Abstract
Patients are increasingly seeking uterus-preserving, minimally invasive treatments for symptomatic uterine fibroids. This has led to a greater use of nonresective treatments such as uterine artery embolization (UAE), focused ultrasound (FUS) and more recently, radiofrequency ablation (RFA) of fibroids. This systematic review, following PRISMA guidelines, examines the change in uterine and fibroid volumes associated with UAE, FUS, and RFA. Pubmed and MedlinePlus databases were searched from 1956 to 2016. The keywords used were ‘radiofrequency ablation,’ ‘magnetic resonance guided focused ultrasound,’ ‘ultrasound guided focused ultrasound’, ‘uterine artery embolization,’ ‘uterine fibroid embolization,’ and ‘leiomyoma’ or ‘fibroid’. Publications with at least 20 patients were included. Data were collected and analyzed using Microsoft Excel® (Microsoft Corporation, Redmond, WA) software. Eighty-one relevant papers were identified: 52 related to UAE, 11 to RFA, 17 to FUS, 1 compared UAE and FUS. We report the published uterine volume and fibroid volume changes seen in these studies at 1 to 36 months. The pooled fibroid volume reductions at six months seen with RFA were 70%, UAE 54% and FUS 32%. All three types of nonresective treatment result in fibroid volume reduction. However, fibroid volume reduction is most marked with RFA, with UAE resulting in the next most volume reduction. Additional larger cohort studies, including those that are randomized and/or comparative, would enable definitive conclusions. This is the first systematic review comparing uterine and fibroid volume reduction after RFA, UAE and MRgFUS.
Introduction
Uterine fibroids may be present in over 70% of the premenopausal population [Citation1,Citation2], with a prevalence that increases with age [Citation3]. It is estimated that as many as 50% of leiomyomas are symptomatic [Citation4] and represent the most common indication for hysterectomy in many countries. Fibroids account for approximately 240 000 cases, or 40% of all hysterectomies performed annually in the United States [Citation5] and nearly 20 000 inpatient admissions in the United Kingdom [Citation6]. Uterus-sparing treatment options have been increasing in usage because of many women’s desire to preserve fertility and/or their uteri, as well as to avoid major surgery; income, education and insurance type also may play a role in the choice of fibroid treatment [Citation7]. In a prospective study on management options chosen by 933 women with symptomatic leiomyomas, only 16% had hysterectomy, 27% had at least one uterus-sparing procedure, and 57% chose nonprocedural interventions [Citation8].
Uterus-sparing procedures include myomectomy (transabdominal, laparoscopic or hysteroscopic routes), radiofrequency ablation (RFA), uterine artery embolization (UAE), and focused ultrasound (FUS), which can be performed under magnetic resonance guidance (MRgFUS) or ultrasound guidance (USgHIFU). Of note, alternatives to hysterectomy that leave treated fibroids in-situ (e.g., RFA, UAE and FUS) are effective with regard to durable symptom relief if and only if there is some degree of reduction in total fibroid volume [Citation9]. While data from studies involving MRgFUS have suggested that nonperfused volumes >20% are associated with sustained symptom relief at 24 months, it is also expected that higher percentages of fibroid ablation are more likely to result in improved clinical outcomes [Citation10–12]. Similarly, the percentage of fibroid tissue that is infarcted during UAE is also predictive of clinical outcome [Citation13]. With this understanding that greater ablation is associated with higher treatment success, the aim of this systematic review is to compare and contrast the existing literature on fibroid and uterine volume changes among current nonresective uterus-sparing treatment modalities: RFA, UAE and FUS. This is the first systematic review of its kind. There has not previously been a study that collates data from primary studies on fibroid or uterine volume changes with these three uterine sparing, nonresective treatments nor have these modalities been compared to one in another in this way. The aim is to identify all reported fibroid and uterine volume changes with RFA, UAE and FUS with the hope to inform the clinician and patient as part of the decision making process regarding the best treatment option.
