Abstract
Purpose: To retrospectively evaluate the accuracy of a novel software platform for assessing completeness of percutaneous thermal ablations.
Materials & methods: Ninety hepatocellular carcinomas (HCCs) in 50 patients receiving percutaneous ultrasound-guided microwave ablation (MWA) that resulted in apparent technical success at 24-h post-ablation computed tomography (CT) and with ≥1-year imaging follow-up were randomly selected from a 320 HCC ablation database (2010–2016). Using a novel volumetric registration software, pre-ablation CT volumes of the HCCs without and with the addition of a 5 mm safety margin, and corresponding post-ablation necrosis volumes were segmented, co-registered and overlapped. These were compared to visual side-by-side inspection of axial images.
Results: At 1-year follow-up, CT showed absence of local tumor progression (LTP) in 69/90 (76.7%) cases and LTP in 21/90 (23.3%). For HCCs classified by the software as “incomplete tumor treatments”, LTP developed in 13/17 (76.5%) and all 13 (100%) of these LTPs occurred exactly where residual non-ablated tumor was identified by retrospective software analysis. HCCs classified as “complete ablation with <100% 5 mm ablative margins” had LTP in 8/49 (16.3%), while none of 24 HCCs with “complete ablation including 100% 5 mm ablative margins” had LTP. Differences in LTP between both partially ablated HCCs vs completely ablated HCCs, and ablated HCCs with <100% vs with 100% 5 mm margins were statistically significant (p < .0001 and p = .036, respectively). Thus, 13/21 (61.9%) incomplete tumor treatments could have been detected immediately, were the software available at the time of ablation.
Conclusions: A novel software platform for volumetric assessment of ablation completeness may increase the detection of incompletely ablated tumors, thereby holding the potential to avoid subsequent recurrences.
Introduction
One essential factor affecting the success of thermal ablation is the ability to incorporate the whole tumor into the necrosis volume and create an adequate safety (or ‘peri-ablational’) margin [Citation1,Citation2], measured as the distance from the initial tumor boundaries to the border of the post-treatment ablation zone [Citation3]. Indeed, it has been determined that whenever the size of the ablation zone (defined as homogeneously non-enhancing attenuation volume) is larger than the size of the original tumor volume, with a concentric safety margin greater than 5 mm [Citation4], the ablation can be considered successful [Citation5] with an extremely low probability of local tumor progression (LTP) [Citation2,Citation6–8]. Moreover, ablation is usually considered complete when no LTP (defined as residual or new area of contrast enhancement in arterial phase, marginally or internally to the ablation zone with early wash-out [Citation9]) is detectable on at least 1-year follow-up CT scan [Citation3].
Despite the importance in determining accurate post-ablation margins for all three dimensions of a treated tumor, few studies have reported methods for the assessment of ablative margins. In the earliest report, pre-ablation scans achieved with CECT or CEMRI were fused with post-ablation scans achieved with CEUS using built-in software of US machines for a rough manual measurement of the ablative margin [Citation10]. In two other studies, ablative margin assessment was performed by side-by-side juxtaposition of pre- and post-ablation CT scans with automatic software rigid registration and final manual adjustments [Citation11–12]. While these studies represented advances towards a more quantitative approach to ablation assessment, such an approach not only potentially permits ambiguity in measurement, but is also rather challenging even for experienced interventional oncologists, given the need to fully compare the pre- and post-ablation findings in all three dimensions.
In an attempt to surmount these issues, for this study, a software has been developed for segmentation, rigid and non-rigid co-registration and volume analysis (Ablation-fitTM, R.A.W. Srl, Milan, Italy) for the assessment of ablation completeness or the prediction of occurrence and exact location of LTP and report upon an initial retrospective use of this platform in 90 HCCs from 50 patients who were treated with microwave ablation (MWA) and followed for a minimum of 1 year. Thus, the aim of the study is to retrospectively evaluate the accuracy of a novel software platform for assessing completeness of percutaneous thermal ablations.
