Abstract
Objective: To assess the safety and efficacy of ultrasound-guided microwave ablation (MWA) in the treatment of patients who develop secondary hyperparathyroidism (SHPT) after renal transplantation (RT).
Methods: In total, nine patients, each with symptomatic SHPT caused by RT and at least one enlarged parathyroid gland, underwent MWA via hydrodissection. Intact parathyroid hormone (i-PTH), serum calcium, serum phosphorus, creatinine and blood urea nitrogen concentrations, before and after MWA, were assessed and compared.
Results: Complete ablation was achieved in all patients for a total of 14 ablated parathyroid glands. The mean follow-up time was 17.2 ± 1.7 months post-operation. The mean maximum diameter of the parathyroid glands was 1.3 ± 0.4 cm (range: 0.4–2.0 cm). The ablation power implemented was 30 W and the mean time for each parathyroid gland to achieve complete ablation was 287.5 ± 83.4 s. The mean i-PTH, serum calcium and phosphorus concentrations at one day post-MWA (69.6 pg/mL, 2.23 ± 0.29 mmol/L, 1.2 2 ± 0.48 mmol/L, respectively) were significantly lower than those before MWA (780.0 pg/mL, 2.62 ± 0.32 mmol/L, 1.39 ± 0.61 mmol/L, respectively; p < .01), whereas the creatinine and blood urea nitrogen concentrations before and after MWA did not differ significantly from each other (p > .05). No significant differences were found between the biomarker concentrations observed at one day post-MWA and at the follow-ups (p > .05). No major operation-related complications occurred.
Conclusion: Ultrasound-guided MWA is a safe and effective technique for destroying parathyroid gland tissue in patients who develop SHPT after RT and its clinical effects are long-lasting.
Introduction
Secondary hyperparathyroidism (SHPT) is a condition in which an excess of parathyroid hormone (PTH) is produced due the enlargement and hyperactivity of parathyroid glands, usually as a response to hypercalcemia, and it frequently occurs after renal transplantation (RT). It is a condition that increases the risk of developing a variety of kidney-related disorders, such as renal osteodystrophy, calcific uremic arteriolopathy (a syndrome of vascular calcification, thrombosis and skin necrosis) and vascular calcification. SHPT can also cause graft dysfunctions, such as tubulointerstitial calcification in renal allografts, which negatively affects patients’ prognoses [Citation1–3].
Although SHPT progression and its complications can be managed with medicine, including oral vitamin D sterols [Citation4], intravenous vitamin D analogs [Citation5,Citation6] and cinacalcet [Citation7–9], parathyroidectomy is the standard treatment [Citation10,Citation11]. However, most patients either are unsuitable candidates for parathyroidectomy, or simply refuse to undergo the procedure.
Imaging-guided percutaneous ablation is a minimally invasive alternative to highly invasive surgeries and it is gradually being accepted into clinical settings due to its potential advantages over classic surgical treatments, such as having reduced risks, side effects and costs, faster recovery times, and more effective clinical results. Its modalities include radiofrequency ablation, microwave ablation (MWA), ethanolinjection ablation, acetic acid injection ablation, laser ablation and high-intensity focused ultrasound ablation [Citation12–23]. Among these modalities, MWA has been used to treat hyperparathyroidism due to its abilities in inducing high intratumoral temperatures, quickly reaching these high temperatures and producing high ablation volumes. MWA is also less dependent on the electrical conductivities of tissues and less limited by the electrical impedances of tumor tissues than other percutaneous ablation modalities [Citation13,Citation14,Citation20,Citation22]. Therefore, the purpose of this study was to evaluate the feasibility, safety and efficacy of MWA, since we believed MWA to be a viable technique for patients who develop SHPT after RT.
Materials and methods
Patient population
In total, nine patients who developed severe SHPT after RT and were treated at the Interventional Ultrasound Center between 1 January 2016 and 31 December 2017 were included in this study. This retrospective pilot study protocol was approved by the Human Ethics Review Committee of the hospital and written informed consent was obtained from every participant.
Definition of hyperparathyroidism and inclusion criteria
Hyperparathyroidism, in patients who have developed the condition after RT, was defined as having an intact parathyroid hormone (i-PTH) concentration greater than the upper normal limit (300 pg/mL, according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative) for 6 months [Citation15].