Materials and methods
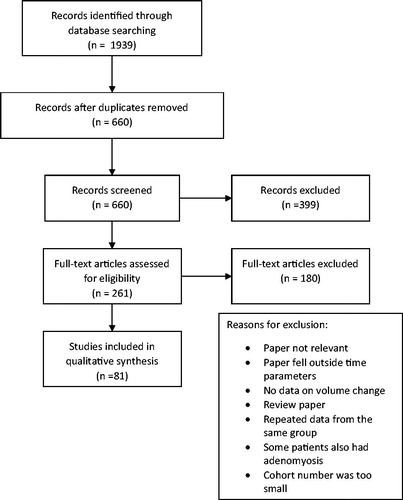
PRISMA guidelines were used to perform this systematic review. The PICO model was used to formulate the search criteria. The population were patients having treatments for their fibroids. The interventions were RFA, UAE and FUS. The outcome was change in fibroid or uterine volume. PubMed and MedlinePlus databases were searched from October 1956 to September 2016. Two authors, S.K. and M. T, independently searched the databases to identify all relevant papers. The keywords used were ‘radiofrequency ablation,’ ‘magnetic resonance-guided focused ultrasound,’ ‘ultrasound-guided focused ultrasound’, ‘uterine artery embolization,’ ‘uterine fibroid embolization,’ and ‘leiomyoma’ or ‘fibroid.’ Initially, all identified paper titles and abstracts were reviewed and any abstract that may have had relevant data had a full paper review by the authors. All papers were reviewed by 2 of the 3 authors MT, KS and CP using an established standardized scoring system. The NIH quality assessment tool for observational cohort and cross-sectional studies were used. Relevant papers which scored a value of good or fair were included. It was agreed that any papers where there was disagreement about inclusion would be discussed; however, there were no papers where there was disagreement. Publications in the English language that were retrospective, prospective, and case series that had at least 20 patients were included. If the study reported on more than one treatment, each treatment arm had to include 20 or more patients. Any papers that provided data on change in uterine or fibroid volumes at any time, following treatment in patients who had been treated with UAE, RFA or FUS, were included. Papers that included patients who also had adenomyosis were excluded. Studies from the same research group were compared to ensure that there was no repetition of data.
Measurements by ultrasound scan, MRI or CT scan were included and analyzed together. Radiofrequency ablation via the laparoscopic or transcervical routes were included, as was data with and without concurrent imaging guidance. For MRgFUS studies, data on nonperfused fibroid volumes and fibroid intensity on T2-weighted MRI images were also collected, when available (nonperfused fibroid volume refers to the volume of a fibroid that has been devascularized secondary to the treatment, and thus does not demonstrate contrast enhancement on MR imaging). Nonperfused volume (NPV) is usually provided as the percentage of fibroid volume that lacks contrast enhancement. The signal intensity of the fibroid is determined by its appearances on T2-weighted MRI scan; hyperintense lesions are brighter than skeletal muscle, isointense appear equivalent and hypointense are darker. Signal intensity relates to blood flow within the fibroid with hyperintense fibroids being the most vascular. Articles that focused on pregnant patients and patients without leiomyomas were excluded. Data were collected and analyzed using Microsoft Excel® (Microsoft Corporation, Redmond, WA) software.
Results
The literature search yielded 660 abstracts; 579 of these were excluded using the criteria as detailed in the methodology. summarizes the results of the literature search and the selection process for eligibility. Of the 81 remaining papers, 11 related to RFA, 52 to UAE and 17 to FUS (three of these ultrasound-guided and the remaining magnetic resonance guided) and 1 paper directly compared MRgFUS and UAE. shows a summary of the basic demographic data for each treatment modality. The mean age stated in the studies ranged from 32.4 to 52 years.
Table 1. Summary of basic demographic data for RFA, UAE and FUS.
There was heterogeneity in the reporting of data between the papers. A percentage volume reduction of the uterus or fibroids was stated or could be calculated from all the included papers. However, not all publications provided a standard deviation or range for this data. Based on the published data available, in view of this heterogeneity, a meta-analysis could not be performed. In some studies, fibroid volume reduction was measured only in the dominant fibroid and in others, all treated fibroids were included. The results here show these categories combined as reduction in size of fibroids.