Materials and methods
Institutional Review Board approval was waived for this retrospective study; every patient signed an informed consent before ablation.
Inclusion criteria for this study were: (A) MWA of one or more HCC nodules (0.3–5 cm); (B) pre-ablation, 24 h post-ablation and 12-m follow-up CT scans; and (C) technical success (tumor covered completely by the ablation zone [Citation3]) based upon the 24-h post-ablation CT by 2 radiologists with more than 15 years of experience (-initials blinded for review-, LS, TI).
The cohort was comprised of 90 HCCs with imaging and/or histologic diagnosis in 50 patients (median age = 72 years, range = 61–86 years, females/males = 11/39) randomly selected using an online random number generator paired to the ordinal numbers of patients’ database. Tumor size was 2.7 cm ± 2 cm (mean ± standard deviation). Six (6.5%) HCCs had maximum axial diameter <1 cm, 46 (51%) from 1.1 to 2 cm, 27 (30%) from 2.1 to 3 cm and 11 (12.5%) from 3.1 to 4.5 cm. Forty one patients had HCV-related cirrhosis, 2 HBV-related cirrhosis and the remaining 7 steatohepatitis.
All tumors were ablated with high-power (140 W, 2.45 GHz) MW generator (AMICA, HS Hospital Service, Aprilia, Italy) with 14-gauge, internally cooled, coaxial antennas based upon recommended manufacturer guidelines (40–70 Watt power, 3–12 min time) [Citation13,Citation14]. Procedures were performed under ultrasound (US) guidance using moderate analgesic sedation (i.e. medazolam, fentonyl, and propofol administered by a licensed anesthesiologist).
Pre- and post-ablation CT examinations were performed with a 40-slice scanner (Somatom Sensation 40, Siemens, Healthcare, Erlangen, Germany) after intravenous injection of 100–120 ml of contrast agent (Iomeron 350, Bracco Imaging, Milan, Italy). Scans were obtained during expiration breath-hold, in arterial (using a bolus-tracking technique; 15–25 s delay), portal-venous (75 s) and delayed (180 s) vascular phases, with the following scanning parameters: 120 kVp, 200 mA with care dose 4 D, 1.5–5 mm slice thickness.
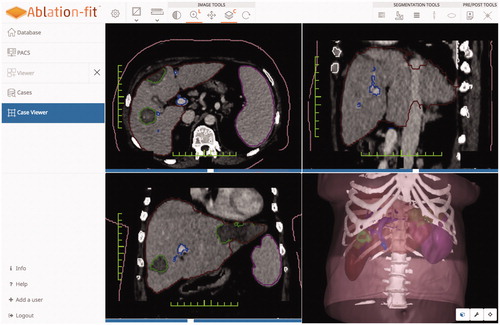
The platform (Ablation-fitTM, R.A.W. Srl, Milan, Italy) is a stand-alone software aimed at planning, verifying and managing cross-sectional images of patients undergoing interventional oncology procedures. Using CT scans in DICOM format, the software automatically segments and reconstructs hepatic parenchyma and its blood vessels, spleen, and ribs, and semi-automatically segments and reconstructs both focal liver lesions and ablation induced necrosis showing them with differentiated colors (), The software contours all of this anatomic information not only in axial, sagittal, and coronal planes for 2 D visualization, but also three-dimensionally. After automatic or semi-automatic segmentation, it is possible to manually modify each organ segmentation in the 2 D axial visualization and consequently the three-dimensional mapping of the scan changes accordingly.
Figure 1. Result of organs’ segmentations: 2D contours in the axial, sagittal and coronal views and 3D reconstructions.
Once the ablation procedure has been performed, pre- and post-treatment scans are automatically registered using rigid and non-rigid registration: the first one to recover rotation and translation differences between pre and post-treatment images, the second one in order to account for non-rigid liver deformations due to patient’s respiration and movement [Citation15,Citation16].