Participants were included in this study based on a set of inclusion criteria, which were as follows: (1) has a clear history of RT treatment; (2) has medication intolerance (uncontrolled SHPT with adequate medical therapy); (3) has an i-PTH concentration of more than, or equal to, 300 pg/mL, which is associated with high cardiovascular mortality rates (hazard ratio: 1.23; 95% confidence interval: 1.12–1.34) and all cause and cardiovascular hospitalization [Citation16]; (4) has at least one enlarged persistent SHPT nodule, with a maximum diameter of more than 0.6 cm and suitable for MWA treatment (no critical main blood vessel, nerve and esophagus structures in the needle’s path), that is detected by ultrasound; (5) has increased 99mTc-sestamibi accumulation in the affected parathyroid gland during both the early and delayed phases; (6) has a prothrombin time of less than, or equal to, 25s; (7) has a prothrombin activity rate of greater than 40%; (8) has a platelet count of greater than or equal to 40 × 109cells/L; (9) has no recurrent laryngeal nerve injuries as confirmed by laryngoscopy (for patients experiencing voice changes following a parathyroidectomy) and (10) has no intractable complications, such as cardiac insufficiency and hypertension, that cannot be controlled with drugs. If patients did not meet these criteria, their MWA was delayed until any abnormal results changed and conformed to the inclusion criteria. If this was not possible, these patients were excluded. Saline was injected into the surrounding parathyroid adenoma capsule with an ultrasound-guided 21-gauge PTC needle to protect nerves, vessels and other important structures from thermal injury.
Ultrasound-guided percutaneous MWA procedure
Ultrasound and MIBI scans were performed to locate and evaluate SHPT nodules and their surrounding anatomical structures before performing MWA [Citation18,Citation19]. The ultrasound examinations were performed with a 10.0-MHz transducer (Aplio 500, Toshiba, Tokyo, Japan) and produced real-time color Doppler images. The MIBI scans (Symbia T2, Siemens, Berlin, Germany) were conducted due to their high sensitivity (85%) and specificity (100%) in diagnosing SHPT [Citation24]. Contrast enhance ultrasound (CEUS) examinations using SonoVue (Bracco Company, Milan, Italy) were performed to evaluate the characteristics of enhancement for planning the distribution of ablation energy.
In order for MWA to be properly performed, patients were first put into the supine position with their necks extended and received minimal to moderate sedation via intravenous midazolam (Roche Laboratories, Nutley, NJ) and fentanyl (Cephalon, Frazer, PA). Then, after sterilizing the surgical site on the neck, we injected a local anesthetic, 2% lidocaine (Synera, Salt Lake City, UT), at the designated MWA puncture site. Next, lidocaine mixed with a normal saline solution (1:3, lidocaine concentration: 0.5%) was injected into the 0.5cm area surrounding the hyperplastic gland capsule to provide heat insulation and further induce local anesthesia by means of lidocaine’s effects on the recurrent laryngeal and vagal nerves [Citation25]. The volume of the lidocaine and saline solution used in this procedure ranged from 60 to 150 ml.
A microwave generator and a 17-gauge internally cooled antenna with a 0.4-cm needle tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China) were used to perform MWA. Typically, parathyroid glands are located posterolateral to the thyroid gland, although ectopic parathyroid glands can be located in the submandibular region, suprasternal fossae and anterior mediastinum [Citation17,Citation20]. Therefore, we inserted the antenna into these locations under ultrasound guidance. A 10-MHz linear probe attached to the antenna was used for parathyroid glands in the neck and a 3.5-MHz convex array probe was used for parathyroid glands in the suprasternal fossa and supra-anterior mediastinum. Moreover, we inserted the antenna laterally for parathyroid glands in the neck and suprasternal fossa and cranio-caudally for parathyroid glands in the supra-anterior mediastinum to avoid the clavicle [Citation26]. Before each ablation, the needle tip’s location in a parathyroid gland was confirmed via ultrasound. The power for ablation was kept at 30 W for the duration of the procedure. The needle tip was held in a quiescent state for 15–25 s, because SHPT glands are usually small, with a maximum diameter of approximately 1 cm, and this was repeated two to four times at intervals of 5 s to prevent heat injury to surrounding critical structures [Citation27]. After the ablation of one area (one ablation unit), the antenna was repositioned, under ultrasound guidance, to continue the ablation of the parathyroid gland. Ablation was terminated when transient hyperechoic echotexture was seen throughout the gland. After ablation, CEUS examinations were used to evaluate the extent of ablation. If a nonenhanced area covered the ablated gland, the ablation was considered complete. If nodular enhancement was observed inside the ablated gland, an additional MWA was performed immediately. After the complete ablation of one side of a parathyroid gland, MWA was continued for the other side. During this procedure, an anesthesiologist was present to assist the radiologist in cases of pain, vasovagal reactions and uncontrollable hematomas pressing on the trachea. The total ablation time was recorded for each patient. At the end of this procedure, a mild compression with bagged normal saline of 4 °C was applied to the site of the needle path for 20 min. All patients remained under observation for 2 h post-operation in case of complications [Citation17,Citation20].