Radiofrequency ablation
Eleven papers reported on changes in uterine or fibroid volume reduction after RFA, mostly with noncommercial, ‘off the shelf’ devices often repurposed from other RFA systems designed for hepatic tumors. Two papers compared two groups. Galen et al. [Citation14] presented multi-center, multi-study data of patients from pilot studies of a commercial laparoscopic RFA system. Yin et al. [Citation15] split their patient group into premenopausal and menopausal cohorts. However, the definition of this was not provided and menopausal women appeared to be menstruating from the study description. Data are also available for two commercial devices. The commercial laparoscopic system (Acessa®, (Halt Medical, Brentwood, CA) was associated with a 45.1% reduction in total fibroid volume at 12 months [Citation16]. A transcervical device (the Sonata® System, Gynesonics, Redwood City, CA), which is in commercial use in Europe and the United States, demonstrated a 66.6% reduction in total fibroid volume at 12 months [Citation17,Citation18]. reports pooled data of fibroid volume reductions from the RFA studies. The means of the studies at each time point were pooled. The minimum and maximum reported fibroid volume reductions are reported as well as the number of studies reporting data at each time point.
Table 2. Pooled data of fibroid volume reductions from the RFA studies.
Four papers reported on uterine volume reductions following RFA. The maximum uterine volume reductions at any time point ranged from 20% to 40%, with the minimum reported reduction of 15%.
Uterine artery embolization
Fifty-two papers reported on changes in fibroid or uterine volume size after treatment with UAE. Four of these compared two groups, both undergoing UAE but with different cohorts, treatment protocols or successful outcome definitions. Toor et al. [Citation19] reported on patients thought to have had a successful embolization against those thought to have had an unsuccessful procedure. Treatment was considered to have failed if a patient’s symptoms worsened or failed to improve, and/or if patients actively sought or had secondary treatment for their fibroids. Bilhim et al. [Citation20] compared UAE with small polyvinyl alcohol particles (350–500 µm) and large (500–700 µm) particles. Kim et al. [Citation21] compared patients who had utero-ovarian anastomoses with those who did not have anastomoses, as demonstrated at angiography. Song et al. [Citation22] compared two embolization particles with one group having nonspherical polyvinyl alcohol particles and the other having gelatin sponge particles. Ikink et al. [Citation23] compared MRgFUS to UAE. reports pooled data of fibroid volume reductions from the RFA studies. The means of the studies at each time point were pooled. The minimum and maximum reported fibroid volume reductions are reported as well as the number of studies reporting data at each time point. No papers reported on fibroid volume reductions at 36 months.
Table 3. Pooled data of fibroid volume reductions from the UAE studies.
Forty-two papers reported on uterine volume reductions following UAE. The maximum uterine volume reductions at any time point ranged from 25% to 75%, with the minimum reported reduction of 13%.
Focused ultrasound
Eighteen papers reported on changes in fibroid or uterine volume size after treatment with FUS, with either MR guidance or real-time sonographic imaging. This includes the paper by Ikink et al. [Citation23] comparing it to UAE. reports pooled data of fibroid volume reductions from the RFA studies, and illustrates the relationship between NPV achieved and fibroid volume reduction in these studies.
Figure 2. The relationship between nonperfused volume (NPV) and fibroid volume reductions in FUS studies.
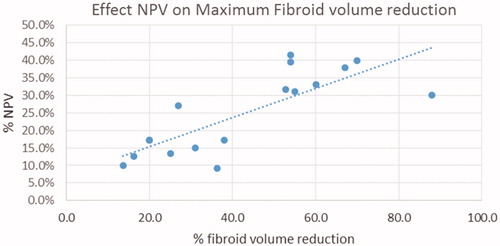
Table 4. Pooled data of fibroid volume reductions from the FUS studies.
Two papers reported on uterine volume reductions following FUS. The maximum uterine volume reductions at any time point ranged from 16% to 28%, with the minimum reported reduction of 11%.
A full table of NPV, fibroid intensity and fibroid volume reduction is available in Table S4.
