Abstract
Background: Microwave ablation (MWA) has several advantages over radiofrequency ablation (RFA) for the treatment of hepatocellular carcinoma (HCC). We aimed to compare the efficacy and safety of MWA with those of RFA for HCC from the perspectives of percutaneous and laparoscopic approaches.
Methods: PubMed/MEDLINE, Embase, the Cochrane library, and China Biology Medicine databases were searched. Studies comparing the efficacy and safety of MWA with those of RFA in patients with HCC were considered eligible. Complete ablation (CA), local recurrence (LR), disease-free survival (DFS), overall survival (OS), and the major complication rate were compared between MWA and RFA.
Results: Four randomized controlled trials and 10 cohort studies were included. For percutaneous ablation, no significant difference was found between MWA and RFA regarding CA, LR, DFS, OS, and the major complication rate. A subgroup analysis of tumors measuring ≥3 cm revealed no difference in CA and LR for percutaneous ablation. For laparoscopic ablation, a significantly lower LR rate and a non-significant trend toward a higher major complication rate were observed for the MWA group (odds ratio [OR] 2.16, 95% confidence interval [CI] 1.16–4.02, p = .01 for LR; OR 0.21, 95% CI 0.04–1.03, p = .05 for major complication rate). CA, DFS, and OS were similar between the two groups.
Conclusions: Percutaneous (P)-MWA had similar therapeutic effects compared with P-RFA for HCC. Patients undergoing laparoscopic MWA had a lower LR rate; however, their major complication rate appeared to be higher. The superiority of MWA over RFA remains unclear and needs to be confirmed by high-quality evidence.
Précis
The efficacy and safety of microwave and radiofrequency ablation were evaluated; however, the superiority of microwave ablation over radiofrequency ablation could not be confirmed due to lack of sufficient evidence.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide [Citation1], and it is effectively treated with radiofrequency ablation (RFA). RFA can be as effective as surgical resection in terms of disease-free survival (DFS) and overall survival (OS) in patients with HCC [Citation2,Citation3]. Meanwhile, microwave ablation (MWA), a recently developed thermal ablation technique, has several advantages over RFA, including faster heating over a larger volume, less susceptibility to heat sinks and local perfusion [Citation4].
Despite the advantages of MWA, its efficacy and safety compared with RFA are under debate. Recent meta-analyses comparing percutaneous-MWA (P-MWA) and percutaneous-RFA (P-RFA) found that the two techniques had similar efficacies [Citation5–7]. However, these studies had several limitations. First, few randomized controlled trials (RCTs) were included, which would inevitably introduce bias [Citation8]. Second, as a result of the improvement of MWA technology and an increase in operators’ experience, the advantages of MWA compared with RFA might be outstanding. Third, a meta-analysis comparing laparoscopic-MWA (L-MWA) and laparoscopic-RFA (L-RFA) has not been performed. For these reasons, it is necessary to evaluate the efficacy and safety of MWA compared with RFA from the perspectives of percutaneous and laparoscopic approaches.
Therefore, the purpose of this meta-analysis was to assess the efficacy and safety of MWA compared with RFA for HCC treatment. To fully assess the role of MWA, articles on laparoscopic ablation were also included. Complete ablation (CA), local recurrence (LR), DFS, OS and the major complication rate were assessed.
Materials and methods
Search strategy and inclusion criteria
A computerized literature search was conducted on PubMed, Embase, the Cochrane Library, and China Biology Medicine databases to identify articles published before May 2018. The search strategy included the following keywords: (‘carcinoma, hepatocellular’ (MeSH term) OR ‘hepatocellular carcinoma’ (text)) AND (‘radiofrequency ablation’ (text) OR ‘radio-frequency therapy’ (text) OR ‘radio-frequency ablation’ (text)) AND (‘microwave coagulation’ (text) OR ‘microwave ablation’ (text) OR ‘microwave therapy’ (text)).
The inclusion criteria for articles were as follows: (1) full text available in English or Chinese; (2) results that provided data on CA, LR, DFS, OS, or complications compared between MWA and RFA for HCC; and (3) RCTs or high-quality cohort studies assessed using the Newcastle-Ottawa scale (NOS) [Citation9]. The reference lists of review articles were checked to identify additional related articles.
The exclusion criteria were as follows: (1) lack of the required data in the results, and no response after attempts to contact the author; (2) duplicate data if recent studies were included; and (3) ablative techniques combined with other locoregional therapies.
