 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction: Microwave ablation (MWA) uses heat to ablate undesired tissue. Development of pre-planning algorithms for MWA of small renal masses requires understanding of microwave-tissue interactions at different operating parameters. The objective of this study was to compare the performance of two MWA systems in in-vivo porcine kidneys.
Methods: Five ablations were performed using a 902–928 MHz system (24 W, 5 min) and a 2450 MHz system (180 W, 2 min). Nonlinear regression analysis of temperature changes measured 5 mm from the antenna axis was completed for the initial 10 s of ablation using the power equation and after the inflection point using an exponential equation. Thermal damage was calculated using the Arrhenius equation. Long and short axis ablation diameters were measured.
Results: The average ‘a’ varied significantly between systems (902–928 MHz: 0.0299 ± 0.027, 2450 MHz: 0.1598 ± 0.158), indicating proportionality to the heat source, but ‘b’ did not (902–928 MHz: 1.910 ± 0.372, 2450 MHz: 2.039 ± 0.366), signifying tissue type dependence. Past the inflection point, average steady-state temperature increases were similar between systems but reached more quickly with the 2450 MHz system. Complete damage was reached at 5 mm for both systems. The 2450 MHz system produced significantly larger short axis ablations (902–928 MHz: 2.40 ± 0.54 cm, 2450 MHz: 3.32 ± 0.41cm).
Conclusion: The 2450 MHz system achieved similar steady state temperature increases compared to the 902–928 MHz system, but more quickly due to higher output power. Further investigations using various treatment parameters and precise thermal sensor placement are warranted to refine equation parameters for the development of an ablation model.
1. Introduction
According to the American Urological Association (AUA) guidelines, the current gold standard of care for small renal masses (SRM) 4 cm or less is the partial nephrectomy, in which the tumor plus a small rim of surrounding healthy tissue is excised [Citation1]. This treatment is a traumatic procedure in which the hilum is clamped to prevent hemorrhage, resulting in warm ischemia [Citation2–5]. A skilled physician has less than 40 min to excise the SRM and perform a renorrhaphy [Citation6]. An alternative therapeutic modality that is less traumatic to the patient would be beneficial, especially for those patients who are elderly, have a solitary kidney, or have other co-morbidities. For such patients, needle-ablative therapies are considered an alternative [Citation1]. Needle-ablative therapies utilize energy emitted from a needlelike probe to ablate the target tumor tissue. The needlelike probe is inserted into the tumor site under image guidance, such as ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI). These therapeutic modalities have the advantage of less trauma, shorter surgical and hospitalization times, reduced cost and lower risk of infection without having to subject the kidney to warm ischemia [Citation7].
Two methods currently dominant in clinical use for SRM are radiofrequency ablation (RFA) [Citation8–13] and cryotherapy (CRYO) [Citation14–17]. RFA uses heat induced by the tissue resistance to RF current; however, the heating is highly susceptible to convective heat loss due to blood flow [Citation18]. CRYO uses cycles of freezing and thawing, but when areas greater than 3 cm are treated, cracking of the ice ball with subsequent hemorrhage can occur, requiring the use of hemostatic agents [Citation16,Citation17].
Microwave ablation (MWA) is a minimally-invasive thermal treatment modality that also uses hyperthermia to treat the target tumor site. Heat is induced by the interaction of the tissue with microwave radiation at 915 MHz or 2450 MHz emitted from a coaxial [Citation19] or triaxial [Citation20] antenna. MWA offers many theoretical advantages over RFA and CRYO. MWA has a larger zone of direct heating and is thus less susceptible to heat sink effects due to blood flow when compared to RFA [Citation20–23]. Unlike RFA, no grounding pads are needed. MWA is also more ergonomic to use than CRYO, requiring smaller, transportable systems with no large gas canisters [Citation23,Citation24].