Comparing modalities
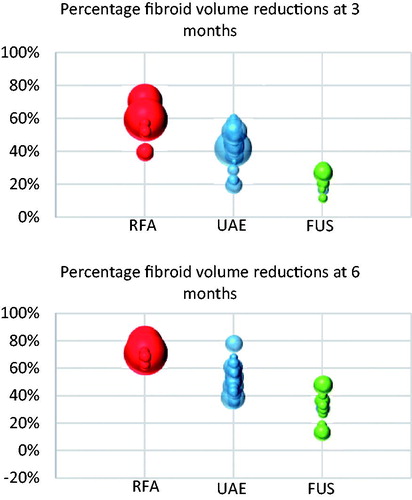
demonstrates percentage fibroid volume reductions at three and six months for each treatment modality.
Figure 3. Bubble charts of fibroid volume reductions at three and six months’ post treatment. Each bubble represents a single study with the size of the bubble representing the N number of the study.
The full set of all collected data is summarized in the appendices. Table S1 shows all data on basic demographic data including starting uterine and fibroid volumes. Table S2 shows data on the fibroid volume reductions and Table S3 on uterine volume reductions. Table S4 reports the available data on NPV and fibroid intensity in the FUS studies.
Discussion
The aim of this systematic review is to compare the published data on fibroid and uterine volume reductions following nonresective, uterus-conserving treatments for fibroids. While the heterogeneity of the data meant that meta-analysis was not possible, meaningful trends can be observed in the collected data.
Fibroid and uterine volume reductions have been demonstrated with UAE, RFA and FUS (MRgFUS and USgHIFU). The fibroid volume reductions appear more marked with RFA and UAE than with FUS, which is clearly demonstrated by both the pooled volume reductions in and as illustrated in . At six months post-treatment, pooled fibroid volume reductions are 70% for RFA, 54% for UAE and 32% for FUS. Only one paper directly compared two of the studied modalities [Citation23]. Ikink and colleagues compared UAE with MRgFUS and found at three-month follow-up that uterine and fibroid percentage volume reductions were significantly greater in the UAE group compared with the MRgFUS cohort. While MRgFUS provides modest fibroid volume reductions relative to those associated with UAE and RFA, MRgFUS may still be associated with symptom relief through 24 months postablation. However, when there is a desire for significant reductions in bulk fibroid volume or for longer-lived symptom relief, MRgFUS may be less effective than other modalities. It should be noted that initial studies using MRgFUS in the United States were limited by Food and Drug Administration (FDA) imposed restrictions regarding the size of the ablation within the fibroids. These restrictions have been since changed, and further data regarding the success of this modality may reflect this.
It is known that with treatment with MRgFUS, higher nonperfused volumes are associated with greater improvements in quality of life scores and symptoms [Citation24]. It has also been seen that larger nonperfused volumes are seen with less vascular fibroids with hypo-intense appearances on T2-weighted MRI [Citation25,Citation26]. In this systematic review, there does seem to be a positive trend between NPV and volume reduction, as illustrated in . Three papers comment on the MRI intensity of the studied fibroids, as shown in Table S4. Funaki et al. [Citation27] note that more signal-intense fibroids are associated with both lower NPVs and lesser degrees of fibroid volume reduction, with some fibroids increasing in size, rather than decreasing, within this cohort.
While reductions in fibroid volumes have been correlated with durable symptom relief after MRgFUS [Citation10], only three of the studied papers looked directly at the link between uterine volume change and symptoms, all involved treatment with UAE. Scheurig et al. [Citation28] showed a moderate positive correlation between the change in symptom severity score and percentage uterine volume reduction. Pron et al. [Citation29], in contrast, found that improvement in abnormal uterine bleeding and life impact scores were not related to uterine volume reductions. They did suggest that this improvement in uterine bleeding may be a result of vascular disruption produced by vessel occlusion. Smith et al. [Citation27] reported contradictory results. They found no association between the changes in symptom severity scores or health related quality of life scores and uterine volume reductions. However, they did find a significant association (p = .01906) between global satisfaction and the percentage change in uterine volumes. It would be logical to expect that greater fibroid and uterine fibroid volumes would be associated with greater symptom improvement; however, the data from this review are insufficient to draw firm conclusions and further studies are needed.