The registration method is implemented using Insight Toolkit (ITK) libraries and Elastix toolbox [Citation17,Citation18]. Normalized Mutual Information was used for registration. Non-linear registration is based on B-splines [Citation17]. Consequently, the software is capable of verifying whether the necrosis entirely surrounds the tumor and the safety margin. An example of 3 D ablation outcomes in shown in . Moreover, calculations of residual unablated volumes of both the target tumors and pre-determined safety margins are instantly and automatically provided (). The calculation of the percentage of ablative margin is: (the volume of the calculated 5 mm ablative margin outside of the achieved ablation zone divided by the calculated volume of 5 mm total ablative margin)*100. Reconstructions and volume analysis were made applying the scientific research version of Ablation-fitTM retrospectively to the pre- and post-ablation CT scans, subsequently to the acquisition of the 1-year follow-up CT scan. A 3 D ablative margin of 5 mm was manually selected, based upon prior literature [Citation2,Citation6].
Figure 2. Categorization of post-ablation outcomes. Top left: Tumor (inner part) entirely included in the necrosis (outer part). Top right: Tumor (inner part) and 5 mm safety margin (middle part) entirely included in the necrosis (outer part). Bottom left: Tumor (inner part) entirely included in the necrosis (outer part), 5 mm safety margin (middle part) partially included in the necrosis. Arrow indicates the safety margin region not included in the necrosis. Bottom right: Tumor (inner part) and 5 mm safety margin (middle part) partially included in the necrosis (outer part). Arrows indicate the tumor region and the safety margin region not included in the necrosis.
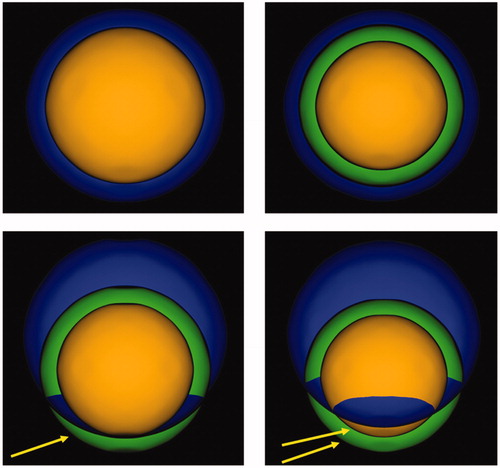
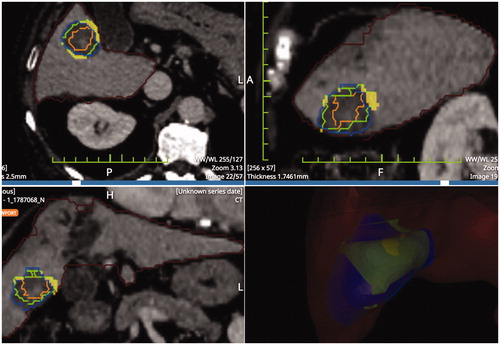
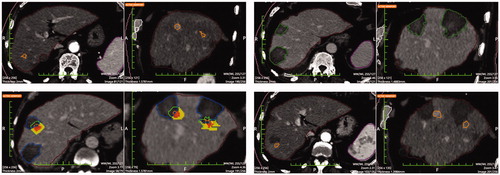
Figure 3. Result of pre- post-ablation registration: HCCs is the inner part, 5 mm margins (middle part) coagulation zone (outer part). The solid portion outside the necrosis is the unablated safety margin. Calculations rendered are: residual unablated nodule 0%, residual unablated 5 mm margin 7.8%.
In the retrospective analysis using the Ablation-fitTM software, results were classified into five categories: incomplete tumor treatment, or complete tumor ablation while achieving either 100%, 90–99%, 50–89%, or 0–49% of the intended 5 mm ablative margin, respectively. The size of the HCCs belonging to each category is reported in . The variables among the categories were compared and significance established using Student’s t-test, Fisher’s exact test, and Chi-square tests. Statistical analysis was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Moreover, correspondence between the location of the local progression and suspect location of incomplete treatment with the software was evaluated.