Clinical data collection and follow-ups
Clinical data were collected for all patients. These data include the i-PTH, serum calcium, phosphate, blood creatinine and blood urea nitrogen concentrations before ablation and at 2 h, 24 h, 7 days, 1 month and 3 months post-ablation. From then on, biomarker concentration data were collected every 3 months. All data were obtained from the same laboratory. i-PTH is the main bioactive product of PTH which shows the best correlation with the production and biologic activity of the hormone. Furthermore, ultrasound examinations were performed by two authors at two and 24 h post-ablation to identify hematomas and any other post-operation complications. Major and minor complications were defined according to the criteria provided by the Society of Interventional Radiology [Citation28]; a major complication being an event that led to substantial morbidity and disability, an increased the level of care, or resulted in hospital admission or a substantially lengthened hospital stay. Any other complications were considered minor [Citation29].
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 for Windows (IBM SPSS Statistics, Armonk, NY). Continuous data were expressed as means ± standard deviation or medians. Continuous variables were analyzed by using t-tests or the Wilcoxon rank-sum test, as applicable. Paired-sample t-tests and paired-sample Wilcoxon signed-rank tests were used to compare pre-ablation and post-ablation treatment outcomes. The relationships between two variables were calculated using the Spearman rank correlation analysis method. A p value of <.01 indicated a significant difference.
Results
Patient and clinical characteristics
The baseline clinical characteristics, nodule parameters, operation data and laboratory test results, before and after MWA, of the enrolled patients are listed in . Nine patients (six men and three women; median age: 49.7 ± 9.6 years; range: 38–63 years) received ultrasound-guided MWA between 1 January 2016 and 31 December 2017. The mean time between RT and ablation was 45.2 ± 34.7 months. All patients had high serum i-PTH concentrations [mean concentration: 780.0 (320.8–1095.2) pg/mL; normal concentration: 15–88 pg/mL], high serum phosphorus concentrations [mean concentration: 1.40 ± 0.61 (0.98–2.49) mmol/L; normal concentration: 0.97–1.62 mmol/L] and high total calcium concentrations [mean concentration: 2.62 ± 0.32 (2.1–3.15) mmol/L; normal concentration: 2.03–2.54 mmol/L] levels. The mean serum i-PTH, calcium and phosphorus concentrations at one day post-MWA (69.6 pg/mL, 2.23 ± 0.29 mmol/L, 1.22 ± 0.48 mmol/L, respectively) were significantly lower than those before MWA (780.0 pg/mL, 2.62 ± 0.32 mmol/L, 1.39 ± 0.61 mmol/L, respectively; p < .01), whereas the creatinine and blood urea nitrogen concentrations before MWA and at one day post-MWA did not significantly differ from each other (p > .05). No significant differences were found between the biomarker concentrations observed at one day post-MWA and those observed at the follow-up periods (p > .05). There was no significant correlation between pre-MWA and post-MWA i-PTH, calcium, and phosphate concentrations (p > .05) (). The Spearman rank correlation analysis of the clinical characteristics at baseline and after MWA treatment showed no significant correlations (p > .05). All patients received long-term medicine treatment with various doses of oral calcitriol [mean dose: 0.25 ± 0.13 (0.125–0.5) mg/day] and/or calcium carbonate [mean dose: 2093.8 ± 900.2 (600–4000) mg/day].