Comparing UAE and RFA, the data suggest that individual fibroid volume reductions are more impressive with RFA but that overall uterine volume reductions are greater with UAE. Analyzing the full data set in appendices, a greater number of larger fibroids are treated in patients undergoing UAE compared to RFA. This is consistent with known practice trends, and the fact that UAE is a global fibroid therapy while RFA (like FUS) is a focal treatment modality. Across all studies, reporting of the median or mean number of fibroids treated was inconsistent and often lacking. However, papers more commonly reported on the percentage of patients with a single-treated fibroid. Of the included UAE studies, 21 papers reported on 23 cohorts of patients. In only four of the 23 cohorts (i.e. 17%) did >60% of the patients have a single fibroid, which implies that patients undergoing UAE tend to have multiple fibroids. In the reported RFA studies, four papers commented on single fibroids, and in three of these, >60% of the patients had a single diagnosed fibroid, implying that the majority of patients undergoing RFA were having treatment to a single fibroid. Uterine and dominant fibroid volumes also seem to be larger in the UAE studies than in the RFA studies. Mean uterine volumes <300 cc were seen in 75% of the studies on RFA but only 9% of the UAE studies. Similarly, mean dominant fibroid volumes of <80 cc were seen in 80% of the RFA studies, but only 4% of the UAE studies.
While volume reductions of both fibroids and uteri after treatment with UAE and RFA tend to materially increase with increasing time from treatment, the fibroid volume reductions following MRgFUS peaked at 6 months. This is consistent with reports that MRgFUS is most effective for women over the age of 45 who are approaching menopause. It is also consistent with a less durable effect of MRgFUS, in line with published data on reintervention rates of at least 50% at five years, [Citation30] even with NPVs greater than 50% [Citation30].
The effect of UAE on fibroid volumes over time is shown in and . UAE was the treatment modality with the greatest number of published studies and is arguably, the modality with which clinicians have the greatest experience. The collected data show consistent reductions in both uterine and fibroid volumes with a maximal reduction of pooled fibroid volume of 70% at 24 months.
RFA also results in consistent reductions in uterine and fibroid volumes. Maximal pooled fibroid volume reductions of 84% were seen at 36 months.
The link between reintervention and uterine or fibroid volume reductions were explored by six studies. Funaki et al. [Citation27] were the only group to address this in patients undergoing MRgFUS. They showed a trend of greater reintervention (21.6%) in patients with lower fibroid volume reductions (9.1%) at six months in women with hyperintense fibroids, but no comment was made on whether this met statistical significance. The remaining studies to investigate reintervention involved patients undergoing UAE. Lohle et al. [Citation31] and Hald et al. [Citation32] both found that reintervention was significantly more likely in patients with lower percentage fibroid or uterine volume reductions. Toor et al. [Citation19] also found that patients who had failed treatment had significantly lower total fibroid volume reductions. Volkers et al. [Citation33], however, used multiple regression analysis and found no association between volume reduction and failure. Dueholm et al. [Citation34] also found that uterine or fibroid volume reductions of less than 45% were not associated with an increased risk of major reintervention (hysterectomy, myomectomy or repeat embolization). Reintervention rates of 20–30% at five years have been reported in the literature [Citation35,Citation36]. None of the included studies correlated volume reductions with reintervention rates after RFA. However, reintervention rates of 11% at 36 months [Citation37] and 8% at 12 months [Citation17] have been reported for Acessa™ and Sonata™, respectively.
This review has focused on volume reductions in fibroids and uteri after nonresective procedures. Medical therapy with a GnRH analog such as leuprolide acetate or with the selective progesterone receptor modulator ulipristal acetate will also result in fibroid volume reduction [Citation38]. It is known that these medications have to be continued in order to see a maintained reduction in fibroid size and as such, have mostly been used in the short-term neoadjuvant setting, given the risks to bone density associated with longer use of GnRH analogues and known association of ulipristal with progesterone receptor modulator-associated endometrial changes. While some medical therapies has been approved for long-term use, many patients will choose the convenience and security of a single treatment such as RFA, UAE or FUS.