MWA cannot be considered a nephron-sparing, gold standard of care for SRM, though, until its oncological outcomes are similar or better than those achieved with a partial nephrectomy. Despite its advantages and feasibility, oncological outcomes using MWA have been inconsistent [Citation21,Citation25–34], in part due to the lack of pre-planning specific to the kidney and the MWA system being used. Microwave-tissue interaction varies as a result of the frequency-dependence of dielectric properties [Citation24]. For instance, at 37 °C, the permittivity and electric conductivity are 58.6 and 1.40 S/m at 915 MHz and 52.7 and 2.43 S/m at 2450 MHz [Citation35]. Aside from frequency, different aspects of energy-delivery for each system, including the energy-delivery algorithm and antenna construction, will also vary and are usually designed in order to maximize energy-delivery within the tissue while minimizing charring. Therefore, each system would require their own treatment pre-planning algorithm that would predict the optimal treatment parameters necessary to achieve a nephron-sparing treatment of various sized tumors. Optimal pre-planning, in turn, requires that the relationship between these treatment parameters (output power, irradiation time) and the temperature profile and ablation geometry within the kidney be defined for each MWA system employed.
The purpose of this study is to analyze and compare the performance in in-vivo porcine kidneys of two commercially available MWA systems. Our investigation highlights temperature distribution and ablation parameters that must be taken into account when developing pre-planning models for each system. We purposely chose two systems with different central operating frequencies (915 and 2450 MHz). Due to their operational differences (frequency, power, irradiation time, treatment algorithm, coaxial antenna design, etc.), each system used in this investigation was set to the maximum power recommended by the manufacturers for treatment of renal tissue. Rate of heating and time to cell damage in the region near the antenna tip were assessed and the ablation sizes compared.
2. Methods and materials
2.1. In-vivo animal procedures
Nine female Yorkshire pigs, ranging from 35 to 45 kg, were treated under an IACUC approved protocol. Each animal was subjected to general anesthesia via Isoflurane inhalation at 2–4%, in conjunction with telazol (1.4 mg/kg), and xylazine (2 mg/kg). Once anesthetized, each kidney was exposed via a midline incision. The exposed kidney was placed into an acrylic immobilization apparatus which allowed a microwave antenna to be inserted into the kidney orthogonal to fiber optic thermal sensors (). The antenna needle was inserted into the kidney at either the upper or lower pole, parallel to the Brodel’s line, and between the cortex surface and the collecting system boundary. The fiber optic thermal sensors were connected to a Focal Pointe or m33300 (LumaSense, Inc., Santa Clara, CA) fiber optic thermal monitoring system and inserted into the kidney through a grid on the surface of the apparatus. Temperatures during ablations and for five minutes after treatment were measured with the fiber optic thermal sensors (Fluoroptic Temperature Probe, LumaSense, Inc., Santa Clara, CA) placed in 5 mm intervals from the antenna axis (). It is assumed that the thermal sensors located at 5 mm were located within the direct heating zone and temperature changes are due to absorption of microwave energy [Citation36].
Figure 1. MWA in in-vivo porcine kidneys with fiber optic thermal sensors inserted through the acrylic apparatus perpendicular to the microwave antenna axis plane.
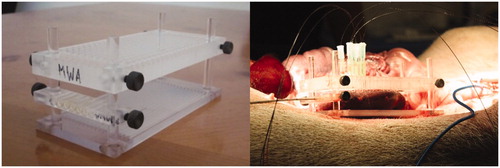
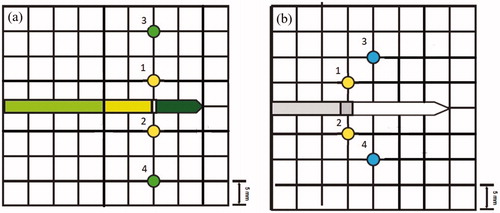
Figure 2. Location of the fiber optic thermal sensors relative to the MWA antenna axis (center of grid) for the (a) 902–928 MHz system and the (b) 2450 MHz system.
Both kidneys of each animal were ablated, with up to four ablations per animal (up to two per kidney). After ablation of both kidneys, the animal was euthanized, and the kidneys harvested for gross analysis of ablation zones. The temperatures recorded from each thermal sensor were stored and analyzed.
2.1.1. 902–928 MHz system procedure
Five ablations were performed with the AveCure MW system (Medwaves, San Diego, CA) which offers frequency variability from 902 to 928 MHz to minimize reflective power losses. The system consists of a generator and a 16-gauge single, straight coaxial antenna with a 2 cm active tip and a thermal sensor located at the proximal side of the emitting region of the antenna. The needle was inserted 4.0 cm into the kidney and fiber optic thermal sensors were placed at 5 and 15 mm from the antenna axis (). The recommended power control mode was used with a maximum output power of 24 W, a threshold temperature of 106 °C and a treatment time of 5 min. The chosen parameters were based on the manufacturer’s maximum parameter recommendations for treatment of renal tissue. In power mode, microwave energy is emitted until the antenna’s temperature sensor detects a local temperature equal to that of the threshold temperature. The irradiation is then paused until the temperature decreases to 30 °C below the threshold temperature, at which point the irradiation resumes. This continues until the total treatment time is reached. The term ‘902–928 MHz system’ is used to refer to the AveCure MW System in this text.