Table 1 Size of HCCs in the 1-yr follow-up CT and in the five categories of Ablation-fitTM analysis.
Results
At 1-year follow-up, CT showed absence of local tumor in 69/90 (76.7%) cases and local tumor progression in 21/90 (23.3%).
In the retrospective analysis using the Ablation-fitTM software, complete tumor ablation was achieved for 73/90 (81.1%) HCCs. In 24/73 (32.9%) of these, 100% of the desired 5 mm margin was ablated. On 1-year follow-up CT none of them developed LTP (0/24) (. 90–99% of the 5 mm margin was ablated for 17/73 (23.3%) HCCs with 1-year follow-up CT demonstrating LTP in only one of 17 (5.9%) HCCs. Complete tumor ablation with a 50–89% or 0–49% 5 mm margin ablated were noted for 30/73 (41.1%) and 2/73 (2.7%) HCCs, respectively. On 1-year follow-up CT, LTPs occurred in 5/30 (16.7%) and 2/2 (100%) cases, respectively.
Figure 4. Top left: Pre-ablation HCC segmentation. Top right: Post-ablation segmentation of the necrosis volume. Bottom left: pre- post-ablation registration; the residual unablated nodule percentage is 0% and the residual unablated 5 mm margin is 12.5% (the solid portion outside the necrosis is the unablated safety margin). Bottom right: 1-year follow-up CT with necrosis segmentation showing no-LTP.
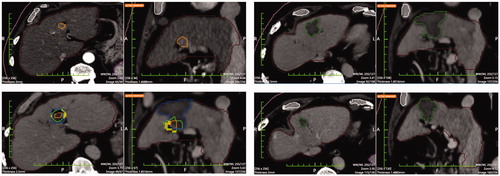
‘Incomplete tumor treatment’ (i.e. software demonstration that portions of the HCCs were not enveloped within the volume of necrosis) occurred in 17/90 (18.9%) HCCs. On 1-year follow-up CT, LTP was detected in 13/17 (76.5%) of these cases. Moreover, all 13 (100%) of these LTPs occurred in the exact location where residual non-ablated tumor was identified by Ablation-fitTM (. Ablation-fitTM software had a sensitivity of 62% and a specificity of 94%.
Figure 5. Top left: Pre-ablation segmentation of two HCCs in the same patient. Top right: Post-ablation segmentation of the coagulation volumes achieved. Bottom left: pre- post-ablation registration. Calculations rendered are the following: residual unablated tumor 78.9% and 100% respectively and residual unablated 5 mm margin 77.6% and 93.0% (the inner solid portion outside the necrosis is the unablated tumor and the outer solid portion outside the necrosis is the unablated safety margin). Bottom right: 1-year follow-up CT with LTPs segmentations. LTPs developed in the exact location where residual unablated tumor was shown in the pre-post-ablation registration (bottom left image).
The difference in LTP occurrence among the five groups was statistically significant (both overall Chi-square and Fisher’s exact tests with p < .01). A statistically significant worse outcome in LTP was noted between HCCs ablated without a margin vs. any margin achieved (p < .01 all comparisons). Moreover, achieving a 100% 5 mm margin produced better outcomes than <90% 5 mm margins (p = .036 and p < .01, for 50–90% and <50%, respectively). However, results were not statistically significant between achieving a 90–100% 5 mm margin and ablation of the entire margin (p < .24). In addition, no statistically significant correlation between size of HCC and outcome of ablation was found both on the 1-year follow-up CT and on the retrospective assessment with Ablation-fitTM (; Chi-square = 0.585; p = 1).