Table 1. Baseline clinical characteristics of RT patients with SHPT treated with MWA.
Table 2. Laboratory data of RT patients with SHPT before or after MWA.
Five patients had one hyperplastic parathyroid gland, three had two glands and one had three glands. Of these, one parathyroid gland was located behind the middle of left thyroid, five parathyroid glands were located behind the lower pole of left thyroid, two parathyroid glands were located behind the upper pole of left thyroid, two parathyroid glands located behind the lower pole of right thyroid and four parathyroid glands located behind the upper pole of right thyroid. The maximum diameter of the parathyroid glands ranged from 0.4 to 2.0 cm (mean maximum diameter: 1.3 ± 0.4 cm). The pre-operation CEUS examinations showed uniform hyperenhancement in the arterial phase in six parathyroid glands and nonuniform hyperenhancement in eight parathyroid glands. The hyperenhancement observed was consistent with high parathyroid function.
Technical outcomes of MWA
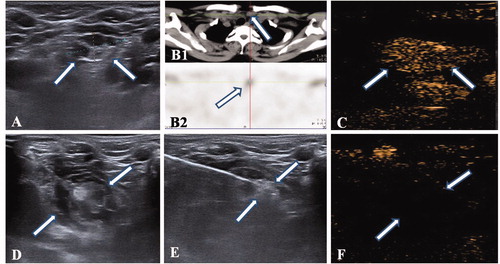
Complete ablation was achieved in all patients for a total of 14 ablated parathyroid glands. All patients were discharged at one day post-ablation. The mean follow-up time was 17.2 ± 1.7 months post-ablation. All 14 glands were treated with MWA by 10 sessions. The mean ablation time was 287.5 ± 83.4 s (range: 120–435 s) for a single parathyroid gland, and a single puncture site yielded 1–9 ablation units (mean ablation unit per puncture site: 6.1 ± 1.8). After MWA, all glands showed nonenhancement during the post-operation CEUS examination, which indicated complete ablation. shows a percutaneous MWA procedure. A hematoma formed during the ablation procedure of the patient with three hyperplastic parathyroid glands (10%), but it was treated successfully with local compression. No major operation-related complications occurred.
Figure 1. MWA of SHPT nodule in a patient who had undergone successfully renal transplantation. (A) The nonuniform hypoechoic nodule (Arrow) was disclosed by preablation ultrasound in neck. (B1) The CT scan showed there is a small nodule (Arrow) locating in the right front of trache, which corresponds to the one in ultrasound; (B2) The nodule has radioactivity concentration (Arrow) in late phase of MIBI scan. (C) An uniform hyperenhancement of nodule (Arrow) was displayed in CEUS preablation. (D) The spacer fluid was injected surrounding the nodule, which displayed non echo area (Arrow). (E)The hyperechoic emerging inside nodule (Arrow) during ablation. (F) A non-enhancement area covered the nodule (Arrow) after ablation, which suggested a complete ablation.
Discussion
Successful RT corrects many bone and mineral metabolism abnormalities caused by end-stage renal disease. However, these types of abnormalities do not always completely resolve in a significant number of patients, even after several years of restored renal function, and these abnormalities may result in the redevelopment of hyperparathyroidism [Citation30]. PTH, blood calcium and phosphorus concentrations usually increase considerably within the first year following RT, and high levels of PTH are associated with increased graft loss and mortality rates [Citation31]. Moreover, persistent secondary and tertiary hyperparathyroidism following transplantation has been observed in 32.9% of patients [Citation32]. In addition, hyperparathyroidism also aggravates hypercalcemia, which, in turn, can cause conditions, such as renal vasoconstriction and tubulointerstitial calcification, that have deleterious effects on allografts [Citation3]. Hyperparathyroidism also commonly leads to poor bone and cardiovascular outcomes, such as the loss of bone mass density, and increased fracture, calcification, and vascular event rates [Citation1,Citation33]. As for hyperparathyroidism treatment, current guidelines recommend the use of vitamin D and its analogs to correct post-transplantation hyperparathyroidism, but their use is often precluded by hypercalcemia [Citation34]. Surgical parathyroidectomy is also considered an effective approach, but it is associated with the same risks as anesthesia and the risk of nerve and vessel damage, incomplete resections, and permanent hypoparathyroidism [Citation35]. Recurrent diseases are also more difficult to approach surgically and the re-exploration of the neck in these situations is associated with an increased risk of complications.