In this study, CA was defined as no enhancement in ablated areas after ablation. LR was defined as any new lesion inside or adjacent to the ablated zone. DFS was defined as the length of time that the patient survived without any signs of HCC after ablation. OS was defined as the length of time from the start of ablation to the date of the death or the last follow-up. According to the Clavien-Dindo Classification [Citation10], complications of grade 3 or higher were defined as major complications.
The quality of the included studies was assessed independently by two authors (T.WC, D.QW) according to the Cochrane Collaboration’s tool [Citation11] for RCTs and the NOS for cohort studies.
Statistical analyses
Reviewer Manager 5.3 (Cochrane Informatics) was used for pooling data. Data of CA, LR, DFS, OS and the major complication rate were analyzed and presented as odds ratios (ORs) and 95% confidence intervals (CIs). Heterogeneity was assessed by means of Cochrane’s Chi-square test, with a value of >50% suggestive of significant heterogeneity. A fixed-effects model was used if heterogeneity was significant; otherwise, the random-effects model was used. A p values <.05 was considered statistically significant.
Results
Literature search and characteristics of included studies
shows the search strategy used in this meta-analysis. Initially, we identified 450 potentially relevant studies. After a preliminary review, 418 papers were excluded because they were considered irrelevant studies, case reports, animal studies, comment letters, or reviews. Among 32 potentially relevant papers, we excluded five studies in which patients were treated with embolization combined with MWA or RFA [Citation12–16] and another seven because of insufficient data [Citation17–23]. Six studies were excluded because of duplicate data [Citation24–29]. For studies with duplicate data, reports with the lower number of patients and incomplete data were excluded. Finally, four RCTs [Citation30–33] and 10 cohort studies [Citation25,Citation34–42] with 1816 patients were included. All four RCTs focused on percutaneous ablation. Among the 10 cohort studies, four [Citation35,Citation38,Citation40,Citation41] compared L-MWA and L-RFA.
The main characteristics of the included studies are reported in . The recruitment period ranged from 1997 to 2015. The baseline background characteristics of patients, including age, Child-Pugh class, tumor size, and tumor location, were comparable.
Table 1. Characteristics of the included studies.
The 10 cohort studies were assigned 8–9 points upon NOS evaluation. The RCTs had a high risk of performance bias because blinding of physicians was difficult as different devices were used during the therapy. Initial follow-up was performed with computed tomography or magnetic resonance imaging, the data of which were objective. Therefore, detection bias in the outcome evaluation was low. Details on the quality assessment are presented in Table S1 and Figure S1.
Complete ablation rate
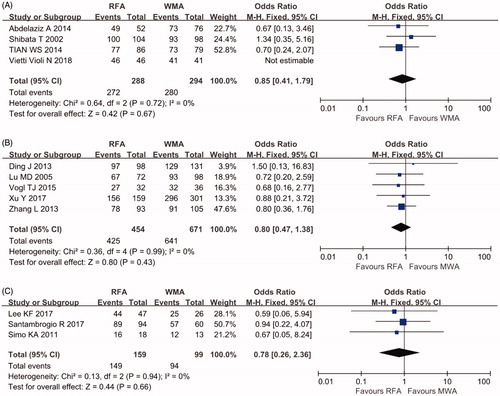
As shown in , no significant difference in the CA rate was found between the P-RFA and P-MWA groups (OR 0.85, 95% CI 0.41–1.79, p = .67 in RCTs; OR 0.8, 95% CI 0.47–1.38 p = .43 in cohort studies). No evidence of heterogeneity was found in these studies (I2=0%). There was also no significant difference in the CA rate between the L-RFA and L-MWA groups (OR 0.78, 95% CI 0.26–2.36, p = .66).
Local recurrence rate
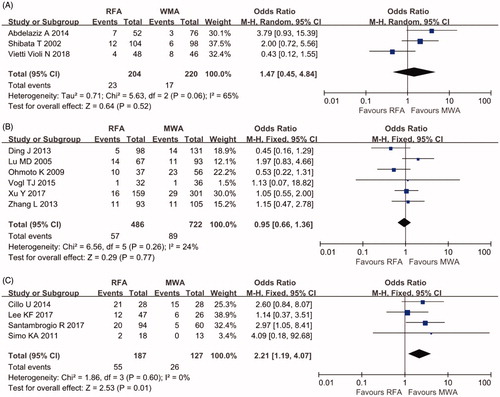
Three RCTs with 424 individuals and 10 cohort studies with 1522 individuals reported the LR rate. Heterogeneity was found among the RCTs (I2=65%), and the random-effects model yielded an OR of 1.47 for the RCTs (95% CI 0.45–4.84, p = .52). No significant difference in the LR rate was found in the meta-analysis of cohort studies using percutaneous ablation (OR 0.95, 95% CI 0.66–1.36, p = 0.77, I2=24%). However, a significantly lower LR rate was found in patients treated with L-MWA (OR 2.21, 95% CI 1.19–4.07, p = .01, I2=0%) ().