Discussion
In this retrospective analysis it has been demonstrated that using a segmentation/registration software it is possible to achieve detection of undertreated tumor leading to a LTP in the follow up studies. Main causes of LTP may be untreated satellite lesions, too small to be detected on imaging prior to ablation, or insufficient ablation margins. Regardless, the need for achieving complete treatment in a single ablation session, thereby avoiding local re-treatments is paramount and further justified by recent studies that demonstrate adverse effects caused by incomplete treatments and persistence of tumor remnants [Citation19–21]. Indeed, thermal ablation can activate hepatocyte growth factor/c-Met pathway and vascular endothelial growth factor stimulation, prompting distant tumor growth and leading to aggressive recurrences [Citation22,Citation23]. In addition, for currently unclear mechanisms, an insufficient treatment of HCC by ablation that enables survival of some cells can induce further malignant transformation [Citation24]. As a consequence, the aim of any ablation treatment should be to kill the totality of malignant cells in one session, through the achievement of ablation volumes exceeding the target lesion volume of at least 5 mm in all three-dimensions. Documentation of intra-procedural treatment adequacy could potentially reduce LTP and recurrence rates [Citation2,Citation4,Citation6].
Nowadays, the most common method to assess the technical success of ablations is subjective matching of the imaging scans acquired before and after ablation. The pre- and post-ablation scans are loaded on to two different screens, the central slices of the tumor and of the necrosis are identified with visual inspection by the clinician to determine whether the necrosis entirely surrounds the tumor [Citation25]. Nonetheless, this assessment is sometimes cumbersome and not objective, as it relies just on operator expertise, and suffers from a lack of 3 D representation. As a consequence, other methods for the assessment of treatment outcome have been developed. Intraoperative contrast-enhanced ultrasound (CEUS) (or CEUS fused with CT [Citation12]) led to good results [Citation26–29], but it suffers from the low spatial resolution of sonography and the lack of 3 D representation. Registration and fusion of other imaging techniques (e.g. CT, MRI and PET [Citation30–33]) and coronal reformations using modern CT machines [Citation34] have been used.
The clinical use of Ablation-fitTM software can offer significant advantages for the assessment of the outcome of thermal ablations. Indeed, this study confirmed that even highly experienced interventional oncologists overestimated the number of technical successes achieved as Ablation-fitTM showed that 17/90 (18.9%) of those cases initially thought of as adequately treated were not even entirely ablated, and actually in 13 of these 17 cases (76.5%) LTP developed at 1-year following CT scan. A key point of Ablation-fitTM software is that the registration algorithms are fully automatic and fast (less than 3 min to complete the entire procedure) and take into account liver deformations and different breathing phases between pre- and post-treatment CT scans. Furthermore, deformable registration is applied, giving both 2 D and 3 D visual representation of the treatment outcome and calculations of the residual tumor volume plus residual ablative margin volume. Hence, unlike systems previously described in the literature, the described software tool allows one to change from a standard 2 D subjective evaluation based upon a two-screens slice-by-slice comparison of pre- and post-ablation scans to an objective evaluation of the outcome of treatments based upon a 3 D comparison of pre- and post-ablation scans. Moreover with appropriate modifications similar registration strategies could be employed for other modalities such as cone beam CT [Citation15].
The extent or three-dimensional completeness of ablative margins achieved has been clearly demonstrated to be a crucial determinant of the outcome of ablations. As it has been shown previously for HCCs and colorectal metastases [Citation4,Citation6,Citation8–10,Citation12,Citation13] using conventional evaluation techniques, a statistically significant difference between complete tumor ablation with less than 100% margin and complete tumor ablation including 100% margin has been demonstrated – even when using the smallest ablative margin currently considered acceptable (i.e. 5 mm). Additionally, in this study, sub-set statistical analysis demonstrated completeness of the ablative margin turned out to be more strictly related to the ablation outcome than to the size of HCCs both on the 1-year follow-up CT and on the retrospective assessment with Ablation-fitTM. Moreover, the accuracy of Ablation-fitTM software was further proven as there was exact spatial correspondence between the site of incomplete ablation clearly depicted retrospectively by the software and the site of LTP development on the 1-year follow-up CT.