Ultrasound-guided MWA was verified as feasible, safe and effective for destroying parathyroid gland tissue and maintaining normal blood calcium and phosphorus concentrations [Citation20]. Most patients that develop hyperparathyroidism due to RT are not good candidates for parathyroidectomy due to their inability to tolerate anesthesia. Minimally invasive ultrasound-guided MWA would be a better alternative in this case.
In our study, ultrasound-guided MWA was successfully performed in all patients, who all showed a high tolerance for this technique, and were all discharged at one day post-ablation. A hematoma did form during one of the ablation procedures, but was treated successfully with local compression. Furthermore, no nerve and vessel damage, skin burns, and uncontrolled bleeding occurred, and the lack of a significant difference between mean pre-MWA and post-MWA creatinine and blood urea nitrogen concentrations indicate no decline in renal function. These results suggested ultrasound-guided MWA is a safe, feasible and minimally invasive technique for the treatment of SHPT caused by RT.
In addition, ultrasound-guided MWA is associated with the long-term statistically significant decrease of PTH and calcium concentrations, and the normalization of phosphate concentrations. However, it was surprising that the decrease PTH and calcium concentrations at one day post-ablation lasted through the follow-up period. Although there was a slight increase, the post-MWA i-PTH concentrations were also within the normal range [Citation36]. A similar phenomenon was also observed with the phosphate concentrations. A reduction of PTH is believed to improve allograft, bone and cardiovascular outcomes in RT patients and an increase of phosphate concentrations toward the normal range may also be beneficial because post-transplantation hypophosphatemia, caused by the phosphaturic effect of PTH, is associated with a decrease in bone mineral density and low turnover bone disease [Citation37,Citation38]. These results indicate that ultrasound-guided MWA is a useful and efficient approach for treating SHPT caused by RT, and its clinical effects are long-lasting.
The results of this study should be interpreted with caution in light of several limitations. Firstly, this study is limited by its retrospective nature and the fact that the results are based on patients from one institution. Secondly, the sample size was relatively small, and the follow-up is limited. A larger sample and longer follow-up period are required to fully clarify the efficacy of ultrasound-guided MWA in treating SHPT caused by RT. Thirdly, the 99mTc-sestamibi sequence test was not repeated during the period to check the efficiency of MWA.
In conclusion, this pilot study provides initial evidence that ultrasound-guided MWA is safe and effective for treating patients who have developed SHPT after RT. This study may also help to standardize this minimally invasive alternative technique for patients with refractory drug-resistant SHPT or for patients who refuse and/or are not able to undergo parathyroidectomy. A further prospective study that enrolls a large sample size should be carried out to provide a more definitive conclusion.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Perrin P, Caillard S, Javier RM, et al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant. 2013;13:2653–2663.
- Mazzaferro S, Pasquali M, Taggi F, et al. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol. 2009;4:685–690.
- Gwinner W, Suppa S, Mengel M, et al. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant. 2005;5:1934–1941.
- Komaba H, Moriwaki K, Goto S, et al. Cost-effectiveness of cinacalcet hydrochloride for hemodialysis patients with severe secondary hyperparathyroidism in Japan. Am J Kidney Dis. 2012;60:262–271.
- Sezer S, Tutal E, Bal Z, et al. Differential influence of vitamin D analogs on left ventricular mass index in maintenance hemodialysis patients. Int J Artif Organs. 2014;37:118–125.
- Akizawa T, Akiba T, Hirakata H, et al. Comparison of paricalcitol with maxacalcitol injection in Japanese hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial. 2015;19:225–234.
- Zhang Q, Li M, You L, et al. Effects and safety of calcimimetics in end stage renal disease patients with secondary hyperparathyroidism: a meta-analysis. PloS One. 2012;7:e48070.
- Akizawa T, Kido R, Fukagawa M, et al. Decreases in PTH in Japanese hemodialysis patients with secondary hyperparathyroidism: associations with changing practice patterns. Clin J Am Soc Nephrol. 2011;6:2280–2288.