Disease-free survival and overall survival
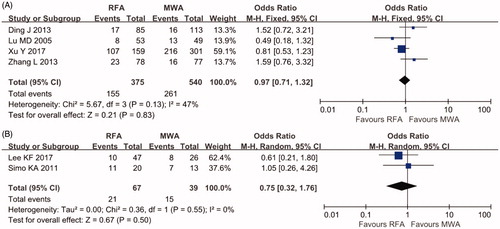
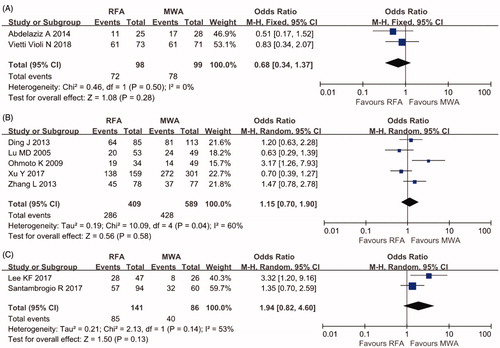
DFS at three years was reported by four cohort studies of percutaneous ablation with 915 patients and two cohort studies of laparoscopic ablation with 106 patients. A low grade of heterogeneity was found for these studies (I2=47%; I2=0%, respectively); hence, the fixed-effects model was used. No significant difference in the LR rate was found (OR 0.97 95% CI 0.71–1.32, p = .83 for percutaneous ablation; OR 0.75, 95% CI 0.32–1.76, p = .50 for laparoscopic ablation) ().
As shown in , OS at three years was available for two RCTs with 197 patients and seven cohort studies with 1225 patients. There was no significant difference in OS rate between the two treatment groups (OR 0.68, 95% CI 0.34–1.37, p = .28, I2=0% in RCTs; OR 1.15, 95% CI 0.70–1.90, p = 0.58, I2=60% in cohort studies of percutaneous ablation; OR 1.94, 95% CI 0.82–4.60, p = 0.13, I2=53% in cohort studies of laparoscopic ablation).
Major complication rate
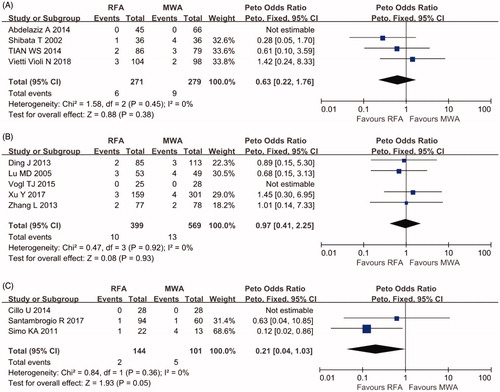
Four RCTs with 550 patients and eight cohort studies with 1213 patients reported major complications with details. No perioperative deaths related to the therapy were reported. As shown in , no significant difference in the major complication rate was found between the RCTs and cohort studies of percutaneous ablation (OR 0.63, 95% CI 0.22–1.76, p = .38, I2=0%; OR 0.97, 95% CI 0.41–2.25, p = .93, I2=0%, respectively). For cohort studies of laparoscopic ablation, the OR was 0.21 (95% CI 0.04–1.03, p = .05, I2=0%). The major complication rate was higher after L-MWA without reaching the significance threshold.
Subgroup analysis
Subgroup analysis of tumors ≥3 cm revealed no difference in CA and LR rates between P-RFA and P-MWA (OR 1.09, 95% CI 0.30–3.93, p = .90, I2=0%; OR 0.85, 95% CI 0.35–2.10, p = .73, I2=0%, respectively) (Figure S2).
Discussion
Thermal ablative therapies have become important alternative treatments for HCC. In our study, there were no significant differences in CA, LR, DFS, OS and the major complication rate between P-MWA and P-RFA. A significantly lower LR rate and a non-significant trend toward a higher rate of major complications were found in the L-MWA group compared with the L-RFA group.