2.1.2. 2450 MHz system procedure
Five ablations were performed with the Acculis 2450 MHz MTA System (Denmead, Hampshire UK) consisting of a single saline-cooled (22 °C) 1.8 mm diameter coaxial antenna with a 2 cm emitting region coupled to a generator set at maximum output power of 180 W. Irradiation time was 2 minutes. The needle was inserted 4.0 cm into the kidney. The output power of 180 W was the maximum the generator was capable of at the time of this study and the 2-min irradiation time chosen was recommended by the manufacturer for treatment of renal tissue. Fiber optic thermal sensors were placed at 5 and 10 mm from the antenna axis (). The term ‘2450 MHz system’ is used to refer to the Acculis MTA System in this text.
2.2. Data analysis
2.2.1. Temperature analysis
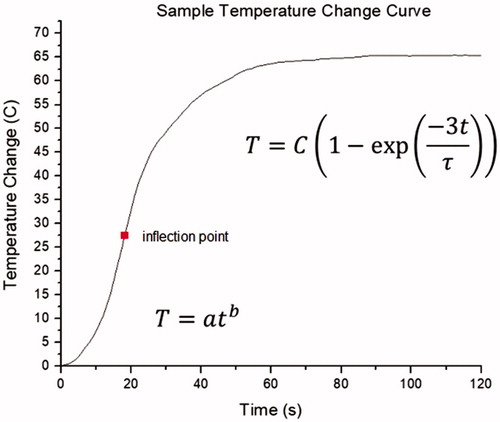
A non-linear regression analysis using the Levenberg–Marquardt algorithm (Origin 8, Northampton, MA) was performed for temperatures measured from two fiber optic thermal sensors located 5 mm from the puck region on either side of the antenna axis (thermal sensors 1 and 2 in ). Analysis was performed in two parts: within the initial 10 s of ablation and after the inflection point in the temperature measurements (). Regression for the initial 10 s was performed using a power equation:
(1)
(1)
where ΔT is the temperature increase above baseline temperature [°C], t is the time [s], and a and b are equation parameters. Regression for the temperature changes after the inflection point was performed using an exponential equation:
(2)
(2)
where C represents the steady-state temperature increase above the temperature at inflection [°C], and τ is the time constant needed to reach 95% of the steady-state temperature [s].
Figure 3. Example of the temperature change as a function of time during MWA in in-vivo porcine kidneys. The initial temperature rise can be described by a power equation. Following the inflection point, an exponential equation best fits the temperature changes. (Adapted from temperatures measured at 5 mm from the MWA antenna during an ablation using the Acculis 2450 MHz system).
2.2.2. Thermal damage
Thermal coagulation is a strong biomarker for irreversible cell damage. The percentage of cell damage due to increases in temperature over time for temperatures measured at 5 mm (both systems) and either 10 mm (2450 MHz) or 15 mm (902–928 MHz) from the antenna axis on both sides (thermal sensors 3 and 4 in ) of the antenna axis was calculated using the Arrhenius equation:
(3)
(3)
where Co and Ct are the quantity of cells alive prior to heating and during heating at time t, respectively, A is the frequency constant [s−1], is the temperature as a function of time [K],
is the energy of activation [J·mol−1], and R is the universal gas constant [8.314 J·mol−1K−1]. The frequency constant (A) and the energy of activation (
) depend on the tissue type being examined. The values of the frequency constant and energy of activation for renal cells are 1.48 × 1060 s−1 and 399.55 kJ/mol, respectively [Citation37]. Complete cell damage is assumed at 99.99%.
2.2.3. Ablation geometry
After ablation, the kidneys were dissected for gross analysis of the coagulation zones. The hemorrhagic and necrotic lesion dimensions were measured along the long and short axis diameters using a caliper. The long axis diameter was defined as the ablation length along the axis of the MWA antenna and the short axis diameter as perpendicular to the antenna. The circularity, which is a measure for the roundness of the ablation zone, was calculated as the ratio of the short axis diameter to the long axis diameter. A circularity of 1 describes perfect roundness, a circularity <1 describes an ellipsoid with the long axis along the antenna axis and a circularity >1 describes a flattened sphere [Citation20].
