higher ORR and DCR than sorafenib alone. To further evaluate the efficacy of the combined therapy, we compared PFS and OS between the cryoablation + sorafenib and sorafenib-only groups and found significant differences in PFS and OS between these two groups (PFS: 20 months vs. 12 months; OS: 36 months vs. 29 months). This finding suggests that the combined therapy has better response, improves prognosis, and prolongs survival time compared with sorafenib alone. Possible reasons for this effect are that (1) cryoablation can effectively reduce tumor load within a short time, increase ORR and DCR, and effectively control tumor progression in patients with advanced RCC; (2) cryoablation results in the necrosis of a large number of tumor cells in the target area and may increase the permeability of tumors and surrounding tissues, thus increasing the sensitivity of residual tumor cells to targeted drugs. Hyperthermia has been shown to enhance drug delivery [Citation32], so the changes induced by cryotherapy may improve the uptake of sorafenib, although this association requires further investigation. (3) The kidney is an immunogenic organ, and immunotherapy plays an important role in the treatment of renal cancer. We, therefore, speculate that cryoablation may influence the immunogenicity and drug resistance of residual tumor cells, thereby changing or prolonging the effective time of targeted drug therapy. In the current study, we also found that PFS and OS were significantly higher in patients undergoing complete cryoablation than in those undergoing incomplete cryoablation. Therefore, within the controllable range of side effects caused by cryoablation therapy, complete cryoablation is recommended for advanced RCC with relatively small tumor diameter, and further cryoablation treatments are recommended for tumors with large diameters to achieve complete ablation.
To further clarify whether cryoablation can improve immunological function in patients with advanced RCC, we analyzed serum indicators related to immune function of two groups of patients. In the cryoablation + sorafenib group, the proportion of Treg cells was significantly decreased and the proportions of NK, CD3 + T, CD4 + T, and CD4 + T/CD8 + T cells were significantly increased after treatment compared with before treatment. By contrast, in the sorafenib-only group, there were no significant differences in the proportions of T lymphocyte subsets and Treg cells before and after treatment. These findings suggest that cryoablation enhanced tumor immunity more than sorafenib alone.
In the cryoablation + sorafenib group, serious complications such as abdominal hemorrhage, tumor rupture, and renal failure after cryoablation were not observed, but adverse events such as hematuria, lumbar pain, and mild renal dysfunction were reported. However, most adverse events resolved after symptomatic treatment. Adverse events in the cryoablation + sorafenib group were relatively less severe than those in the sorafenib-only group. There were no significant differences in the incidence and severity of adverse events such as hand–foot skin reactions, elevated blood pressure, and gastrointestinal discomfort between the cryoablation + sorafenib and sorafenib-only groups, possibly because cryoablation treatment enhanced patient immunity, increasing the tolerance to drug side effects. There was no significant difference in quality of life before and after treatment in each group, and no significant difference in ECOG score after treatment was observed between the cryoablation + sorafenib and the sorafenib-only groups. These findings suggest that cryoablation combined with sorafenib can greatly increase efficacy without impairing patients’ quality of life.
Conclusion
Cryoablation combined with sorafenib for the treatment of advanced RCC that cannot be surgically resected can reduce tumor load, improve anti-tumor immunosuppressive status, effectively increase ORR and DCR, maintain quality of life, and prolong patient survival with an acceptable safety profile. As a potential new treatment strategy for advanced RCC, cryoablation combined with sorafenib, therefore, requires further investigation in future randomized controlled trials.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the Cancer Hospital of Tianjin Medical University, China. All included patients provided signed informed consent prior to participation in the study.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Qin C, Cao Q, Li P, et al. The influence of genetic variants of sorafenib on clinical outcomes and toxic effects in patients with advanced renal cell carcinoma. Sci Rep. 2016;6:20089.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490.
- Drucker BJ. Renal cell carcinoma: current status and future prospects. Cancer Treat Rev. 2005;31:536–545.
- Kim SH, Kim S, Nam BH, et al. Primary tumor characteristics are important prognostic factors for sorafenib-treated patients with metastatic renal cell carcinoma: a retrospective multicenter study. Biomed Res Int. 2017;2017:9215930.
- Ye DW, Zhang HL. Critical appraisal of sorafenib in the treatment of Chinese patients with renal cell carcinoma. Onco Targets Ther. 2014;7:925–935.
- Hutson TE. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist. 2011;16:14–22.