MWA and RFA are commonly used for the treatment of HCC. They differ in their mechanism of action: RFA uses electric current, whereas MWA uses electromagnetic energy. However, the principle underlying these two treatment methods is similar, in that high temperatures are induced in localized areas to coagulate tissue. MWA has several advantages over RFA. The heat-sink effect, a phenomenon that occurs when thermal energy is dispersed from the target lesion due to blood flow in the adjacent vessels, is an important disadvantage of RFA. Consequently, the shape and size of the ablation zone in RFA may be unpredictable, and such limitations can lead to an inadequate ablation zone. For MWA, the time needed for ablation is less than that required for RFA. Moreover, MWA can deliver higher temperatures in the ablation zone. These two features of MWA lead to a more predictable ablation zone. Several studies [Citation29,Citation43,Citation44] have found that MWA produced significantly larger zones than RFA. Therefore, the apparent superiority of MWA over RFA has made it an alternative method to RFA, and it may potentially be a more powerful technique than RFA.
In our study, CA, LR, DFS, OS and the major complication rate between P-MWA and P-RFA were not significantly different. In subgroups with a tumor size ≥3 cm, no difference was detected in CA and LR rates between P-MWA and P-RFA. Similar results were reported previously [Citation5,Citation45]. Although MWA has been shown to have certain theoretical advantages over RFA, these advantages have not been confirmed in clinical practice. There may be several reasons for the reportedly conflicting results. First, microwave devices in some clinical trials have not been perfect. Different types of available MWA machines may have varying efficacies, which could lead to a failure of MWA to achieve the expected results. Second, the tumors involved in the research articles were often smaller than 3 cm or the number of patients included was small; consequently, the advantages of MWA failed to be demonstrated.
To fully assess the role of MWA, we included articles on laparoscopic as well as percutaneous ablation. Laparoscopic approaches for the ablation of HCC are viable in a proportion of patients who are unsuitable for hepatic resection or percutaneous ablation. A laparoscopic approach provides access to tumors that are located underneath the liver capsule, adjacent vessels, gallbladder, or diaphragm and that may be difficult to reach. Our study revealed a lower incidence of LR in the L-MWA group. This result may be interpreted as evidence of the superiority of MWA over RFA, which was mentioned above. However, RCTs comparing L-MWA and L-RFA have not been conducted. Selection bias and heterogeneity of the device used must be taken into consideration. Consequently, robust data are needed to demonstrate this result.
The major complication rate of MWA and RFA remains controversial. A recent meta-analysis found that the major complication rate of MWA was higher than that of RFA, although the results did not reach statistical significance. Similar results were observed in our study. Given the fact that MWA is less affected by the heat-sink effect, MWA can produce more uniform and larger tumor necrosis. However, these characteristics, in turn, are associated with a theoretically increased risk of damaging neighboring organs, especially vascular and biliary structures [Citation46]. A larger ablation area might account for a higher complication rate [Citation36,Citation47]. However, according to previously published data, the incidence of vascular and biliary complications caused by MWA and RFA appeared to be similar and infrequently reported [Citation48–50]. It should be noted that the majority of clinical studies are retrospective. This raises the possibility of under- and misreporting of clinical complications. Insufficient clinical data make it difficult to compare the incidence of vascular and biliary complications between the two groups. Therefore, high-quality evidence is needed to compare the complication rates of MWA and RFA.
This study had some limitations. First, many included studies were conducted over different time periods and with different ablation devices. The evolving ablation technology and the increase in the experience of operators affected the results. Second, most of the studies involved HCC with nodules less than 3 cm in size, and it was difficult to compare MWA with RFA according to tumor location. Therefore, the advantages of MWA in terms of generating larger ablation areas and avoiding the heat-sink effect were difficult to demonstrate. Third, the small number of included articles were limited in their significance. Additional evidence is needed to confirm our findings.
In conclusion, our study showed that P-MWA had similar therapeutic effects compared with P-RFA for HCC. Patients undergoing L-MWA had a lower LR rate but appeared to have a higher rate of major complications. The superiority of MWA over RFA still needs to be confirmed by high-quality research.
Tables S1 and Figures S1-S2
Download PDF (275.5 KB)Acknowledgments
We thank the authors and participants of the included studies for their important contributions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- El-Serag HB. Advances in the management of hepatocellular carcinoma. Clin Adv Hematol Oncol. 2017;15:2–6.
- Cho YK, Rhim H, Noh S. Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol. 2011;26:1354–1360.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802.
- Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: an updated review. WJGPT. 2016;7:477–489.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344.
- Chinnaratha MA, Chuang MY, Fraser RJ, et al. Percutaneous thermal ablation for primary hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:294–301.
- Huo YR, Eslick GD. Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis. J Vasc Interv Radiol. 2015;26:1139–1146.
- Huang Q, Yang H, Lin QN, et al. 'Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis': two issues should be noted. Int J Hyperthermia. 2016;32:345.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196.