Although these results are promising, future studies must include defining the optimal ablation margin, which may very well be different for different tumors and organs. Likewise, it will be important to take into consideration other effects of MW ablation on surrounding tissues that may alter the amount of measured coagulation. Most specifically, several previous studies [Citation35–37] demonstrate in ex-vivo tissues that microwave ablation produces significant tissue contraction particularly in the central ablation zone and in the radial direction, thus making ablation zones measured post-treatment markedly smaller than the pretreatment tissue dimensions. Additionally, some (but likely less) shrinkage has been reported for RF ablation [Citation38]. Thus, although actual shrinkage parameters are not fully elucidated for clinical practice, the size of the ablation volumes calculated by the software may be underestimated, with the value of the 5 mm margin selected for this study consequently representing a larger effective area of treatment: this could also explain why in this study the difference in outcomes between achieving a 90–100% 5 mm margin and ablation of the entire margin was not statistically significant.
Limitations of this study are mainly due to its retrospective nature and to a selected cohort of only apparently technically successful cases. The results of this study will need confirmation from future prospective studies in larger and more varied cohort of cases covering a wide range of tumor types, organs, and ablation devices. Likewise, this study only evaluated CT imaging follow-up. Thus, the development of Ablation-fitTM software that will enable to also segment and non-rigidly register pre- and post-ablation MRI scans in DICOM format is in the process.
In conclusion, this retrospective evaluation demonstrates that if Ablation-fitTM software could have been used at the time of ablation, a significant percentage of incomplete tumor treatments could have been detected immediately at the time of treatment. Further studies are needed to verify the potential for software detection of undertreated tumors with CT scans acquired immediately after ablation.
Statement of informed consent
Informed consent was obtained from all individual participants included in the study.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Disclosure statement
Marco Solbiati, Alessandro Rotilio, Katia M Passera and Ilaria Marre are employees of R.A.W. Srl and developers of the described technique. Riccardo Muglia, Tiziana Ierace, and Luigi Solbiati declare they have no conflict of interest to disclose. S. Nahum Goldberg performs unrelated consulting for Angiodynamics and Cosman Instruments. The authors declare they have received no funding.
References
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29:268–275.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. JVIR. 2014;25:1691–1705.
- Teng W, Liu K-W, Lin C-C, et al. Insufficient ablative margin determined by early computed tomography may predict the recurrence of hepatocellular carcinoma after radiofrequency ablation. Liver Cancer. 2015;4:26–38.
- Park M, Rhim H, Kim Y, et al. Spectrum of CT findings after radiofrequency ablation of hepatic tumors. Radiographics. 2008;28:379–390.
- Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–488.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175.
- Kim Y-S, Lee WJ, Rhim H, et al. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758–765.
- Filippiadis DK, Kelekis NL. Percutaneous radiofrequency ablation for the treatment of hepatocellular carcinoma: long-term follow up, efficacy and prognostic factors. Ann Gastroenterol. 2013;26:368–370.
- Bo X-W, Xu H-X, Guo L-H, et al. Ablative safety margin depicted by fusion imaging with post-treatment contrast-enhanced ultrasound and pre-treatment CECT/CEMRI after radiofrequency ablation for liver cancers. BJR. 2017;90: 20170063.
- Makino Y, Imai Y, Igura T, et al. Utility of computed tomography fusion imaging for the evaluation of the ablative margin of radiofrequency ablation for hepatocellular carcinoma and the correlation to local tumor progression. Hepatol Res. 2013;43:950–958.
- Tomonari A, Tsuji K, Yamazaki H, et al. Feasibility of fused imaging for the evaluation of radiofrequency ablative margin for hepatocellular carcinoma. Hepatol Res. 2013;43:728–734.