2.2.4. Statistical analysis
Quantitative data are presented as mean ± standard deviation. The average overall maximum temperatures were determined for each system. Maximum temperatures, equation parameters, thermal damage and ablation zone sizes and circularity were compared between the two MWA systems. Results were compared using unpaired t-tests (p < 0.05).
3. Results
All animals survived the procedures. Two animals experienced complications during the ablation procedure. During trial 1 of the 902–928 MHz experiments, the animal had a full bladder and was experiencing tachycardia until the contents of the bladder were partially drained. During trial 5 of the 902–928 MHz experiments, the animal exhibited a faint pulse.
3.1. Temperature changes during ablation
The mean maximum temperatures achieved overall at 5 mm from the antenna axis were 98.12 ± 4.82 °C for the 902–928 MHz system and 105.99 ± 2.17 °C for the 2450 MHz system, which are significantly different (p = 0.0115).
3.1.1. Non-Linear regression for initial 10 s
The average maximum temperature measured after 10 s was higher for the 2450 MHz system (51.64 ± 15.31 °C) when compared to the 902–928 MHz system (37.09 ± 1.06 °C) (). The power equation (EquationEquation (1)(1)
(1) fit the data for both systems well (R > 0.99), although the 902–928 MHz system generated a much slower rise in temperature than the 2450 MHz system, as can be seen in a comparison of the scale of the temperature rises in the sample curves in . The b equation parameters were similar between both systems. The difference in the a equation parameters were statistically significant between systems (p = 0.0195), with the a parameter being larger for the 2450 MHz system than the 902–928 MHz system at 5 mm from the antenna axis.
Figure 4. Sample non-linear regression of temperature increases using the power equation (EquationEquation (1)(1)
(1) ) for the initial 10 s of MWA using the (a) 902–928 MHz system and the (b) 2450 MHz system at 5 mm from the microwave antenna axis.
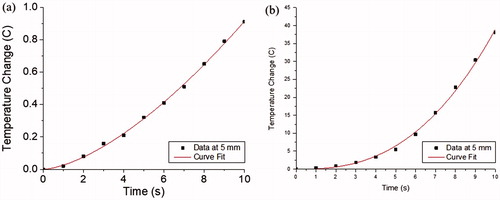
Figure 5. Non-linear regression of temperature increases at 5 mm during MWA using the (a) 902–928 MHz system and the (b) 2450 MHz system performed after the inflection point (EquationEquation (2)(2)
(2) ).
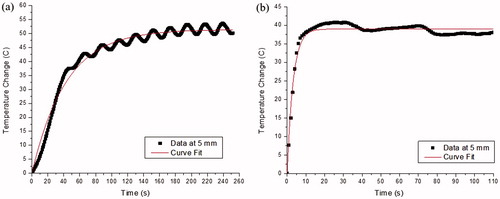
Table 1. Non-linear regression parameters for temperature changes during the initial 10 s of ablation at 5 mm from the antenna axis.
3.1.2. Non-linear regression after inflection point
EquationEquation (2)(2)
(2) proved to be a good fit for temperature changes induced by both the 902–928 MHz system (R = 0.93 ± 0.07) and the 2450 MHz system (R = 0.98 ± 0.01) (). Although the average temperatures at the inflection point were higher for the 2450 MHz system, the average steady-state temperature increase parameter, C, values were similar between both systems at 5 mm (). The value of τ represents the time to reach 95% of the asymptotic temperature change. The τ value for the 902–928 MHz system is larger than for the 2450 MHz system and the differences are statistically significant (p = 0.0015). The 2450 MHz system uses higher power settings than the 902–928 MHz system, and temperature changes were achieved more rapidly.
Table 2. Non-linear regression parameters for temperature changes following the time of inflection at 5 mm from the antenna axis.
3.2. Thermal damage
Complete cell damage (99.99%) was achieved at 5 mm during irradiation using both systems. With the exception of a single thermal sensor during one trial, complete cell damage was achieved at each thermal sensor location at 10 mm using the 2450 MHz system experiments. Only two of the thermal sensors located at 15 mm reached complete cell damage during two trials using the 902–928 MHz system. A summary of the time to damage is given in . The time necessary to achieve complete cell damage was significantly less with the 2450 MHz system than with the 902–928 MHz system (p = 0.0004).