- Soliman AR, Maamoun HA, Soliman MA, et al. Cinacalcet versus parathyroidectomy in the treatment of secondary hyperparathyroidism post renal transplantation. Rom J Intern Med. 2016;54:184–189.
- Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339.
- Younes M, Belghali S, Zrour-Hassen S, et al. Complete reversal of tumoral calcinosis after subtotal parathyroidectomy in a hemodialysis patient. Joint Bone Spine. 2008;75:606–609.
- Kim BS, Eom TI, Kang KH, et al. Radiofrequency ablation of parathyroid adenoma in primary hyperparathyroidism. J Med Ultrason (2001). 2014;41:239–243.
- Diao Z, Liu X, Qian L, et al. Efficacy and its predictor in microwave ablation for severe secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2016;32:614–622.
- Zhao J, Qian L, Zu Y, et al. Efficacy of ablation therapy for secondary hyperparathyroidism by ultrasound guided percutaneous thermoablation. Ultrasound Med Biol. 2016;42:1058–1065.
- Chen HH, Lin CJ, Wu CJ, et al. Chemical ablation of recurrent and persistent secondary hyperparathyroidism after subtotal parathyroidectomy. Ann Surg. 2011;253:786–790.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia. 2016;32:180–186.
- Noussios G, Anagnostis P, Natsis K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Exp Clin Endocrinol Diabetes. 2012;120:604–610.
- Roy M, Mazeh H, Chen H, et al. Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. World J Surg. 2013;37:102–106.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282:576–584.
- Adda G, Scillitani A, Epaminonda P, et al. Ultrasound-guided laser thermal ablation for parathyroid adenomas: analysis of three cases with a three-year follow-up. Horm Res. 2006;65:231–234.
- Cao XL, Cheng ZG, Yu XL, et al. Ultrasound-guided percutaneous microwave ablation of parathyroid adenoma. J Vasc Interv Radiol. 2016;27:1929–1931.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24:2052–2058.
- Yang J, Hao R, Yuan L, et al. Value of dual-phase (99m)Tc-sestamibi scintigraphy with neck and thoracic SPECT/CT in secondary hyperparathyroidism. AJR Am J Roentgenol. 2014;202:180–184.
- Wang R, Jiang T, Chen Z, et al. Regression of calcinosis following treatment with radiofrequency thermoablation for severe secondary hyperparathyroidism in a hemodialysis patient. Intern Med. 2013;52:583–587.
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12:525–540.
- Monchik JM, Donatini G, Iannuccilli J, et al. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006;244:296–304.
- Cardella JF, Kundu S, Miller DL, et al.; Society of Interventional R. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–S191.
- Saad WE, Wallace MJ, Wojak JC, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21:789–795.
- Torres A, Lorenzo V, Salido E. Calcium metabolism and skeletal problems after transplantation. J Am Soc Nephrol. 2002;13:551–558.
- Gomes LK, Custodio MR, Contieri FL, et al. Persistent disorders of mineral metabolism after one year of kidney transplantation. J Bras Nefrol. 2016;38:282–287.
- Al-Moasseb Z, Aitken E. Natural History of Serum Calcium and Parathyroid Hormone Following Renal Transplantation. Transplant Proc. 2016;48:3285–3291.
- Pihlstrom H, Dahle DO, Mjoen G, et al. Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation. 2015;99:351–359.
- Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311.
- Torres PU, Prie D, Beck L, et al. New therapies for uremic secondary hyperparathyroidism. J Ren Nutr. 2006;16:87–99.
- Urena P, Jacobson SH, Zitt E, et al. Cinacalcet and achievement of the NKF/K-DOQI recommended target values for bone and mineral metabolism in real-world clinical practice–the ECHO observational study. Nephrol Dial Transplant. 2009;24:2852–2859.
- Weisinger JR, Carlini RG, Rojas E, et al. Bone disease after renal transplantation. Clin J Am Soc Nephrol. 2006;1:1300–1313.
- Messa P, Cafforio C, Alfieri C. Clinical impact of hypercalcemia in kidney transplant. Int J Nephrol. 2011;2011:906832.