- Higgins JPTGS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011.
- Thornton LM, Cabrera R, Kapp M, et al. Radiofrequency vs microwave ablation after neoadjuvant transarterial bland and drug-eluting microsphere chembolization for the treatment of hepatocellular carcinoma. Curr Probl Diagn Radiol. 2017;46:402–409.
- Abdelaziz AO, Abdelmaksoud AH, Nabeel MM, et al. Transarterial chemoembolization combined with either radiofrequency or microwave ablation in management of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2017;18:189–194.
- Sheta E, El-Kalla F, El-Gharib M, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28:1198–1203.
- Ginsburg M, Zivin SP, Wroblewski K, et al. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol. 2015;26:330–341.
- Veltri A, Gazzera C, Calandri M, et al. Percutaneous treatment of Hepatocellular carcinoma exceeding 3 cm: combined therapy or microwave ablation? Preliminary results. Radiol Med. 2015;120:1177–1183.
- Mironov O, Jaberi A, Beecroft R, et al. Retrospective single-arm cohort study of patients with hepatocellular adenomas treated with percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2018;41:935–941.
- Francica G, Meloni MF, de Sio I, et al. Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: safety and efficacy. Eur J Radiol. 2016;85:739–743.
- Chinnaratha MA, Sathananthan D, Pateria P, et al. High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practice. Eur J Gastroenterol Hepatol. 2015;27:349–354.
- Ei S, Hibi T, Tanabe M, et al. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol. 2015;22:1294–1300.
- Yi Y, Zhang Y, Wei Q, et al. Radiofrequency ablation or microwave ablation combined with transcatheter arterial chemoembolization in treatment of hepatocellular carcinoma by comparing with radiofrequency ablation alone. Chin J Cancer Res. 2014;26:112–118.
- Sakaguchi H, Seki S, Tsuji K, et al. Endoscopic thermal ablation therapies for hepatocellular carcinoma: a multi-center study. Hepatol Res. 2009;39:47–52.
- Loriaud A, Denys A, Seror O, et al. Hepatocellular carcinoma abutting large vessels: comparison of four percutaneous ablation systems. Int J Hyperthermia. 2018;34:1171–1178.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Thermal ablation therapy for hepatocellular carcinoma: comparison between radiofrequency ablation and percutaneous microwave coagulation therapy. Hepatogastroenterology. 2006;53:651–654.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Radiofrequency ablation versus percutaneous microwave coagulation therapy for small hepatocellular carcinomas: a retrospective comparative study. Hepatogastroenterology. 2007;54:985–989.
- Xu HX, Xie XY, Lu MD, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53–61.
- Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer-Am Cancer Soc. 2009;115:1914–1923.
- Kuang M, Xie XY, Huang C, et al. Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg. 2011;15:2165–2171.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22:1983–1990.
- Vietti VN, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317–325.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337.
- Tian WSKMLM. A randomised comparative trial on liver tumors treated with ultrasound-guided percutaneous radiofrequency versus microwave ablation. Chin J Hepatobiliary Surg. 2014;20:119–122.
- Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc. 2014;28:3429–3434.
- Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054–1060.
- Simo KA, Sereika SE, Newton KN, et al. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104:822–829.
- Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. Plos One. 2013;8:e76119.
- Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379–1384.
- Cillo U, Noaro G, Vitale A, et al. Laparoscopic microwave ablation in patients with hepatocellular carcinoma: a prospective cohort study. HPB (Oxford). 2014;16:979–986.
- Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging. 2015;40:1829–1837.
- Lee KF, Wong J, Hui JW, et al. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: a retrospective comparative study. Asian J Surg. 2017;40:301–308.
- Santambrogio R, Chiang J, Barabino M, et al. Comparison of laparoscopic microwave to radiofrequency ablation of small hepatocellular carcinoma (≤3 cm). Ann Surg Oncol. 2017;24:257–263.
- Xu Y, Shen Q, Wang N, et al. Microwave ablation is as effective as radiofrequency ablation for very-early-stage hepatocellular carcinoma. Chin J Cancer. 2017;36:14.
- Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37:2967–2973.
- Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol. 2009;24:223–227.
- Kuang M, Lu MD, Xie XY, et al. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna-experimental and clinical studies. Radiology. 2007;242:914–924.
- Mann CD, Metcalfe MS, Lloyd DM, et al. The safety and efficacy of ablative techniques adjacent to the hepatic vasculature and biliary system. Anz J Surg. 2010;80:41–49.
- Ding J, Jing X, Liu J, et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68:608–615.
- Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. 2014;83:552–558.