Table 3. Time to achieve 99.99% cell damage at each thermal sensor location during MWA using a 902–928 MHz and a 2450 MHz system.
3.3. Ablation geometry
Ablation zones were elliptical in shape. Mean long and short axis diameters were 3.33 ± 0.67 cm and 2.40 ± 0.54 cm using the 902–928 MHz system and 4.09 ± 0.89 cm and 3.32 ± 0.41 cm for the 2450 MHz system. Only the short axis diameters were significantly different between the two systems (p = 0.016). Mean circularity values were 0.72 ± 0.06 for the 902–928 MHz system and 0.83 ± 0.12 for the 2450 MHz system and were not significantly different between the systems ().
Table 4. Ablation size and circularity following MWA using a 902–928 MHz and a 2450 MHz system.
4. Discussion
MWA at both 915 and 2450 MHz is currently utilized as a minimally-invasive treatment for small renal tumors, but it cannot yet be considered a gold standard of care until the oncological and physiological outcomes are similar to or better than current extirpative techniques. This can be achieved when the optimal treatment parameters necessary to attain a desired ablation size with minimal risk of collateral damage can be determined. However, due to the difference in frequency and energy delivery used by each MWA system, predicting the optimal system-specific treatment parameters requires knowledge of the relationship between the treatment parameters and the resulting temperature distribution and thermal ablation. This relationship is expected to be MWA system dependent.
In this investigation, we aimed to determine the difference in performance of two MWA systems operating at different frequencies and treatment parameters, the Acculis 2450 MHz system and the MedWaves AveCure 902–928 MHz system, by measuring and analyzing the temperatures within the surrounding in-vivo porcine kidneys during MWA and the resulting ablation zones. Aside from both being coaxial, the antennas for each system are designed differently with only the 2450 MHz system using cooling to limit antenna shaft heating and tissue charring. In-vivo porcine kidneys were chosen as a good mimic of human in-vivo kidneys due to their similarity in shape and structure. The systems also utilize their own algorithms within their generators in order to maximize the amount of energy delivered to the tissue through their antennas while minimizing charring. In order to compare the effects of the different systems, the treatment parameters were chosen based on the manufacturer’s recommendations for ablation of renal tissue.
Power reflected back along the antenna was not directly monitored during each ablation, but it was observed that the power delivered to the tissue was slightly lower than the selected output power. For example, the display of the 2450 MHz system generator showed the selected power along with actual output power, indicating that the system measure reflected power. It was observed that the actual output power was lower than the selected output power of 180 W. The 902–928 MHz system also measured reflected power. The system is designed so that ablation is paused if reflected power is greater than 12 W. If this occurs, the user selects the option to resume the ablation. This system also modulates power based on the temperature detected by the thermal sensor located on the antenna, resulting in an average output power lower than 24 W.
The temperature distribution in tissue due to absorption of microwave energy can be obtained by solving the Pennes bioheat equation [Citation38]:
(4)
(4)
where ρ [kg/m3] is mass density, c [J/kg K] is specific heat capacity, k [W/m K] is thermal conductivity, T [K] is temperature, Q [W/m3] is the source term due to absorbed electromagnetic energy, Qp is heat loss due to microvascular blood perfusion and Qm is metabolic heat generation, which is generally negligible.
For irradiation time much less than the thermal relaxation time, the diffusion term of the bioheat equation can be considered negligible. If the source term is assumed to be constant with time and there is no change in the electric field, the bioheat equation becomes a linear equation, given by the following [Citation39]:
(5)
(5)
where To [K] is the baseline initial temperature prior to ablation. Under this assumption, it would be expected that the temperature increases within the energy field would behave linearly.
However, a linear response was not observed during any of the trials at both 902–928 MHz and 2450 MHz. Assuming no measurement artifact from the fiber-optic thermal sensors, the initial temperature change within the first 10 s of MWA at 5 mm from the antenna axis proved to be non-linear. The relationship between the change in temperature and time, as given by EquationEquation (1)(1)
(1) , proved to be quite accurate with our experimental findings. Using the Levenberg-Marquardt regression algorithm, the curve fit using EquationEquation (1)
(1)
(1) had an average R-squared value of 0.99. The best fit of a power function has also observed in other MWA investigations [Citation36], indicating the source term cannot be assumed to be constant. According to our non-linear regression analysis, the a equation parameter was significantly larger using the 2450 MHz system, yet the b equation parameter varied little between systems. This indicates that the a parameter is proportional to the source term, while the b parameter is proportional to the tissue properties.