This study did not directly compare the differences in symptom improvement, reintervention rates or pregnancy outcomes among the three treatment modalities, although these will be important considerations for patients and their clinicians. In particular, effects on fertility and pregnancy following RFA have not yet been systematically studied, although case series do suggest that RFA may be compatible with future fertility [Citation39–42]. Reports of the effects of UAE on fertility and fecundity have been contradictory, and there is a risk of altered ovarian blood supply and premature ovarian failure in these patients [Citation43–46]. On the other hand, MRgFUS has been approved in women who desire fertility in the United States. As an ablative treatment for fibroids, the compatibility of MRgFUS with future pregnancy suggests that RFA might similarly be found to be suitable as its evidence base increases [Citation18].
Although publication bias cannot be absolutely excluded, the inclusion of all identified studies that reported on volume reductions should ensure that these data is as comprehensive as possible.
Conclusions
Women with symptomatic fibroids are increasingly seeking minimally invasive, uterus-sparing treatment options. This systematic review aimed to summarize the reductions in fibroid and uterine volumes with three such treatments, UAE, RFA and FUS. All three modalities result in fibroid and uterine volume reduction, but these were most significant with RFA, followed by UAE. This pattern was seen consistently at all time points. At six months, pooled fibroid volume reductions were 70% with RFA, 54% with UAE and 32% with FUS. The effects of RF and UAE seem to be prolonged with reductions in fibroid volumes at least up to 24 months post-treatment. A meta-analysis with original data from the authors would help to statistically analyze the trends observed in this review. Furthermore, a randomized controlled trial with head-to-head comparators of various fibroid interventions is needed to establish which the more effective treatment is and whether different patient groups see better results with either treatment, so that clinicians may best advise their patients.
Tables S1-S4
Download PDF (575.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–438.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107.
- Lurie S, Piper I, Woliovitch I, et al. Age-related prevalence of sonographicaly confirmed uterine myomas. J Obstet Gynaecol. 2005;25:42–44.
- Vilos GA, Allaire C, Laberge PY, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2015;37:157–181.
- Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229–234.
- Fernandez H, Farrugia M, Jones SE, et al. Rate, type, and cost of invasive interventions for uterine myomas in Germany, France, and England. J Minim Invasive Gynecol. 2009;16:40–46.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67–77.
- Jacoby VL, Jacoby A, Learman LA, et al. Use of medical, surgical and complementary treatments among women with fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:220–225.
- Stewart EA, Rabinovici J, Tempany CM, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–29.
- Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–287.
- Fennessy FM, Tempany CM, McDannold NJ, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery-results of different treatment protocols. Radiology. 2007;243:885–893.
- Morita Y, Ito N, Hikida H, et al. Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids - early experience. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):199–203.
- Kroencke TJ, Scheurig C, Poellinger A, et al. Uterine artery embolization for leiomyomas: percentage of infarction predicts clinical outcome. Radiology. 2010;255:834–841.
- Galen DI, Pemueller RR, Leal JG, et al. Laparoscopic radiofrequency fibroid ablation: phase II and phase III results. JSLS. 2014;18:182–190.
- Yin G, Chen M, Yang S, et al. Treatment of uterine myomas by radiofrequency thermal ablation: a 10-year retrospective cohort study. Reprod Sci. 2015;22:609–614.
- Chudnoff SG, Berman JM, Levine DJ, et al. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121:1075–1082.
- Brölmann H, Bongers M, Garza-Leal J, et al. The FAST-EU trial: 12-month clinical outcomes of women after intrauterine sonography-guided transcervical radiofrequency ablation of uterine fibroids. Gynecol Surg. 2016;13:27–35.
- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the sonata system. Curr Obstet Gynecol Rep. 2017;6:67–73.
- Toor SS, Tan KT, Simons ME, et al. Clinical failure after uterine artery embolization: evaluation of patient and MR imaging characteristics. J Vasc Interv Radiol. 2008;19:662–667.
- Bilhim T, Pisco JM, Duarte M, et al. Polyvinyl alcohol particle size for uterine artery embolization: a prospective randomized study of initial use of 350-500 mum particles versus initial use of 500-700 mum particles. J Vasc Interv Radiol. 2011;22:21–27.
- Kim HS, Tsai J, Patra A, et al. Effects of utero-ovarian anastomoses on clinical outcomes and repeat intervention rates after uterine artery embolization. J Vasc Interv Radiol. 2006;17:783–789.