- Amabile C, Ahmed M, Solbiati L, et al. Microwave ablation of primary and secondary liver tumors: ex vivo, in vivo, and clinical characterization. Int J Hyperthermia. 2017;33:34–42.
- Cavagnaro M, Amabile C, Bernardi P, et al. A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans Biomed Eng. 2011;58:949–959.
- Solbiati M, Passera KM, Goldberg SN, et al. A novel CT to cone-beam registration method enables immediate real-time intraprocedural three-dimensional assessment of ablative treatments of liver malignancies. Cardiovasc Intervent Radiol. 2018;41:1049–1057.
- von Siebenthal M, Székely G, Lomax AJ, et al. Systematic errors in respiratory gating due to intrafraction deformations of the liver. Med Phys. 2007;34:3620–3629.
- Klein S, Staring M, Murphy K, et al. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205.
- Shamonin DP, Bron EE, Lelieveldt BP, et al. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer's disease. Front Neuroinform. 2014;7:50.
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2007;47:82–89.
- Yan K, Chen MH, Yang W, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–347.
- Lee HY, Rhim H, Lee MW, et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23:190–197.
- Ahmed M, Kumar G, Moussa M, et al. Hepatic radiofrequency ablation-induced stimulation of distant tumor growth is suppressed by c-Met inhibition. Radiology. 2016;279:103–117.
- Kang TW, Lim HK, Cha DI. Aggressive tumor recurrence after radiofrequency ablation for hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:95–101.
- Obara K, Matsumoto N, Okamoto M, et al. Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol Int. 2008;2:116–123.
- Zhang NN, Lu W, Cheng XJ, et al. High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015;70:1237–1243.
- Claudon M, Dietrich CF, Choi B, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210.
- Guibal A, Bertin C, Egels S, et al. Contrast-enhanced ultrasound (CEUS) follow-up after radiofrequency ablation or cryoablation of focal liver lesions: treated-area patterns and their changes over time. Eur Radiol. 2013;23:1392–1400.
- Persichetti A, Bizzarri G, Guglielmi R, et al. Ultrasound-guided laser ablation for local control of neck recurrences of medullary thyroid cancer. A feasibility study. Int J Hyperthermia. 2018;1–5. doi: 10.1080/02656736.2018.1508759.
- Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209–216.
- Meloni MF, Andreano A, Zimbaro F, et al. Contrast-enhanced ultrasound: roles in immediate post-procedural and 24-h evaluation of the effectiveness of thermal ablation of liver tumors. J Ultrasound. 2012;15:207–214.
- Di Mauro E, Solbiati M, De Beni S, et al. Virtual navigator real-time ultrasound fusion imaging with positron emission tomography for liver interventions. Conf Proc IEEE Eng Med Biol Soc. 2013;1406–1409. doi: 10.1109/EMBC.2013.6609773.
- Giesel FL, Mehndiratta A, Locklin J, et al. Image fusion using CT, MRI and PET for treatment planning, navigation and follow up in percutaneous RFA. Exp Oncol. 2009;31:106–114.
- Mauri G, Cova L, De Beni S, et al. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143–151.
- Motoyama T, Ogasawara S, Chiba T, et al. Coronal reformatted CT images contribute to the precise evaluation of the radiofrequency ablative margin for hepatocellular carcinoma. Abdom Imaging. 2014;39:262–268.
- Amabile C, Farina L, Lopresto V, et al. Tissue shrinkage in microwave ablation of liver: an ex vivo predictive model. Int J Hyperthermia. 2017;33:101–109.
- Farina L, Weiss N, Nissenbaum Y, et al. Characterization of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia. 2014;30:419–428.
- Liu D, Brace CL. CT imaging during microwave ablation: analysis of spatial and temporal tissue contraction. Med Phys. 2014;41:113303–113309.
- Ganguli S, Brennan DD, Faintuch S, et al. Immediate renal tumor involution after radiofrequency thermal ablation. J Vasc Interv Radiol. 2008;19:412–418.