Following the non-linear temperature rise in accordance with EquationEquation (1)(1)
(1) , an inflection point was reached. Beyond this inflection point, the temperature continues to increase, yet the rate begins to slow in accordance with EquationEquation (2)
(2)
(2) . According to the Levenberg-Marquardt regression algorithm, the curve fit of the temperature change as a function of time after the inflection point exhibited average R-squared values of 0.93 and 0.98 for the 902–928 MHz and 2450 MHz systems, respectively. The average steady-state temperature increases from the temperature at inflection were similar between the two systems, signifying that the steady-state temperature changes reached after a long irradiation time would be similar. This is further indicated by the near-similar actual average maximum temperatures at 902–928 MHz (98.12 °C) and 2450 MHz (105.99 °C). However, the average time constant, τ, was significantly different between the 902–928 MHz (184.7 s) and 2450 MHz (47.8 s) systems, signifying that the temperature rise at the onset of irradiation is greater with the 2450 MHz system.
In many cases, the maximum temperatures reached for both systems were greater than the calculated steady-state temperature found by adding the temperature at inflection to the steady-state temperature increase. Temperatures using 902–928 MHz system would oscillate around the calculated steady state temperature because of the power-switching algorithm used by the system operating in power mode. Temperatures at 2450 MHz would eventually decrease with time following the initial rapid rate of heating due to heat conduction and then hover around the calculated steady-state value.
The 2450 MHz system achieved more rapid increases in temperature over a shorter period of time when compared to the 902–928 MHz system. The resulting temperature gradients within the tissue are much larger with the 2450 MHz system and limit the ability to directly compare the two systems beyond 5 mm from the antenna axis where heat conduction is the predominant source of temperature elevations in the tissue. At 5 mm, the thermal sensors are assumed to be within the energy field where temperature changes result from absorption of the microwave energy (direct heating zone).
One of the theoretical benefits of MWA over other thermal ablative modalities, such as RFA, is that it is not impacted by the heat sink effect due to the large zone of direct heating created by the energy field [Citation20]. This advantage is only applicable in the regions nearest to the antenna where the field penetrates. Further from the antenna axis, changes in the tissue temperature occur as a result of heat conduction and are subject to the heat sink effect, in which the blood can carry the heat away from the tissue. This concept was demonstrated through the case of the animal with a faint pulse. The decreased rate of blood flow through the kidney resulted in larger ablation diameters. Higher temperatures further from the antenna axis were most likely induced because lower volumes of blood were carrying away heat. Studies comparing MWA in ex-vivo and in-vivo kidneys have shown similar results, with larger ablation in ex-vivo kidneys due to lack of perfusion [Citation40]. Therefore, it is important to account for blood flow through both large and small vessels when predicting temperature profiles and ablation zones during MWA
Arrhenius analysis considers temperature change history to determine the tissue damage percentage at any given time during the ablation. Complete damage (99.99%) was achieved within the irradiation time at 5 mm for both systems, but the 2450 MHz system reached complete cell damage significantly faster. In an attempt to measure the extent of cell damage further from the antenna axis for both system, temperatures were also measured at 10 (2450 MHz) and 15 mm (902–928 MHz). Different distances were used under the assumption that the microwaves at a frequency of 902–928 MHz would travel further into the tissue [Citation24]. According to our Arrhenius equation analysis at these locations, one temperature measurement location at 15 mm from the antenna axis did not reach 99.9% cell damage within the 5-min irradiation time, while its conjugate location achieved cell damage only shortly after the irradiation time. This observation strongly indicates the possibility that the average ablation short axis dimension achieved at 902–928 MHz falls within 3 cm diameter, as confirmed by gross examination of the short axis ablation diameter. Complete damage was reached at both temperature measurement locations at10 mm within the 2-min irradiation time using the 2450 MHz system. This corresponds with the average short axis diameter exceeding well past 2 cm. Both systems produced ablation zones that were ellipsoid in shape. The long axis diameters were similar for each system, due to both microwave antenna having the same emitting length of 2 cm.