- Song YG, Jang H, Park KD, et al. Non spherical polyvinyl alcohol versus gelatin sponge particles for uterine artery embolization for symptomatic fibroids. Minim Invasive Ther Allied Technol. 2013;22:364–371.
- Ikink ME, Nijenhuis RJ, Verkooijen HM, et al. Volumetric MR-guided high-intensity focused ultrasound versus uterine artery embolisation for treatment of symptomatic uterine fibroids: comparison of symptom improvement and reintervention rates. Eur Radiol. 2014;24:2649–2657.
- Healey S, Buzaglo K, Seti L, et al. Ovarian function after uterine artery embolization and hysterectomy. J Am Assoc Gynecol Laparosc. 2004;11:348–352.
- Park MJ, Kim YS, Rhim H, et al. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US therapy. J Vasc Interv Radiol. 2014;25:231–239.
- Zhao WP, Chen JY, Chen WZ. Effect of biological characteristics of different types of uterine fibroids, as assessed with T2-weighted magnetic resonance imaging, on ultrasound-guided high-intensity focused ultrasound ablation. Ultrasound Med Biol. 2015;41:423–431.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34:584–589.
- Scheurig C, Gauruder-Burmester A, Kluner C, et al. Uterine artery embolization for symptomatic fibroids: short-term versus mid-term changes in disease-specific symptoms, quality of life and magnetic resonance imaging results. Hum Reprod. 2006;21:3270–3277.
- Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120–127.
- Quinn SD, Vedelago J, Gedroyc W, et al. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:247–251.
- Lohle PN, Boekkooi FP, Smeets AJ, et al. Limited uterine artery embolization for leiomyomas with tris-acryl gelatin microspheres: 1-year follow-up. J Vasc Interv Radiol. 2006;17:283–287.
- Hald K, Noreng HJ, Istre O, et al. Uterine artery embolization versus laparoscopic occlusion of uterine arteries for leiomyomas: long-term results of a randomized comparative trial. J Vasc Interv Radiol. 2009;20:1303–1310.
- Volkers NA, Hehenkamp WJ, Birnie E, et al. Uterine artery embolization in the treatment of symptomatic uterine fibroid tumors (EMMY trial): periprocedural results and complications. J Vasc Interv Radiol. 2006;17:471–480.
- Dueholm M, Langfeldt S, Mafi HM, et al. Re-intervention after uterine leiomyoma embolisation is related to incomplete infarction and presence of submucous leiomyomas. Eur J Obstet Gynecol Reprod Biol. 2014;178:100–106.
- Moss JG, Cooper KG, Khaund A, et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011;118:936–944.
- Spies JB, Myers ER, Worthington-Kirsch R, et al. The FIBROID Registry: symptom and quality-of-life status 1 year after therapy. Obstet Gynecol. 2005;106:1309–1318.
- Berman JM, Guido RS, Garza Leal JG, et al. Three-year outcome of the Halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. J Minim Invasive Gynecol. 2014;21:767–774.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421–432.
- Berman JM, Bolnick JM, Pemueller RR, et al. Reproductive outcomes in women following radiofrequency volumetric thermal ablation of symptomatic fibroids. a retrospective case series analysis. J Reprod Med. 2015;60:194–198.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid: a case report. J Reproduct Med. 2012;57:159–163.
- Garza-Leal JG. Normal pregnancy outcome after transcervical radiofrequency ablation of uterine fibroids. J Minim Invasive Gynecol. 2015;22:S237–S2S8.
- Keltz J, Levie M, Chudnoff S. Pregnancy outcomes following direct uterine fibroid thermal ablation: a review of the literature. J Minim Invasive Gynecol. 2017;24:538–545.
- Goldberg J, Pereira L. Pregnancy outcomes following treatment for fibroids: uterine fibroid embolization versus laparoscopic myomectomy. Curr Opin Obstet Gynecol. 2006;18:402–406.
- Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869–872.
- McLucas B. Pregnancy following uterine artery embolization: An update. Minim Invasive Ther Allied Technol. 2002;100(5 Pt 1):869–872.
- Pisco JM, Duarte M, Bilhim T, et al. Pregnancy after uterine fibroid embolization. Fertil Steril. 2010.