- Procopio G, Derosa L, Gernone A, et al. Sorafenib as first- or second-line therapy in patients with metastatic renal cell carcinoma in a community setting. Future Oncol. 2014;10:1741–1750.
- Sheng X, Chi Z, Cui C, et al. Efficacy and safety of sorafenib versus sunitinib as first-line treatment in patients with metastatic renal cell carcinoma: largest single-center retrospective analysis. Oncotarget. 2016;7:27044–27054.
- Mai H, Huang J, Zhang Y, et al. In-vivo relation between plasma concentration of sorafenib and its safety in Chinese patients with metastatic renal cell carcinoma: a single-center clinical study. Oncotarget. 2017;8:43458–43469.
- Molina AM, Motzer RJ. Clinical practice guidelines for the treatment of metastatic renal cell carcinoma: today and tomorrow. Oncologist. 2011;16:45–50.
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. JCO. 2009;27:3312–3318.
- Rini B, Szczylik C, Tannir NM, et al. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: a randomized, double-blind, placebo-controlled, phase 2 study. Cancer. 2012;118:6152–6161.
- Krokidis ME, Kitrou P, Spiliopoulos S, et al. Image-guided minimally invasive treatment for small renal cell carcinoma. Insights Imaging. 2018;9:385–390.
- Kapoor A, Wang Y, Dishan B, et al. Update on cryoablation for treatment of small renal mass: oncologic control, renal function preservation, and rate of complications. Curr Urol Rep. 2014;15:396.
- Aoun HD, Littrup PJ, Jaber M, et al. Percutaneous cryoablation of renal tumors: is it time for a new paradigm shift? J Vasc Interv Radiol. 2017;28:1363–1370.
- Lalloue F, Ruffion A, Valette PJ, et al. Cryotherapy percutaneous for renal tumors: our center's beginning experience. Prog Urol. 2016;26:310–318.
- Pal SK, Vogelzang NJ. Sequential treatment strategies and combination therapy regimens in metastatic renal cell carcinoma. Clin Adv Hematol Oncol. 2013;11:146–155.
- Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012;53:217–228. PubMed PMID: 22536463; PubMed Central PMCID: PMCPMC3332131.
- Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2015. J Natl Compr Canc Netw. 2015;13:151–159. FebPubMed PMID: 25691606.
- Tan X, Liu Y, Hou J, et al. Targeted therapies for renal cell carcinoma in Chinese patients: focus on everolimus. Onco Targets Ther. 2015;8:313–321.
- Zhang HL, Qin XJ, Wang HK, et al. Clinicopathological and prognostic factors for long-term survival in Chinese patients with metastatic renal cell carcinoma treated with sorafenib: a single-center retrospective study. Oncotarget. 2015;6:36870–36883.
- Chang X, Wang Y, Yu HP, et al. CT-guided percutaneous cryoablation for palliative therapy of gastric cancer liver metastases. Cryobiology. 2018;82:43–48.
- Song KD. Percutaneous cryoablation for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:509–515.
- Gao W, Guo Z, Shu S, et al. The application effect of percutaneous cryoablation for the stage IIIB/IV advanced non-small-cell lung cancer after the failure of chemoradiotherapy. Asian J Surg. 2018;pii:S1015-9584;30507–90507.
- Lin M, Liang SZ, Wang XH, et al. Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non-small cell lung cancer. Immunol Res. 2017;65:880–887.
- Liang X, Yang XL, Guo Z. Effect on cryoablation of high-risk and locally recurrent prostate cancer: a Meta-analysis. Zhonghua Yi Xue Za Zhi. 2017;97:1975–1980.
- Li Z, Fu Y, Li Q, et al. Cryoablation plus chemotherapy in colorectal cancer patients with liver metastases. Tumour Biol. 2014;35:10841–10848.
- Yang Y, Lu Y, Wang C, et al. Cryotherapy is associated with improved clinical outcomes of Sorafenib therapy for advanced hepatocellular carcinoma. Cell Biochem Biophys. 2012;63:159–169.
- Ni H, Yang M, Guo Z, et al. Sorafenib combined with cryoablation to treat unresectable hepatocellular carcinoma. Chin J Cancer Res. 2011;23:188–193.
- Wang C, Lu Y, Wang H, et al. Transarterial chemoembolization with/without cryotherapy is associated with improved clinical outcomes of sorafenib for the treatment of advanced hepatocellular carcinoma. Exp Ther Med. 2012;4:188–196.
- Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17:738–750.