Faster heating rates resulting in more rapid cell damage and larger short axis ablation diameters using the 2450 MHz system can be most likely attributed to the higher output power used. This suggests that the performance of an MWA system is a consequence of a combination of ablation parameters, such as the frequency, antenna length and design, power output, irradiation time, and generator ablation algorithms.
Some of the data displayed large standard deviations. There are several possible contributing factors to variability in the equation parameters, damage times, and ablation sizes. Temperature changes due to microwave absorption impact tissue properties and water content, contributing to nonlinear temperature changes within the microwave field. Although data is limited for the range of frequencies used in MWA, coefficients of dielectric property changes as a function of temperature up to 60 °C have been determined from measured data for kidney [Citation41]. For example, relative conductivity increases slightly with temperature at 900 MHz with a temperature coefficient of approximately 1.3 [% C−1]. At temperatures above 60 °C, changes to tissue dielectric properties become irreversible. Similar to electromagnetic properties, thermal tissue properties, such as thermal conductivity, specific heat capacity and density, also vary as function of temperature and water content. Energy absorption in tissue is impacted most greatly by water content [Citation24]. As water evaporates, the tissue becomes less lossy, which results in greater penetration of microwaves. As a result, the radiation pattern may change significantly during the course of an ablation. Variations in curve fit equation parameters values may be due to variations in tissue structure and water content in the region near the antenna.
Shifting of the thermal sensor positions relative to the antenna axis may have also contributed to variability in calculated equation parameters. Although the thermal sensors were placed through a tract created by a catheter needle, the optical fibers are flexible and may have shifted during the ablation due to changes in tissue shape and size. The kidney is a soft organ and was compressed slightly to fit securely into the holding apparatus. As the tissue was heated, it contracted, causing displacement of the sensors relative to the antenna. In some instances, it was seen that the catheter holding the thermal sensors were pushed out of the kidney. More rigid thermal sensors, such as metal thermocouples are available, but their use disrupts the microwave field and introduces artifacts into the temperature data, a phenomenon known as the thermocouple effect [Citation42].
5. Conclusions
The 2450 MHz system operated at a higher output power and achieved more rapid increases in temperature over a shorter period of time when compared to the 902–928 MHz system, resulting in larger temperature gradients and ablation diameters. In addition to differences in MWA system designs, there are differences in tissue interaction between 915 MHz and 2450 MHz frequencies which need to be considered during preplanning. Effective preplanning algorithms that take into account antenna design, frequency, power, treatment time, and location of tumor are essential for nephron-sparing ablation of small renal masses during MWA. There may be several other benefits to the development of a comprehensive computer model, including exploring device designs, parameter selections, different treatment delivery strategies, and inter-patient variation in tissue/tumor properties. This would aid in the development of robust microwave delivery. Further investigations using various treatment parameters and precise placement of thermal sensors are warranted to refine equation parameters and apply equations to the development of an ablation model.
Acknowledgment
We would like to acknowledge Medwaves, San Diego, CA for providing the AveCure system and antennas and Microsulis for providing the Acculis system and antennas.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271.
- Allaf ME, Bhayani SB, Rogers C, et al. Laparoscopic partial nephrectomy: evaluation of long-term oncological outcome. J Urol. 2004;172:871–873.
- Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46.
- Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177:70–74.
- Patel MN, Menon M, Rogers CG, editors. Robotic partial nephrectomy: a comparison to current techniques. Urologic Oncology: Seminars and Original Investigations; 2010: Elsevier.
- Ramanathan R, Leveillee RJ. A review of methods for hemostasis and renorrhaphy after laparoscopic and robot-assisted laparoscopic partial nephrectomy [journal article]. Curr Urol Rep. 2010;11:208–220.
- Kutikov A, Kunkle DA, Uzzo RG. Focal therapy for kidney cancer: a systematic review. Curr Opin Urol. 2009;19:148–153.
- Bird VG, Carey RI, Ayyathurai R, et al. Management of renal masses with laparoscopic-guided radiofrequency ablation versus laparoscopic partial nephrectomy. J Endourol. 2009;23:81–88.
- Carey RI, Leveillee RJ. Direct real-time temperature monitoring for laparoscopic and CT-guided radiofrequency ablation of renal tumors between 3 and 5 cm. J Endourol. 2007;21:807–813.
- Gervais DA, Arellano RS, Mueller PR. Percutaneous radiofrequency ablation of renal cell carcinoma. Eur Radiol. 2005;15:960–967.
- Gervais DA, McGovern FJ, Arellano RS, et al. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424.
- Wingo MS, Leveillee RJ. Central and deep renal tumors can be effectively ablated: radiofrequency ablation outcomes with fiberoptic peripheral temperature monitoring. J Endourol. 2008;22:1261–1268.
- Zagoria RJ. Imaging-guided radiofrequency ablation of renal masses. Radiographics. 2004;24:S59–S71.
- Badger WJ, de Araujo HAM, Kuehn DM, et al. Laparoscopic renal tumor cryoablation: appropriate application of real-time ultrasonographic monitoring. J Endourol. 2009;23:427–430.
- Hinshaw JL, Shadid AM, Nakada SY, et al. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR Am J Roentgenol. 2008;191:1159–1168.
- Hruby G, Edelstein A, Karpf J, et al. Risk factors associated with renal parenchymal fracture during laparoscopic cryoablation. BJU Int. 2008;102:723–726.
- Lehman DS, Hruby GW, Phillips CK, et al. Laparoscopic renal cryoablation: efficacy and complications for larger renal masses. J Endourol. 2008;22:1123–1128.
- Gervais DA, McGovern FJ, Wood BJ, et al. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–672.
- Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61–67.
- Laeseke PF, Lee FT, Sampson LA, et al. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009;20:1224–1229.
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72:124–131.
- Liang P, Wang Y, Zhang D, et al. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol. 2008;180:844–848.
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25:S69–S83.
- Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng. 2010;38:65–78.
- Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology. 2011;77:792–797.
- Castle SM, Salas N, Leveillee RJ. Radio-frequency ablation helps preserve nephrons in salvage of failed microwave ablation for a renal cancer in a solitary kidney. Urol Ann. 2013;5:42.
- Guan W, Bai J, Liu J, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012;106:316–321.
- Carrafiello G, Mangini M, Fontana F, et al. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:367–374. 2010;/04/01 English.
- Genson P-Y, Mourey E, Moulin M, et al. Image-guided percutaneous microwave ablation of small renal tumours: short- and mid-term outcomes. Quant Imaging Med Surg. 2015;5:649–655.
- Horn JC, Patel RS, Kim E, et al. Percutaneous microwave ablation of renal tumors using a gas-cooled 2.4-GHz probe: technique and initial results. J Vasc Interv Radiol. 2014;25:448–453.
- Lin Y, Liang P, Yu X-l, et al. Percutaneous microwave ablation of renal cell carcinoma is safe in patients with a solitary kidney. Urology. 2014;83:357–363.
- Moreland AJ, Ziemlewicz TJ, Best SL, et al. High-powered microwave ablation of T1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol. 2014;28:1046–1052.
- Wells SA, Wheeler KM, Mithqal A, et al. Percutaneous microwave ablation of T1a and T1b renal cell carcinoma: short-term efficacy and complications with emphasis on tumor complexity and single session treatment [journal article]. Abdom Radiol. 2016;41:1203–1211.
- Yu J, Zhang G, Liang P, et al. Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radial nephrectomy for small renal cell carcinoma. Abdom Imaging. 2015;40:3248–3256.
- Gabriel S, Lau R, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41:2271.
- Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol. 2013;36:505–511.
- He X, McGee S, Coad JE, et al. Investigation of the thermal and tissue injury behaviour in microwave thermal therapy using a porcine kidney model [Article]. Int J Hyperthermia. 2004;20:567–593.
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1:93–122.
- Salas N Jr, Manns F, Milne PJ, et al. Thermal analysis of laser interstitial thermotherapy in ex vivo fibro-fatty tissue using exponential functions. Phys Med Biol. 2004;49:1609.
- Marcelin C, Leiner J, Nasri S, et al. In vivo percutaneous microwave ablation in kidneys: correlation with ex vivo data and ablation work. Diagn Interv Imaging. 2018;99:3–8.
- Duck FA. Physical properties of tissue: a comprehensive reference book. San Diego (CA): Academic Press Inc.; 1990.
- Manns F, Milne PJ, Gonzalez-Cirre X, et al. In situ temperature measurements with thermocouple probes during laser interstitial thermotherapy (LITT): quantification and correction of a measurement artifact. Lasers Surg Med. 1998;23:94–103.
